Abstract
Arterial wave reflections have been associated with mortality in an ethnically homogenous Asian population. It is unknown whether this association is present in a multiethnic population or whether it is independent of subclinical atherosclerosis. We hypothesized that reflection magnitude (RM, defined as the ratio of the amplitude of the backward wave [Pb] to that of the forward wave [Pf]) is associated with all-cause mortality in a large multiethnic adult community-based sample. We studied 5984 participants enrolled in the Multi-Ethnic Study of Atherosclerosis who had analyzable arterial tonometry waveforms. During 9.8±1.7 years of follow-up, 617 deaths occurred, of which 134 (22%) were adjudicated cardiovascular deaths. In Cox-proportional hazards models, each 10% increase in RM was associated with a 31% increased risk for all-cause mortality (HR=1.31; 95%CI=1.11–1.55; P=0.001). This relationship persisted after adjustment for various confounders and for markers of subclinical atherosclerosis (HR=1.23; 95%CI=1.01–1.51, P=0.04), including the coronary calcium score, ankle-brachial index, common carotid intima-media thickness and ascending thoracic aortic Agatston score. Pb was independently associated with all-cause mortality in a similarly adjusted model (HR per 10 mmHg-increase in Pb=2.18, 95%CI=1.21–3.92, P=0.009). RM (HR=1.71, 95%CI 1.06–2.77, P=0.03) and Pb (HR=5.02, 95%CI=1.29–19.42, P=0.02) were mainly associated with cardiovascular mortality. In conclusion, RM is independently associated with all-cause mortality in a multi-ethnic population initially free of clinically-evident cardiovascular disease. This relationship persists after adjustment for a comprehensive set of markers of subclinical atherosclerosis.
Keywords: Reflection magnitude, mortality, wave reflections, atherosclerosis
Introduction
As the pulse wave generated by the left ventricle travels away from the heart, it is partially reflected due to interactions with the elastic and muscular arteries, generating multiple reflected waves that travel back towards the heart.1 These waves arise at multiple sites in the arterial tree, where changes in geometry and/or stiffness occur, thus being influenced by arterial properties. Reflected waves summate in transit and form a composite wave.2 When this wave arrives back at the left ventricle during late systole, it increases ventricular afterload and has been shown to adversely impact left ventricular remodeling as well as systolic and diastolic function.3–14 We have recently shown that reflection magnitude (RM), defined as the ratio of the amplitude of the backward wave to that of the forward wave, is strongly predictive of incident heart failure and is also predictive of a combined endpoint of cardiovascular events.15
Wang et al. recently used a triangular-flow approach in a homogenous population and demonstrated that the amplitude of the backward wave (Pb) was predictive of both cardiovascular and all-cause mortality in men and women.16 However, it is unknown whether this relationship is independent of atherosclerotic disease at baseline or the occurrence of incident heart failure. Furthermore, the study by Wang et al. enrolled only Asian participants.
In this study, we tested the hypothesis that reflection magnitude is a predictor of all-cause mortality in a multiethnic sample free of clinically evident cardiovascular disease at baseline, and that such a relationship is independent of subclinical atherosclerosis or clinical manifestations of heart failure in a multiethnic sample.
Methods
Study Population
The Multi-Ethnic Study of Atherosclerosis (MESA) enrolled 6,814 men and women aged 45–84 years from 6 centers across the United States to ensure inclusion of subjects from diverse ethnic backgrounds. Subjects self-reported their ethnicity as African-American, Asian-American (predominantly Chinese), Caucasian, or Hispanic. All subjects were free of cardiovascular disease by self-report at the time of inclusion. Subjects were enrolled between 2000–2002 and contacted every 9–12 months for assessment of clinical endpoints. All subjects were followed through December 31, 2011. Information was collected from death certificates, hospital medical records, and autopsy reports. In the case of out-of-hospital deaths, interviews or questionnaires were administered to physicians, relatives, or friends.17 Follow-up telephone interviews were completed in 92% of living participants, and medical records were obtained for 98% of hospital admissions.18 The study was approved by the institutional review boards of participating centers, and all participants signed informed consent.
Event Adjudication
Trained personnel abstracted data from the medical record to ascertain cardiovascular events. Cardiovascular and non-cardiovascular deaths were then adjudicated by a MESA study committee using standardized definitions (the reader is referred to web appendix from reference19). Cardiovascular deaths include death from atherosclerotic coronary heart disease (fatal myocardial infarction), stroke, deaths from vascular aneurysms, death due to valvular heart disease, death due to congestive heart failure resulting in shock or low-output states, death following a cardiac procedure such as coronary revascularization, or death due to pulmonary embolism. If none of the above causes were determined, or a strong history of another likely cause of death was present, deaths were coded as non-cardiovascular. For non-cardiovascular deaths, city or state records were sought to determine the underlying cause.
Data Collection
Standardized questionnaires were administered at the time of enrollment. Resting blood pressure was obtained in the right arm in triplicate after resting for 5 minutes in the seated position. An automated oscillometric device (Dinamap-Pro100, GE Medical Systems, Waukesha, Wisconsin) was used to obtain the readings, with the second and third readings averaged to obtain the recorded pressure. Serum cholesterol and C-reactive protein levels were obtained after a 12-hour fast.18 NT-pro-BNP levels were measured from frozen samples drawn at enrollment and stored at −70°C. Samples were analyzed using a commercially available immunoassay from Roche Diagnostics Corporation (Roche Diagnostic Elecsys proBNP Assay, Indianapolis, Indiana, USA).20 Diabetes mellitus was defined as a fasting glucose ≥126 mg/dL or use of diabetic medications. Family history of myocardial infarction was determined as occurrence in any first-degree relative (parent, sibling, child).
Hemodynamic Measurements
Radial arterial waveform recordings were obtained at the baseline visit in the supine position. In all study centers, thirty-seconds of data was recorded using the HDI/PulseWave-CR2000 tonometry device (Hypertension Diagnostics, Eagan, Minnesota) and digitized at 200 Hz for offline processing. Custom-designed software was written in MatLab (The Mathworks, Natick, Massachusetts) for analysis of waveforms and to generate an averaged waveform for each individual. A generalized transfer function was subsequently applied to radial artery pressure waveforms to arrive at the central pressure waveform.21 All pressure waveforms were visually inspected by an investigator (JAC) for quality and physiologic consistency. We excluded averaged waveforms that met any of the following criteria: (1) A non-physiologic appearance (usually from bigeminy, trigeminy, or contamination of the signal average by aberrantly recorded complexes); (2) Cardiac cycle duration variation ≥10%; (3) Pulse height (beat-to-beat pulse pressure) variation ≥20%; (4) Less than 10 adequately recorded cycles available for signal averaging. In addition, we excluded cases in which clear identification of key landmark points in the pressure waveform, required for wave separation using an averaged physiologic flow approach, was not possible.
The same physiologic flow waveform was applied to each individual’s central pressure waveform to separate the forward-traveling (Pf) and backward-traveling (reflected) (Pb) waves, as previously described in detail.22 RM was calculated as:
Assessment of Subclinical Atherosclerosis
Trained technicians performed B-mode ultrasound of both common carotid arteries. Maximum common carotid intima-media thickness (IMT) was calculated as the mean of the maximum IMT of the near and far walls bilaterally.23 Coronary artery calcium (CAC) was measured using computed tomography and referenced to a phantom of known calcium concentration that was included in the field of view. Each participant was scanned twice to determine the average phantom-adjusted Agatson score.23 During these scans, calcification within the thoracic aorta was also measured and quantified as for CAC.24 The ankle brachial index (ABI) was determined for each lower extremity using a hand-held Doppler probe. The numerator was set as the higher of the two pressures between the dorsalis pedis and posterior tibial arteries for each leg. The denominator was the higher brachial artery pressure between both arms. The lower ABI of the two lower extremities was recorded for each patient.25, 26
Statistical Methods
Baseline characteristics of the cohort are presented as mean±standard deviation (SD) or as medians with interquartile ranges as appropriate. The cohort was divided into quintiles based on RM and Pb. Kaplan-Meier mortality curves were generated and assessed using the log-rank test. We used Cox proportional hazards modeling to assess the relationship between reflection magnitude and mortality. Unadjusted Cox proportional hazards models were built to assess the risk increase for all-cause mortality, cardiovascular mortality, and non-cardiovascular mortality, per 10% change in RM. Additional models were then constructed with increasing adjustments for potential confounders. In addition, we built models that adjusted for the presence of subclinical atherosclerosis, assessed by a combination of atherosclerotic markers in different vascular territories (coronary calcium score, ankle-brachial index, common carotid intima-media thickness and ascending thoracic aortic Agatston score). To assess whether the association between reflection magnitude and death was independent of incident heart failure, we built models that censored participants at the time of the first heart failure event, thereby predicting only those individuals whose death was not preceded by clinical HF. Analogous models were also constructed for the absolute magnitude of the forward (Pf) and backward (Pb) waves. Variables that were not normally distributed were log transformed before entering the models if a multiplicative relationship with mortality was present. A two-tailed Type I error rate of ≤0.05 was considered to be statistically significant. Statistical analyses were performed using STATA 13 (StataCorp, College Station, Texas).
Results
Baseline demographic, laboratory, anthropomorphic, and clinical data of our sample are presented in Table 1. Of 6,336 participants who underwent radial tonometry, 5,989 cases had reliable data for RM computations. Follow-up information on vital status was missing in 5 subjects, leaving a final cohort size of 5984 subjects.
Table 1.
Baseline Demographic, Clinical, Laboratory, and Anthropomorphic Data for Study Participants
| Variable | Overall Population (n=5984) |
|---|---|
| Age (±SD) | 61.9±10.2 |
| Male (%) | 2877 (48%) |
| Follow-up Years, mean±SD | 9.8±1.7 |
| Average Reflection Magnitude, mean±SD | 0.84±0.05 |
| Ethnicity (%) | |
| White | 2247 (38%) |
| Black | 1630 (27%) |
| Chinese | 730 (12%) |
| Hispanic | 1382 (23%) |
| Body Measurements, mean±SD | |
| Height (cm) | 166.4±10.0 |
| Weight (kg) | 78.7±17.3 |
| BMI (kg/m2) | 28.3±5.5 |
| Family History of Heart Attack, n (%) | 2361 (42%) |
| Hypertension, JNC VI Criteria, n (%) | 2675 (45%) |
| Hypertensive Medication Use, n (%) | 2217 (37%) |
| Systolic BP (mm Hg), mean ±SD | 126.5±21.3 |
| Diastolic BP (mm Hg), mean±SD | 72.1±10.2 |
| Heart Rate (bpm), mean±SD | 64.2±10.1 |
| Current Smoking, n (%) | 2162 (36%) |
| Cholesterol Profile (mg/dL), mean±SD | |
| Total Cholesterol | 194.1±35.5 |
| LDL Cholesterol | 117.1±31.4 |
| HDL Cholesterol | 50.8±14.7 |
| Triglycerides | 132.0±83.1 |
| Statin Use, n (%) | 876 (15%) |
| Diabetes, n (%) | 764 (13%) |
| Estimated GFR (mL/min/1.732), mean±SD | 78.2±16.2 |
| Urine Albumin (μg)/Creatinine (mg) Ratio, median (IQR) | 5.3 (3.3, 10.9) |
| C-Reactive Protein (mg/L), median (IQR) | 1.88 (0.82, 4.19) |
| NT-pro-BNP (pg/mL), median (IQR) | 52.64 (23.39, 109.1) |
| Markers of Subclinical Atherosclerosis | |
| Ankle-Brachial Index, mean (±SD) | 1.12±0.12 |
| Mean Agatston Coronary Calcium Score, median (IQR) | 0 (0, 81.39) |
| Maximum Common Carotid Intima- Media Thickness mm, mean±SD | 0.87±0.19 |
| Ascending Aortic Agatston Calcium Score, median (IQR) | 0 (0, 0) |
IQR=interquartile range; SD=standard deviation
Subjects were followed for a mean of 9.8±1.7 years (range: 62 days–11.5 years). A total of 617 deaths occurred over the follow-up period, of which 134 (22%) were adjudicated cardiovascular deaths and 460 (75%) were non-cardiovascular (n=327); 23 (4%) deaths were of unknown etiology. Further information regarding cause of death can be found in the supplemental table (Table S1). Four thousand nine-hundred and forty-six (83% of the subjects included in these analyses) had a baseline determination of NT-pro-BNP.
Reflection magnitude and mortality
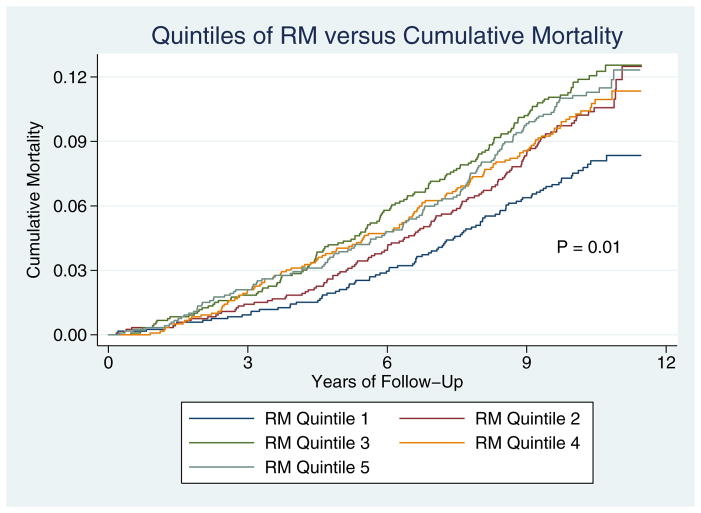
Kaplan-Meier mortality curves for participants in each of the reflection magnitude quintiles are shown in Figure 1. Mortality was significantly different among the five groups (Figure 1, Log-rank test P=0.01).
Figure 1.
Kaplan-Meier Mortality Curves for participants, stratified according to quintiles of reflection magnitude.
Results of proportional hazards models for RM are shown in Table 2. In unadjusted analysis (Model 1), each 10% increase in reflection magnitude was associated with a 31% increase in the risk of all-cause mortality (HR=1.31; 95% CI=1.11–1.55; P=0.001). This was not affected by adjustment for heart rate (Model 2). Similarly after additional adjustment for demographic, clinical, laboratory data, and markers of subclinical atherosclerosis (Model 4), including ankle-brachial index, maximum common carotid intima-media thickness, coronary calcium Agatston score, ascending aortic Agatston calcium score, reflection magnitude remained independently associated with all-cause mortality (HR 1.23, 95% CI 1.01–1.51, P= 0.04). Finally, adjustment was made for NT-pro-BNP in the subset of subjects who had levels drawn at baseline (Model 5). In this model, which included only 4005subjects, the HR for RM did not change appreciably, although the P-value was not nominally significant (HR 1.22, 95%CI 0.98–1.52; P=0.07).
Table 2.
Hazard Ratios and Confidence Intervals for each 10% increase in Reflection Magnitude and Death in Unadjusted and Adjusted Models.
| Model | All-Cause Mortality (617 deaths) | Cardiovascular Mortality (134 deaths) | Non-Cardiovascular Mortality (460 deaths) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | Confidence Interval | P-value | Hazard Ratio | Confidence Interval | P-value | Hazard Ratio | Confidence Interval | P-value | |
| Model 1* | 1.31 | 1.11–1.55 | 0.001 | 1.61 | 1.12–2.31 | 0.01 | 1.28 | 1.06–1.55 | 0.01 |
| Model 2† | 1.32 | 1.12–1.56 | 0.001 | 1.61 | 1.13–2.29 | 0.009 | 1.29 | 1.07–1.56 | 0.008 |
| Model 3‡ | 1.18 | 0.97–1.44 | 0.09 | 1.37 | 0.89–2.11 | 0.15 | 1.18 | 0.94–1.48 | 0.14 |
| Model 4§ | 1.23 | 1.01–1.51 | 0.04 | 1.55 | 0.99–2.44 | 0.06 | 1.21 | 0.96–1.53 | 0.11 |
| Model 5|| | 1.22 | 0.98–1.52 | 0.07 | 1.71 | 1.06–2.77 | 0.03 | 1.16 | 0.90–1.49 | 0.26 |
Model 1 – unadjusted (n=5984, 617 total deaths)
Model 2 – adjusted for heart rate (n=5984, 617 total deaths)
Model 3 – Model 2 + adjustment for age, gender, ethnicity, systolic and diastolic blood pressure, eGFR, urinary albumin/creatinine ratio, total cholesterol, LDL cholesterol, HDL cholesterol, treatment with antihypertensive medications, treatment with statins, current smoking status, BMI, family history of myocardial infarction, diabetes mellitus, C-reactive protein, highest level of education, family income, alcohol use, total calories per day, percent of calories from fat, physical activity (n=4825, total 473 deaths)
Model 4 – Model 3 + adjustment for ankle-brachial index, maximum common carotid intima-media thickness, mean phantom-adjusted Agatston coronary calcium score, ascending thoracic aortic Agatston calcium score (n=4762, 464 deaths)
Model 5 – Model 4 + NT-pro-BNP (n=4005, total 394 deaths)
Associations between reflection magnitude and cardiovascular and non-cardiovascular deaths are also shown in Table 2. While the number of events was small (134 cardiovascular deaths, 460 non-cardiovascular deaths), RM was independently associated with cardiovascular mortality in the adjusted models. No significant relationship between RM and non-cardiovascular mortality persisted after adjustments.
Pb vs. Pf and Mortality
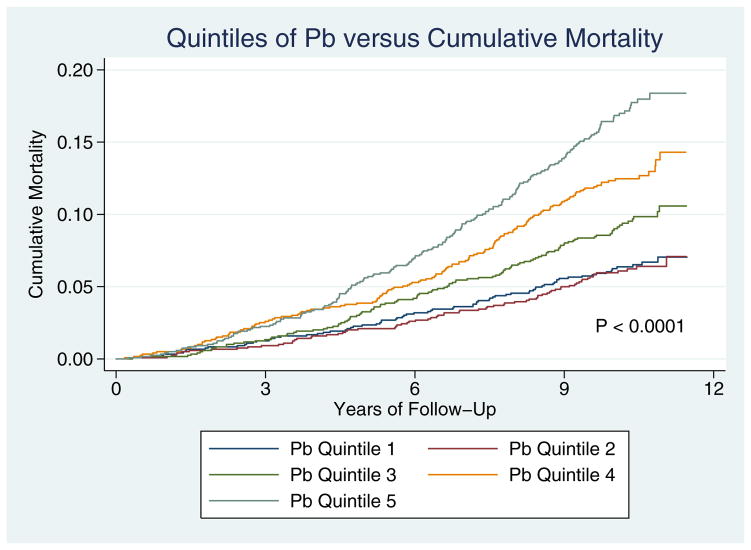
Table 3 presents various models in which the backward (Pb) and forward (Pf) waves were included as separate terms as predictors of all-cause mortality. Hazard ratios are expressed for each 10 mmHg-increase in the amplitude of Pb and Pf. In unadjusted analyses, each 10 mmHg-increase in Pb amplitude resulted in more than a doubling of the risk for all-cause mortality (Model 1, HR=2.02; 95%CI=1.27–3.22; P=0.003). Pb was independently predictive of all-cause death when subsequent adjustments were performed for heart rate (Model 2, HR=2.42; 95%CI=1.51–3.87; P<0.001), clinical, demographic, and laboratory data (Model 3; HR=1.81; 95%CI=1.03–3.16; P=0.04), and markers of subclinical atherosclerosis (Model 4; HR=2.18; 95% CI=1.21–3.92; P=0.009). In the subgroup of subjects who had NT-pro-BNP drawn, Pb continued to be independently predictive of all-cause mortality in the fully-adjusted model (Model 5; HR 2.03, 95% CI=1.08–3.81, P=0.03). Importantly, in this model, Pf demonstrated a negative relationship with mortality risk (HR 0.55, 95% CI=0.32–0.93, P=0.03), indicating that it is the difference between Pf and Pb that is primarily related to mortality. Figure 2 shows mortality curves based on quintiles of Pb; mortality rates were significantly different between groups (P<0.0001).
Table 3.
Hazard Ratios and Confidence Intervals for each 10 mm Hg increase in the magnitude of the Forward (Pf) and Backward (Pb) Wave and Death in Unadjusted and Adjusted Models.
| Model | All-Cause Mortality (617 deaths) | Cardiovascular Mortality (134 deaths) | Non-Cardiovascular Mortality (460 deaths) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | Confidence Interval | P-value | Hazard Ratio | Confidence Interval | P-value | Hazard Ratio | Confidence Interval | P-value | |
| Model 1* | |||||||||
| Pb | 2.02 | 1.27–3.22 | 0.003 | 3.37 | 1.29–8.79 | 0.01 | 1.92 | 1.11–3.32 | 0.02 |
| Pf | 0.84 | 0.56–1.25 | 0.38 | 0.61 | 0.27–1.41 | 0.25 | 0.84 | 0.53–1.34 | 0.47 |
| Model 2† | |||||||||
| Pb | 2.42 | 1.51–3.87 | <0.001 | 4.50 | 1.72–11.78 | 0.002 | 2.22 | 1.28–3.85 | 0.005 |
| Pf | 0.78 | 0.52–1.16 | 0.22 | 0.55 | 0.24–1.25 | 0.15 | 0.79 | 0.50–1.27 | 0.33 |
| Model 3‡ | |||||||||
| Pb | 1.81 | 1.03–3.16 | 0.04 | 2.92 | 0.89–9.62 | 0.08 | 1.86 | 0.97–3.58 | 0.06 |
| Pf | 0.71 | 0.45–1.14 | 0.16 | 0.50 | 0.19–1.35 | 0.17 | 0.70 | 0.41–1.21 | 0.20 |
| Model 4§ | |||||||||
| Pb | 2.18 | 1.21–3.92 | 0.009 | 4.20 | 1.20–14.69 | 0.03 | 2.17 | 1.10–4.26 | 0.03 |
| Pf | 0.56 | 0.35–0.92 | 0.02 | 0.35 | 0.12–0.98 | 0.05 | 0.57 | 0.33–1.01 | 0.05 |
| Model 5|| | |||||||||
| Pb | 2.03 | 1.08–3.81 | 0.03 | 5.02 | 1.29–19.42 | 0.02 | 1.85 | 0.89–3.84 | 0.10 |
| Pf | 0.55 | 0.32–0.93 | 0.03 | 0.25 | 0.08–0.78 | 0.02 | 0.61 | 0.33–1.13 | 0.11 |
Model 1 – unadjusted (n=5984, 617 total deaths)
Model 2 – adjusted for heart rate (n=5984, 617 total deaths)
Model 3 – Model 2 + adjustment for age, gender, ethnicity, systolic blood pressure, eGFR, urinary albumin/Cr ratio, total cholesterol, LDL cholesterol, HDL cholesterol, treatment with antihypertensive medications, treatment with statins, current smoking status, BMI, family history of myocardial infarction, diabetes mellitus, C-reactive protein, highest level of education, family income, alcohol use, total calories per day, percent of calories from fat, physical activity (n=4825, total 473 deaths)
Model 4 – Model 3 + adjustment for ankle-brachial index, maximum common carotid intima-media thickness, mean phantom-adjusted Agatston coronary calcium score, ascending thoracic aortic Agatston calcium score (n=4762, 464 deaths)
Model 5 – Model 4 + NT-pro-BNP (n=4005, total 394 deaths)
Figure 2.
Kaplan-Meier Mortality Curves for participants, stratified according to quintiles of Pb.
Models were also created for cardiovascular and non-cardiovascular mortality (Table 3). Each 10 mm Hg increase in Pb amplitude was associated with an increased risk for cardiovascular mortality (Model 5: HR 5.02, 95%CI 1.29–19.42, P=0.02). In the same model, each 10 mm Hg increase in Pf was associated with a decreased risk: HR 0.25, 95% CI=0.08–0.78, P=0.02). Interestingly, a relationship between Pb and non-cardiovascular mortality was also evident, though non-significant in some of the adjusted models.
Heart Failure
Given the known association between reflected waves and heart failure, additional models were created that censored individuals at the time of the development of incident heart failure (n=208). In the adjusted model, RM was no longer predictive of total mortality (HR 1.16, 95%CI 0.94–1.44; P=0.17). Pb, on the other hand, remained significantly predictive of all-cause mortality (HR 1.91, 95% CI 1.02–3.57, P=0.04). This relationship was attenuated when NT-pro-BNP levels were added to the model (Each 10mm Hg increase in Pb: HR 1.72, 95%CI 0.88–3.38, P=0.11), though the power for this analysis was markedly decreased due to the number of subjects who had NT-pro-BNP levels drawn (Table 4).
Table 4.
Association between RM, Pb, and Pf in Models that Censor Individuals who Develop Incident Heart Failure
| Variable | Hazard Ratio | 95% CI | P-value |
|---|---|---|---|
| RM, per 10% increase | |||
| Adjusted Model* | 1.16 | 0.94–1.44 | 0.17 |
| Adjusted Model + NT-pro-BNP† | 1.13 | 0.89–1.42 | 0.32 |
| Pb, per 10 mm Hg increase | |||
| Adjusted Model‡ | 1.91 | 1.02–3.57 | 0.04 |
| Adjusted Model + NT-pro-BNP§ | 1.72 | 0.88–3.38 | 0.11 |
| Pf′, per 10 mm Hg increase | |||
| Adjusted Model‡ | 0.62 | 0.37–1.04 | 0.07 |
| Adjusted Model + NT-pro-BNP§ | 0.62 | 0.35–1.09 | 0.10 |
Adjustment for heart rate, age, gender, ethnicity, systolic and diastolic blood pressure, eGFR, urinary albumin/Cr ratio, total cholesterol, LDL cholesterol, HDL cholesterol, treatment with antihypertensive medications, treatment with statins, current smoking status, BMI, family history of myocardial infarction, diabetes mellitus, C-reactive protein, highest level of education, family income, alcohol use, total calories per day, percent of calories from fat, physical activity, ankle-brachial index, maximum common carotid intima-media thickness, mean phantom-adjusted Agatston coronary calcium score, ascending thoracic aortic Agatston calcium score (n=4762, 406 deaths)
n=4005, 343 deaths
Adjustment for heart rate, age, gender, ethnicity, systolic blood pressure, eGFR, urinary albumin/Cr ratio, total cholesterol, LDL cholesterol, HDL cholesterol, treatment with antihypertensive medications, treatment with statins, current smoking status, BMI, family history of myocardial infarction, diabetes mellitus, C-reactive protein, highest level of education, family income, alcohol use, total calories per day, percent of calories from fat, physical activity, ankle-brachial index, maximum common carotid intima-media thickness, mean phantom-adjusted Agatston coronary calcium score, ascending thoracic aortic Agatston calcium score (n=4762, 406 deaths)
n=4005, 343 deaths
Discussion
In this study, we demonstrate that in a large multi-ethnic population of adults free of clinically evident cardiovascular disease at baseline, RM independently predicts all-cause mortality. Furthermore, this association is independent of the presence of subclinical atherosclerosis at baseline. We also demonstrate that when the forward and backward (reflected) waves are assessed as predictors of death, Pb and Pf demonstrate opposite hazard ratios, indicating that it is their difference (i.e., magnitude of reflected vs. forward waves) that is primarily related to mortality. On further analyses, the relationships between RM, Pb, and Pf were most clear in their impact on cardiovascular mortality. Finally, when subjects who developed heart failure were censored, Pb remained predictive of all-cause mortality.
With each cardiac cycle, blood is ejected from the left ventricle into the arterial tree where complex hemodynamic parameters regulate the rise in arterial pressure in response to the stroke volume. As the pulse wave travels further from the heart, it encounters points of impedance mismatch (i.e. points where the opposition to pulsatile blood flow changes). When the forward-traveling pulse wave encounters such a site, a portion of the wave is reflected back towards the central aorta. Reflected waves summate during transit to form a relatively discrete composite wave arriving at the proximal aorta.27
Reflection sites along the arterial tree include arterial segments where the arterial diameter decreases, branching points, points of turn or tortuous geometry in conduit vessels, as well as the interfaces between large conduit vessels and the more distal muscular arteries.28 While the literature often describes the bifurcation with the iliac arteries as the main reflection site,28 in reality, there are myriad reflection sites throughout the circulatory system, generating many discrete waves that merge to form a discrete reflected wave.29 Therefore, RM is a composite index influenced by both central and peripheral arterial structure and function and may represent a marker of overall arterial health. In this regard, its relevance to human health may extend beyond its well-known effects on left ventricular afterload, remodeling, and function.
Previously, Wang et al. studied RM in a cohort of 1,272 Taiwanese subjects followed for a median of 15 years.16 These authors noted significant correlations between the magnitude of the reflected wave and increased left ventricular mass, carotid intima-media thickness, and decreased renal function. In this ethnically homogenous population, the magnitude of the reflected wave was also associated with increased cardiovascular mortality in both men and women. The authors conclude by stating that wave reflections may be a clinically relevant marker of vascular aging. Our study extends this work and is novel for several additional reasons: (1) We studied a large multi-ethnic population; (2) We assessed whether the association between RM, Pb, and mortality is independent of subclinical atherosclerosis, extensively assessed in multiple vascular territories (carotid intima-media thickness, ankle-brachial index, coronary and aortic calcium); (3) We assessed whether these associations are independent of the formerly reported association with incident heart failure.15
We found that every 10% increase in RM, defined as the ratio of the amplitude of the backward pressure wave to the amplitude of the forward pressure wave, was associated with a 18–32% increase in all-cause mortality, depending on the adjustments performed. This relationship was independent of relevant baseline characteristics as well as multiple other confounders. Importantly, we found that comprehensive adjustment for markers of subclinical atherosclerosis in several beds did not appreciably diminish the association between RM and mortality, suggesting that the association between RM and death is not mediated by atherosclerosis to any great extent.
The association between RM and mortality is partially mediated through its known strong association with heart failure risk. This relationship is consistent with the differential impact of late systolic load imposed on the LV by wave reflections, which has been shown to induce LV hypertrophy, fibrosis, and myocardial dysfunction.5, 9, 12, 30, 31 Given the known association between RM and heart failure,15 we performed additional analyses that censored individuals who developed incident heart failure. In these models, although the relationship between RM and total mortality was not significant, an association between Pb and mortality persisted. Overall, these analyses suggest that additional mechanisms, beyond heart failure, may partially underlie the relationship between reflected waves and total mortality. The mechanism of this association remains unknown, perhaps because the parameters that govern it remain incompletely understood.
Previous modeling studies suggest that reflection magnitude is dependent on aortic stiffness, degree of distal aortic reflection, and peripheral resistance, rather than arterial tapering.28 These models, which assume the aorta to be uniform in its properties throughout its length and contain a single reflector at its terminus at the bifurcation, are likely to be oversimplified. Other factors, including alterations in aortic geometry over its length, likely play a significant role in reflection magnitude.28 As has been suggested by other authors, reflection magnitude and Pb may be markers of arterial health and aging.16 This notion is supported by our study, which demonstrates an independent association between these parameters measured at baseline and all-cause mortality over the course of approximately a decade, even after adjustment for multiple confounders and a comprehensive assessment of subclinical atherosclerosis in various territories utilizing currently available non-invasive techniques.
Our study must be interpreted in the context of its strengths and limitations. Strengths of this study include its large multi-ethnic sample, careful follow-up and detailed event adjudication, as well as the comprehensive assessment of subclinical atherosclerosis. Limitations of this study include the small number of deaths, thus precluding further analysis by subgroups such as gender. In addition, we used a physiologic averaged flow waveform for wave separation,22 rather than measured flow. This technique only approximates true RM and likely introduced noise in our computations. This may have underestimated the association between RM and incident risk.
Perspectives
In a large multi-ethnic population of adults free of clinically evident cardiovascular disease at baseline, RM and Pb independently predicted all-cause mortality. This association was independent of the presence of subclinical atherosclerosis at baseline. The association between Pb and mortality was also independent of new-onset heart failure. RM and Pb may thus represent overall markers of arterial health. Further studies are required to assess the precise mechanisms behind their associations with all-cause mortality.
Supplementary Material
Novelty and Significance.
1. What is new?
We investigated the impact of reflected waves on all-cause mortality.
We studied a multiethnic population, free of incident cardiovascular disease.
We demonstrate that the association between reflected waves and mortality is independent of atherosclerosis.
The magnitude of the backward wave is likely more important than the overall ratio.
What is relevant?
Reflected waves represent a novel marker of arterial health.
The findings help us understand the importance of the arterial system and its function, beyond measuring blood pressure alone.
Summary
Reflection waves are independently associated with all-cause mortality. This relationship persisted after adjustment for atherosclerosis.
The magnitude of the backward wave is associated with all-cause mortality even after censoring individuals at the time of a first heart failure event, implying that its association with total mortality is at least partially independent of both atherosclerosis and heart failure.
Reflected waves represent an important marker of vascular health with adverse prognostic implications.
Acknowledgments
Funding/Support: This research was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute and RR-024156 as well as National Institutes of Aging grant 1R21AG043802-01 (JAC). Dr. Zamani is supported in part by the Institute for Translational Medicine and Therapeutics of the University of Pennsylvania (Grant Number UL1RR024134 from the National Center for Research Resources) and by 5-T32-HL007843-17.
Footnotes
Additional Information: A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Additional Contributions: We thank the other investigators, the staff, and the participants of MESA for their valuable contributions.
Conflict of Interest Disclosures: Dr. Chirinos has received minor support (equipment loans) from Atcor Medical, Cardiodynamics, and APC cardiovascular.
References
- 1.Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens. 2005;18:3S–10S. doi: 10.1016/j.amjhyper.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Westerhof N, Westerhof BE. Wave transmission and reflection of waves: “The myth is in their use”. Artery Research. 2012;6:1–6. [Google Scholar]
- 3.Borlaug BA, Melenovsky V, Redfield MM, Kessler K, Chang HJ, Abraham TP, Kass DA. Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. J Am Coll Cardiol. 2007;50:1570–1577. doi: 10.1016/j.jacc.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 4.Gillebert TC, Lew WY. Influence of systolic pressure profile on rate of left ventricular pressure fall. Am J Physiol. 1991;261:H805–H813. doi: 10.1152/ajpheart.1991.261.3.H805. [DOI] [PubMed] [Google Scholar]
- 5.Fukuta H, Ohte N, Wakami K, Asada K, Goto T, Mukai S, Tani T, Kimura G. Impact of arterial load on left ventricular diastolic function in patients undergoing cardiac catheterization for coronary artery disease. Circ J. 2010;74:1900–1905. doi: 10.1253/circj.cj-10-0283. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto J, Westerhof BE, Westerhof N, Imai Y, O’Rourke MF. Different role of wave reflection magnitude and timing on left ventricular mass reduction during antihypertensive treatment. J Hypertens. 2008;26:1017–1024. doi: 10.1097/HJH.0b013e3282f62a9b. [DOI] [PubMed] [Google Scholar]
- 7.Hori M, Inoue M, Kitakaze M, Tsujioka K, Ishida Y, Fukunami M, Nakajima S, Kitabatake A, Abe H. Loading sequence is a major determinant of afterload-dependent relaxation in intact canine heart. Am J Physiol. 1985;249:H747–H754. doi: 10.1152/ajpheart.1985.249.4.H747. [DOI] [PubMed] [Google Scholar]
- 8.Iketani T, Takazawa K, Ibukiyama C. The influence of changes in loading patterns on left ventricular relaxation in humans. Jpn Circ J. 1998;62:581–585. doi: 10.1253/jcj.62.581. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi S, Yano M, Kohno M, Obayashi M, Hisamatsu Y, Ryoke T, Ohkusa T, Yamakawa K, Matsuzaki M. Influence of aortic impedance on the development of pressure-overload left ventricular hypertrophy in rats. Circulation. 1996;94:3362–3368. doi: 10.1161/01.cir.94.12.3362. [DOI] [PubMed] [Google Scholar]
- 10.Kohno F, Kumada T, Kambayashi M, Hayashida W, Ishikawa N, Sasayama S. Change in aortic end-systolic pressure by alterations in loading sequence and its relation to left ventricular isovolumic relaxation. Circulation. 1996;93:2080–2087. doi: 10.1161/01.cir.93.11.2080. [DOI] [PubMed] [Google Scholar]
- 11.Marchais SJ, Guerin AP, Pannier BM, Levy BI, Safar ME, London GM. Wave reflections and cardiac hypertrophy in chronic uremia. Influence of body size. Hypertension. 1993;22:876–883. doi: 10.1161/01.hyp.22.6.876. [DOI] [PubMed] [Google Scholar]
- 12.Saba PS, Roman MJ, Pini R, Spitzer M, Ganau A, Devereux RB. Relation of arterial pressure waveform to left ventricular and carotid anatomy in normotensive subjects. J Am Coll Cardiol. 1993;22:1873–1880. doi: 10.1016/0735-1097(93)90772-s. [DOI] [PubMed] [Google Scholar]
- 13.Wu MS, Chang CY, Chang RW, Chang KC. Early return of augmented wave reflection impairs left ventricular relaxation in aged Fisher 344 rats. Exp Gerontol. 2012;47:680–686. doi: 10.1016/j.exger.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Chirinos JA, Segers P, Rietzschel ER, De Buyzere ML, Raja MW, Claessens T, De Bacquer D, St John Sutton M, Gillebert TC. Early and late systolic wall stress differentially relate to myocardial contraction and relaxation in middle-aged adults: the Asklepios study. Hypertension. 2013;61:296–303. doi: 10.1161/HYPERTENSIONAHA.111.00530. [DOI] [PubMed] [Google Scholar]
- 15.Chirinos JA, Kips JG, Jacobs DR, Jr, Brumback L, Duprez DA, Kronmal R, Bluemke DA, Townsend RR, Vermeersch S, Segers P. Arterial wave reflections and incident cardiovascular events and heart failure: MESA (Multiethnic Study of Atherosclerosis) J Am Coll Cardiol. 2012;60:2170–2177. doi: 10.1016/j.jacc.2012.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang KL, Cheng HM, Sung SH, Chuang SY, Li CH, Spurgeon HA, Ting CT, Najjar SS, Lakatta EG, Yin FC, Chou P, Chen CH. Wave reflection and arterial stiffness in the prediction of 15-year all-cause and cardiovascular mortalities: a community-based study. Hypertension. 2010;55:799–805. doi: 10.1161/HYPERTENSIONAHA.109.139964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 18.Martin SS, Blaha MJ, Blankstein R, Agatston A, Rivera JJ, Virani SS, Ouyang P, Jones SR, Blumenthal RS, Budoff MJ, Nasir K. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease: implications for statin therapy from the multi-ethnic study of atherosclerosis. Circulation. 2014;129:77–86. doi: 10.1161/CIRCULATIONAHA.113.003625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patton KK, Heckbert SR, Alonso A, Bahrami H, Lima JA, Burke G, Kronmal RA. N-terminal pro-B-type natriuretic peptide as a predictor of incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis: the effects of age, sex and ethnicity. Heart. 2013;99:1832–1836. doi: 10.1136/heartjnl-2013-304724. [DOI] [PubMed] [Google Scholar]
- 21.Karamanoglu M, O’Rourke MF, Avolio AP, Kelly RP. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. Eur Heart J. 1993;14:160–167. doi: 10.1093/eurheartj/14.2.160. [DOI] [PubMed] [Google Scholar]
- 22.Kips JG, Rietzschel ER, De Buyzere ML, Westerhof BE, Gillebert TC, Van Bortel LM, Segers P. Evaluation of noninvasive methods to assess wave reflection and pulse transit time from the pressure waveform alone. Hypertension. 2009;53:142–149. doi: 10.1161/HYPERTENSIONAHA.108.123109. [DOI] [PubMed] [Google Scholar]
- 23.Folsom AR, Kronmal RA, Detrano RC, O’Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, Tracy RP, Watson KE, Burke GL. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA) Archives of internal medicine. 2008;168:1333–1339. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Mallah MH, Nasir K, Katz R, Takasu J, Lima JA, Bluemke DA, Hundley G, Blumenthal RS, Budoff MJ. Thoracic aortic distensibility and thoracic aortic calcium (from the Multi-Ethnic Study of Atherosclerosis [MESA]) Am J Cardiol. 2010;106:575–580. doi: 10.1016/j.amjcard.2010.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Criqui MH, McClelland RL, McDermott MM, Allison MA, Blumenthal RS, Aboyans V, Ix JH, Burke GL, Liu K, Shea S. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2010;56:1506–1512. doi: 10.1016/j.jacc.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ix JH, Katz R, Peralta CA, de Boer IH, Allison MA, Bluemke DA, Siscovick DS, Lima JA, Criqui MH. A high ankle brachial index is associated with greater left ventricular mass MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2010;55:342–349. doi: 10.1016/j.jacc.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 28.Westerhof BE, Westerhof N. Magnitude and return time of the reflected wave: the effects of large artery stiffness and aortic geometry. J Hypertens. 2012;30:932–939. doi: 10.1097/HJH.0b013e3283524932. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell GF. Arterial stiffness and wave reflection in hypertension: pathophysiologic and therapeutic implications. Curr Hypertens Rep. 2004;6:436–441. doi: 10.1007/s11906-004-0037-1. [DOI] [PubMed] [Google Scholar]
- 30.Chirinos JA, Segers P, Rietzschel ER, De Buyzere ML, Raja MW, Claessens T, De Bacquer D, St John Sutton M, Gillebert TC, Asklepios I. Early and late systolic wall stress differentially relate to myocardial contraction and relaxation in middle-aged adults: the Asklepios study. Hypertension. 2013;61:296–303. doi: 10.1161/HYPERTENSIONAHA.111.00530. [DOI] [PubMed] [Google Scholar]
- 31.Weber T, Wassertheurer S, Rammer M, Haiden A, Hametner B, Eber B. Wave reflections, assessed with a novel method for pulse wave separation, are associated with end-organ damage and clinical outcomes. Hypertension. 2012;60:534–541. doi: 10.1161/HYPERTENSIONAHA.112.194571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.