Abstract
Aims
Two previous randomized trials found an effect for bupropion in reducing methamphetamine use in the subgroup with lower frequency of methamphetamine use at baseline. This study aimed to replicate these results by comparing bupropion versus placebo in methamphetamine dependent participants with less than daily methamphetamine use at baseline.
Methods
Methamphetamine dependent volunteers reporting methamphetamine use on ≤ 29 of past 30 days were randomized to bupropion 150mg twice daily (N=41) or placebo (N=43) and outpatient counseling for 12 weeks. The primary outcome was the proportion achieving end of treatment (EOT) methamphetamine abstinence (weeks 11 and 12) for bupropion versus placebo. A post hoc analysis compared EOT abstinence by medication adherence assessed via plasma bupropion/hydroxybupropion level.
Results
There was no significant difference in EOT abstinence between bupropion (29%, 12/41) and placebo (14%, 6/43; p = 0.087). Among participants receiving bupropion, EOT abstinence was significantly higher in participants assessed as medication adherent by plasma bupropion/hydroxybupropion levels (54%, 7/13) compared to non-adherent participants (18%, 5/28; p = 0.018). Medication adherence by plasma levels was low (32%).
Conclusions
Bupropion may be efficacious for methamphetamine dependence but only in a highly selected subgroup of medication adherent participants with less than daily baseline methamphetamine use. Even a single objective “snapshot” measure of medication adherence is highly associated with treatment outcomes.
Keywords: methamphetamine, bupropion, clinical trial, medication non-adherence
Introduction
Despite multiple clinical trials of potential medications, no medication has been approved for the treatment of methamphetamine dependence [1]. Behavioral therapies such as cognitive behavioral therapy and contingency management are effective for methamphetamine dependence but response is variable [2–4]. The development of an effective medication for methamphetamine dependence could improve outcomes over existing treatments and reduce the negative health and societal consequences of methamphetamine use including transmission of HIV [5]
Bupropion is a dopamine/norepineprine re-uptake inhibitor approved for treatment of depression and cigarette smoking cessation which has pharmacologic and clinical effects that may be of benefit in methamphetamine dependence. In in vitro studies, bupropion inhibits methamphetamine-induced dopamine release via blocking access of methamphetamine to the dopamine transporter (DAT) [6] and increasing vesicular monoamine transporter-2 (VMAT-2) activity resulting in reduced cytosolic dopamine available for reverse transport on DAT [7]. Bupropion reduced methamphetamine self-administration in rats [8] and non-human primates [9] and blunted the cardiovascular and subjective effects of methamphetamine in a human lab study [10, 11]. Together, these studies suggest that bupropion may be an effective medication for methamphetamine dependence due to its ability to blunt methamphetamine-induced release of catecholamines and the associated reinforcing effects.
In addition to blunting of methamphetamine-induced catecholamine release, bupropion may normalize deficits in dopaminergic functioning seen in methamphetamine users. For example, increased VMAT-2 activity with bupropion, resulting in reductions in cytosolic dopamine accumulation and prevention of reactive oxygen species generation, may reduce the toxic effects of methamphetamine on dopaminergic neurons [7]. Bupropion also increases extracellular dopamine in the striatum and nucleus accumbens in rats [12, 13] and may counteract deficits in dopaminergic systems seen in human methamphetamine users [14]. But DAT occupancy with clinical doses of bupropion in humans is relatively low (approximately 25%) [15, 16] and bupropion failed to significantly increase striatal dopamine in a human PET study [13] suggesting that non-dopaminergic mechanisms may be responsible for bupropion’s clinical effects. Bupropion is also a non-competitive antagonist at nicotinic receptors [17]. Nicotinic antagonists reduce methamphetamine-induced dopamine release and methamphetamine self-administration in preclinical studies [18, 19] and bupropion may reduce methamphetamine use via antagonism at nicotinic receptors. These studies provide a strong rationale for bupropion as a treatment for methamphetamine dependence.
To date, two randomized, double-blind, placebo-controlled clinical trials have assessed bupropion as a treatment for methamphetamine dependence [20, 21]. Both trials failed to find an effect for bupropion in reducing methamphetamine use relative to placebo overall, but did find an effect for bupropion on methamphetamine use in a subgroup of methamphetamine dependent participants with lower baseline frequency of methamphetamine use. Additional analyses of the larger trial found an effect for bupropion except in participants with daily methamphetamine use at treatment baseline [22]. Results of these preliminary trials are encouraging but prospective replication of the observed effect for bupropion in the subgroup with less than daily baseline methamphetamine use is necessary. The objective of the current trial was to determine whether bupropion reduced methamphetamine use or increased treatment retention more than placebo when provided with outpatient cognitive behavioral therapy for 12 weeks among methamphetamine dependent participants with less than daily methamphetamine use at baseline. In addition, as recent stimulant dependence trials have reported poor medication adherence rates [23, 24], we performed a post hoc analysis of treatment outcomes and medication adherence assessed via plasma bupropion/hydroxybupropion levels.
Methods
Study activities occurred at a UCLA outpatient clinical research center in Los Angeles. All activities were approved by the UCLA IRB and an independent Data and Safety Monitoring Board. The trial was registered with ClinicalTrials.gov (NCT00833443).
Study Design
The study design was a randomized, double-blind, placebo-controlled clinical trial comparing bupropion sustained release 150 mg twice daily to matching placebo twice daily, in conjunction with weekly cognitive behavioral therapy, for 12 weeks. Participants were recruited from the community via fliers and advertising in print, radio, and online that directed interested individuals to call the research clinic via a toll free number to schedule a meeting at the clinic with a study physician to complete the informed consent process. Following completion of the informed consent process, participants completed medical and psychological assessments, including a physical exam, labs tests, and EKG, to determine study eligibility during a two-week outpatient screening period. Participants who met all eligibility criteria were then randomized to receive bupropion or placebo using an urn randomization procedure [25] to insure balance between the groups on the following factors: gender, severity of baseline depressive symptoms (Revised Hamilton Rating Scale for Depression score ≤ 17 versus >17), cigarette smoker versus non-smoker, and presence of adult Attention Deficit/Hyperactivity Disorder symptoms assessed via the Adult ADHD Clinical Diagnostic Scale – ACDS [26].
Participants visited the research clinic three times a week to provide urine samples, complete study assessments, receive medication refills, meet with study physicians, and complete cognitive behavioral therapy sessions. Following completion of the 12 week treatment period, participants visited the clinic once weekly for four weeks to complete post-medication medical and safety assessments. Study treatment was provided free of charge and participants received incentives in the form of gift cards for attending study visits.
Participants
Study participants were 84 methamphetamine-dependent, treatment-seeking volunteers who met the following eligibility criteria. Inclusion criteria: (1) 18 years of age or older; (2) meet DSM-IV-TR criteria for methamphetamine dependence; (3) seeking treatment for methamphetamine problems; (4) methamphetamine use on 29 or fewer of the past 30 days at baseline, as determined by timeline follow back; (5) willing and able to comply with study procedures, including genotyping; (6) willing and able to provide written informed consent; (7) if female, not pregnant or lactating and willing to use an acceptable method of barrier birth control (e.g. condoms) during the trial. Exclusion criteria: (1) medical condition that, in the study physician’s judgment, may interfere with safe study participation; (2) current neurological disorder or major psychiatric disorder not due to substance abuse (e.g., schizophrenia, bipolar disorder) as assessed by the SCID or a medical history which would make study compliance difficult or compromise informed consent, or past 30 days history of suicide attempts and/or current serious suicidal intention or plan as assessed by the SCID; (3) on prescription medication contraindicated for use with bupropion; (4) current dependence on cocaine, opiates, alcohol, or benzodiazepines as defined by DSM-IV-TR; (5) history of alcohol dependence within the past three years; (6) history of a seizure disorder; (7) a medical condition (such as serious head injury) that is associated with increased risk of seizures or on medication that lowers the seizure threshold; (8) history of anorexia or bulimia; (9) current hypertension uncontrolled by medication, or any other circumstances that, in the opinion of the investigators, would compromise participant safety; (10) history of sensitivity to bupropion; (11) participating in other clinical trial(s) involving medications.
Study Medication
Bupropion Sustained Release (SR) 150 mg tablets (Zyban®) were purchased from the manufacturer (GlaxoSmithKline Inc., Research Triangle Park, NC) and matching placebo tablets were prepared by Murty Pharmaceuticals Inc. (Lexington, KY). Zyban® tablets were overcoated to mask the manufacturer’s brand name logo and match the placebo tablets. Dissolution testing was performed to ensure that the coating process did not alter the release rate of the Zyban® tablets.
Study medication dosing was bupropion SR 150 mg or placebo once daily for three days followed by bupropion SR 150 mg or placebo twice daily until the final three days of the 12 week medication treatment period when the dose was again reduced to bupropion SR 150 mg or placebo once daily prior to discontinuation. Participants were dispensed two week supplies of study medication in blister packages to aid medication adherence and monitoring. Study physicians met with participants weekly to assess for adverse events, perform pill counts, collect used blister packages and dispense new medication blister packages when needed.
Cognitive Behavioral Therapy Platform
Participants met weekly with a master-level therapist for cognitive behavioral therapy (CBT) sessions. Therapists were trained to provide sessions via a manual that has been used in previous methamphetamine clinical trials [27]. To maintain fidelity of the counseling program, counselors met once weekly with one of the investigators (S.S.) to receive corrective feedback and individual clinical supervision.
Study Assessments
Urine drug screens were collected thrice-weekly from participants and analyzed qualitatively for methamphetamine-metabolites (using threshold of ≥ 300 ng/ml) via point of care immunoassay (CLIAwaived, Inc., San Diego, CA). Periodically a random sample of urine specimens was also sent for qualitative determination of methamphetamine metabolites via gas chromatography–mass spectrometry at a reference laboratory (Foundation Laboratory Inc., Pomona, CA) for quality assurance. A plasma sample was collected during week 6 of the 12 week medication treatment period and plasma bupropion and hydroxybupropion levels were determined via high-pressure liquid chromatography with ultraviolet detection (HPLC/UV) at a clinical reference laboratory (LabCorp, San Diego, CA).
The Structured Clinical Interview for the DSM-IV-TR or SCID [28] was used to assess psychiatric and substance abuse diagnoses. The ASI-Lite [29] and timeline followback [30] were used to assess substance abuse severity. Methamphetamine cravings were assessed on a visual analogue scale and methamphetamine withdrawal was quantified via the Amphetamine Cessation Symptom Assessment [31]. Depressive symptoms were assessed with the Hamilton Rating Scale for Depression [32], ADHD via the Adult ADHD Clinical Diagnostic Scale [26], and impulsivity via the Barratt Impulsiveness Scale Version 11 [33].
Outcome Measures
The primary study outcome was end of treatment methamphetamine abstinence, an outcome that is correlated with lower rates of stimulant use and improved functioning one-year post-treatment in stimulant dependence trials [34]. End of treatment abstinence was defined as none of the available urine drug screens positive for methamphetamine-metabolites during the final two weeks of treatment (weeks 11 and 12) and no more than one of the three possible urine drug screens each week missing. Participants with any urine drug screen during the final two weeks positive for methamphetamine-metabolites or missing two or more specimens in either week were considered non-abstinent. Secondary outcomes included: (1) Treatment Effectiveness Score defined as the mean number of methamphetamine negative urine drug screens for participants in the bupropion versus placebo groups and (2) treatment retention defined as the number of days from randomization/start of medication to the final study visit attended.
Data Analysis
Based on data from a previous trial of bupropion in lower frequency methamphetamine users [22], we estimated that we would need to enroll 80 participants (40 in each group) to detect a between group difference in the primary outcome, end of treatment abstinence, similar to the previous trial (30% versus 5%) with 80% power and alpha = 0.05. All data analyses used an “intention-to-treat” approach among the 84 participants randomized to active medication or placebo. Student’s t test and chi-square analyses were used to compare treatment outcomes, medication adherence rates, and frequency of adverse events between bupropion and placebo groups. Logistic and linear regression models were used to compare outcomes between bupropion and placebo controlling for age, gender, and baseline methamphetamine use. Multiple imputation based Generalized Linear Mixed Model analyses [35] were used to model the overall effect of bupropion on urine drug screen results, controlling for age, gender, time, baseline methamphetamine use, cigarette smoking status, and their interactions, as well as depressive symptoms and cigarette smoking during the trial. A post hoc comparison of outcomes for bupropion participants categorized as medication adherent via week 6 random plasma bupropion/hydroxybupropion levels (bupropion ≥ 50 ng/ml and/or hydroxybupropion ≥600 ng/ml, the minimums for the lab reference range) versus medication non-adherent (plasma levels below reference range or plasma sample missing due to participant dropped/absent during week 6) assessed the impact of medication non-adherence on trial results.
Results
Sample Characteristics
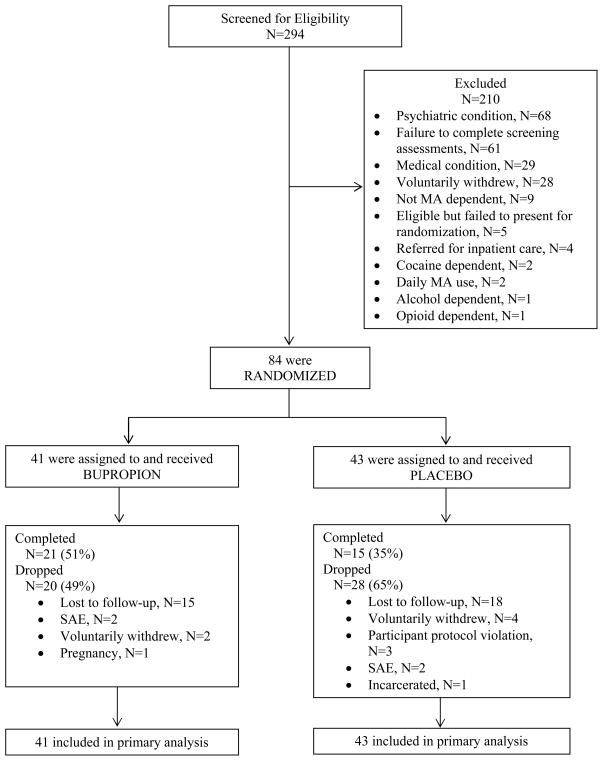
Baseline demographic and clinical characteristics of participants in the bupropion versus placebo groups are shown in Table 1. The flow of participants in the trial is shown in Figure 1.
Table 1.
Baseline demographic and clinical characteristics of methamphetamine dependent participants by treatment condition (mean (standard deviation) or percent (N)).
| Bupropion (N=41) | Placebo (N=43) | |
|---|---|---|
| Age | 38.6 (10.1) | 38.1 (10.3) |
| Gender | ||
| Male | 83% (34) | 79% (34) |
| Female | 17% (7) | 21% (9) |
| Ethnicity | ||
| Hispanic | 44% (18) | 40% (17) |
| White | 37% (15) | 30% (13) |
| African American | 17% (7) | 23% (10) |
| Asian/Pacific Islander | 2% (1) | 7% (3) |
| Days with substance use, past 30 | ||
| Methamphetamine | 10.3 (6.8) | 9.9 (6.1) |
| Marijuana | 5.8 (9.2) | 2.5 (5.8) |
| Alcohol | 4.6 (6.9) | 4.2 (7.0) |
| Cigarette Smoker | ||
| Smoker | 63% (26) | 56% (24) |
| Non-smoker | 37% (15) | 44% (19) |
| Methamphetamine Cravings, Visual Analog Scale | 49.6 (31.5) | 43.1 (33.0) |
| Amphetamine Cessation Symptom Assessment Score | 16.8 (9.8) | 13.8 (11.7) |
| Hamilton Rating Scale for Depression | 6.3 (4.8) | 6.8 (5.1) |
| ADHD | 17% (7) | 12% (5) |
| Barratt Impulsivity Scale 11, Total Score | 67.4 (11.8) | 63.6 (10.8) |
| HIV Positive (self-report) | 22% (9) | 26% (11) |
Figure 1.
CONSORT diagram depicting flow of participants
Primary Outcome: End of Treatment Methamphetamine Abstinence
There was no significant difference in the study’s primary outcome, the proportion of participants with end of treatment methamphetamine abstinence confirmed by urine drug screens during weeks 11 and 12, for bupropion (29%) versus placebo (14%, Table 2). Adjusting for age, gender, and baseline methamphetamine use frequency did not alter the result. Of the 29 (71%) bupropion participants categorized as non-abstinent at end of treatment, 9 (22%) had a urine drug screen positive for methamphetamine in weeks 11/12 and 20 (49%) were assumed to be non-abstinent due to missing urine drug screens in weeks 11/12, while 37 (86%) placebo participants were categorized as non-abstinent of which 10 (23%) had a urine drug screen positive for methamphetamine in weeks 11/12 and 27 (63%) were assumed non-abstinent due to missing urine drug screens in weeks 11/12 (χ2 = 1.67, d.f. = 1, p = 0.20).
Table 2.
Treatment outcomes for bupropion versus placebo
| Bupropion (N=41) | Placebo (N=43) | χ2/t statistic | Degrees of freedom | p value | |
|---|---|---|---|---|---|
| Primary Outcome | |||||
| End of Treatment Abstinence, % (N) | 29% (12) | 14% (6) | 2.92 | 1 | 0.087 |
| Secondary Outcomes | |||||
| Treatment Effectiveness Score, mean (S.D.) | 16.1 (12.7) | 10.6 (11.2) | −2.12 | 82 | 0.037 |
| Days Retained, mean (S.D.) | 61.0 (28.8) | 49.5 (31.7) | −1.73 | 82 | 0.09 |
Secondary Outcomes
The mean Treatment Effectiveness Score for bupropion was significantly higher than for placebo but there was no significant difference in mean days retained in treatment between the two groups (Table 2). Adjusting for age, gender, and baseline methamphetamine use frequency did not alter either result. In a generalized linear mixed effects model predicting the probability of providing methamphetamine positive urine drug screens during the 12 week treatment period, neither the main effect for bupropion (p = 0.22) nor the interaction between bupropion and time (p = 0.08) were statistically significant. There was a significant interaction between bupropion and baseline methamphetamine use (p = 0.02), with a greater reduction in the probability of methamphetamine positive urine tests with bupropion relative to placebo among participants with higher baseline frequency of methamphetamine use. Female gender (p = 0.04) and higher baseline frequency of methamphetamine use (p italic> 0.0001) were significantly associated with testing positive for methamphetamine during treatment.
Post Hoc Analysis: Outcomes by Medication Adherence
Week six plasma samples for bupropion level analysis were available for 63% (26/41) of bupropion participants. Thirteen bupropion participants (32%, 13/41) were assessed as medication adherent on the basis of a week 6 bupropion/hydroxybupropion plasma level above the reference range minimum. The proportion of participants with end of treatment methamphetamine abstinence, the mean Treatment Effectiveness Score, and the mean days retained were all significantly higher for bupropion participants who were medication adherent via week 6 plasma levels compared to those who were non-adherent (Table 3). Medication adherent participants also attended more CBT sessions (mean 10.5 sessions, S.D. 1.5 versus mean 5.8, S.D. 4.0 for non-adherent; t = 4.10, d.f. 39, p = 0.001) than medication non-adherent participants. There were no significant differences in age, gender, baseline methamphetamine use frequency, or medication adherence assessed via pill count between participants assessed as medication adherent via plasma medication levels versus non-adherent (data not shown).
Table 3.
Treatment outcomes for bupropion participants by medication adherence assessed via plasma medication level
| Adherent (N=13) | Non-Adherent (N=28) | χ2/t statistic | Degrees of freedom | p value | |
|---|---|---|---|---|---|
| Primary Outcome | |||||
| End of Treatment Abstinence, % (N) | 54% (7) | 18% (5) | 5.56 | 1 | 0.018 |
| Secondary Outcomes | |||||
| Treatment Effectiveness Score, mean (S.D.) | 23.1 (9.3) | 12.9 (12.9) | 2.54 | 39 | 0.015 |
| Days Retained, mean (S.D.) | 81.7 (4.6) | 51.3 (30.2) | 3.57 | 39 | 0.001 |
Adverse Events
Seventy one percent (71%, 29/41) of bupropion participants reported at least one adverse event compared to 51% (22/43) of placebo participants (χ2 = 3.37, d.f. = 1, p = 0.07). The frequency of reported adverse events was greater for the bupropion group than placebo, but symptoms were generally of mild to moderate severity and typical of treatment with bupropion, including insomnia, feeling “amped up,” depressed mood, and headache.
There were four Serious Adverse Events during the trial, two in participants receiving bupropion and two in participants receiving placebo. One bupropion participant required psychiatric hospitalization for suicidal ideation following binge use of methamphetamine, cocaine, and alcohol and the other bupropion participant was hospitalized for chest pain, shortness of breath, left sided paresthesias, and depressed mood following methamphetamine relapse. One placebo participant required psychiatric hospitalization for depressed mood and suicidal ideation following binge cocaine and alcohol use and the other placebo participant was hospitalized for a liver abscess related to a previous cholecystectomy. None of these were deemed to be due to study medication.
Discussion
Two previous clinical trials of bupropion in methamphetamine dependent participants failed to find an effect for bupropion relative to placebo overall, but did find a moderate sized effect for bupropion in reducing methamphetamine use in the subgroup of participants with lower baseline frequency of methamphetamine use [20, 21]. The current study aimed to replicate these findings prospectively in a sample of methamphetamine dependent participants with less than daily methamphetamine use at baseline. In the current trial, there was no significant difference between bupropion and placebo on the primary study outcome, end of treatment methamphetamine abstinence, but bupropion was significantly superior to placebo on one of the secondary outcomes, Treatment Effectiveness Score or the mean number of methamphetamine-negative urine drug screens. In a post- hoc analysis of participants receiving bupropion, end of treatment abstinence, Treatment Effectiveness Score, and retention were all significantly higher in participants assessed as medication adherent via plasma bupropion/hydroxybupropion levels compared to non-adherent participants, but only 32% (13/41) of bupropion participants were adherent by plasma levels. Together these results suggest efficacy for bupropion in methamphetamine dependence, but only in a highly selected subgroup of medication adherent participants with less than daily baseline methamphetamine use, and as a result the potential effectiveness and clinical utility of bupropion for methamphetamine dependence is likely limited.
Results of this study suggest that current designs for stimulant dependence pharmacotherapy clinical trials may fail to detect medication effects due to high rates of medication non-adherence and new approaches to the early clinical testing of medications for stimulant dependence that address medication non-adherence are needed. Similar to previous studies [23, 24], there was no association between adherence assessed via medication levels and via pill counts highlighting the necessity of including objective measures of medication adherence such as medication levels in pharmacotherapy trials. Medication adherence was assessed in this trial via a single random plasma sample collected during week 6 of the 12 week medication treatment period and samples at additional time points may provide a more sensitive assessment of adherence, but even this single sample, providing a “snapshot” of medication adherence, was significantly associated with treatment outcomes. Measurement of medication levels at even a few time points may be sufficient to assess clinically meaningful differences in adherence while minimizing cost and burden on participants. The twice-daily sustained release formulation of bupropion was used in this trial and adherence may be higher with once-daily extended release bupropion, although adherence was so low (32%) that a change to the once-daily formulation alone is unlikely to produce high levels of adherence. Depot formulations may also improve adherence, such as long-acting injectable naltrexone, although adoption of injectable naltrexone in practice has been low [36] and the development of depot formulations for early clinical testing prior to demonstrating efficacy is likely to be cost-prohibitive. The trial did not include any specific interventions aimed at supporting medication adherence, such as medical management counseling [37], use of an adherence tracer like riboflavin [38], text message reminders [39], or directly observed therapy via cell phone photos [40]. Each of these interventions shows promise but studies to identify the best way to maximize medication adherence in stimulant dependence trials are needed. Early clinical response is associated with subsequent treatment outcomes, including end of treatment methamphetamine abstinence [41], and use of designs such as brief efficacy screening trials [42] combined with intensive medication adherence monitoring/support during early clinical development may be less likely to miss a medication effect due to non-adherence than the 12 week outpatient design used in the current trial. Studies to develop and validate novel clinical trial designs and interventions to increase medication adherence in stimulant dependence trials are needed in order to insure that trials will detect potential medication effects.
This study has several limitations. Rates of missing urine drug screen data were high to due subject attrition and the majority of participants non-abstinent at end of treatment in both bupropion and placebo groups were assumed to be non-abstinent due to missing urine drug screens. Medication adherence analyses were post hoc and it is possible that the association between medication adherence and treatment outcomes are due to chance, better CBT attendance in the medication adherent group, or greater adherence in general among medication adherent participants. Alternatively, clinical improvement as a result of bupropion treatment in adherent participants may have facilitated greater counseling attendance and adherence to other aspects of the trial in the adherent group. The current trial excluded potential participants with daily methamphetamine use on the basis of previous trials showing an effect only in low frequency methamphetamine users, limiting the generalizability of the current results. Neither of the previous trials included an objective measure of medication adherence and the lack of an effect in heavy methamphetamine users could be the result of medication non-adherence, although frequency of baseline methamphetamine use was not associated with medication adherence in the current trial.
In conclusion, bupropion may be efficacious for methamphetamine dependence but only in a subgroup of medication adherent participants with less than daily methamphetamine use at treatment baseline. Outcomes in the placebo group, which received a platform of cognitive behavioral therapy, were poor highlighting the need to identify more effective treatments for methamphetamine dependence. High rates of medication non-adherence in this and other stimulant dependence clinical trials [23, 24] impede the ability of current trial designs to detect medication effects and suggest that non-adherence will be a major obstacle to an effective methamphetamine dependence pharmacotherapy in clinical practice.
Acknowledgments
Declarations of interest: Support for the study was provided by NIDA grants K23 DA023558 to Dr. Heinzerling and T32 DA026400 to Dr. Shoptaw. Drs. Heinzerling, Shoptaw, and Swanson have received previous research funding from MediciNova, Cephalon, Pfizer, and Philip Morris.
The authors wish to thank Raylene Mote, Pharm.D. and Inland Compounding Pharmacy, Loma Linda, CA.
Footnotes
Clinical trial registration: NCT00833443
References
- 1.Brensilver M, Heinzerling KG, Shoptaw S. Pharmacotherapy of amphetamine-type stimulant dependence: an update. Drug Alcohol Rev. 2013;32:449–460. doi: 10.1111/dar.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee NK, Rawson RA. A systematic review of cognitive and behavioural therapies for methamphetamine dependence. Drug Alcohol Rev. 2008;27:309–317. doi: 10.1080/09595230801919494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dean AC, London ED, Sugar CA, Kitchen CM, Swanson AN, Heinzerling KG, et al. Predicting adherence to treatment for methamphetamine dependence from neuropsychological and drug use variables. Drug Alcohol Depend. 2009;105:48–55. doi: 10.1016/j.drugalcdep.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roll JM, Petry NM, Stitzer ML, Brecht ML, Peirce JM, Mccann MJ, et al. Contingency management for the treatment of methamphetamine use disorders. The American journal of psychiatry. 2006;163:1993–1999. doi: 10.1176/ajp.2006.163.11.1993. [DOI] [PubMed] [Google Scholar]
- 5.Vearrier D, Greenberg MI, Miller SN, Okaneku JT, Haggerty DA. Methamphetamine: history, pathophysiology, adverse health effects, current trends, and hazards associated with the clandestine manufacture of methamphetamine. Dis Mon. 2012;58:38–89. doi: 10.1016/j.disamonth.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Simmler LD, Wandeler R, Liechti ME. Bupropion, methylphenidate, and 3,4-methylenedioxypyrovalerone antagonize methamphetamine-induced efflux of dopamine according to their potencies as dopamine uptake inhibitors: implications for the treatment of methamphetamine dependence. BMC research notes. 2013;6:220. doi: 10.1186/1756-0500-6-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rau KS, Birdsall E, Hanson JE, Johnson-Davis KL, Carroll FI, Wilkins DG, et al. Bupropion increases striatal vesicular monoamine transport. Neuropharmacology. 2005;49:820–830. doi: 10.1016/j.neuropharm.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Reichel CM, Murray JE, Grant KM, Bevins RA. Bupropion attenuates methamphetamine self-administration in adult male rats. Drug Alcohol Depend. 2009;100:54–62. doi: 10.1016/j.drugalcdep.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schindler CW, Gilman JP, Panlilio LV, Mccann DJ, Goldberg SR. Comparison of the effects of methamphetamine, bupropion, and methylphenidate on the self-administration of methamphetamine by rhesus monkeys. Exp Clin Psychopharmacol. 2011;19:1–10. doi: 10.1037/a0022432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newton TF, Roache JD, De La Garza R, 2nd, Fong T, Wallace CL, Li SH, et al. Safety of intravenous methamphetamine administration during treatment with bupropion. Psychopharmacology (Berl) 2005;182:426–435. doi: 10.1007/s00213-005-0102-8. [DOI] [PubMed] [Google Scholar]
- 11.Newton TF, Roache JD, De La Garza R, 2nd, Fong T, Wallace CL, Li SH, et al. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacology. 2006;31:1537–1544. doi: 10.1038/sj.npp.1300979. [DOI] [PubMed] [Google Scholar]
- 12.Nomikos GG, Damsma G, Wenkstern D, Fibiger HC. Acute effects of bupropion on extracellular dopamine concentrations in rat striatum and nucleus accumbens studied by in vivo microdialysis. Neuropsychopharmacology. 1989;2:273–279. doi: 10.1016/0893-133x(89)90031-6. [DOI] [PubMed] [Google Scholar]
- 13.Egerton A, Shotbolt JP, Stokes PR, Hirani E, Ahmad R, Lappin JM, et al. Acute effect of the anti-addiction drug bupropion on extracellular dopamine concentrations in the human striatum: an [11C]raclopride PET study. Neuroimage. 2010;50:260–266. doi: 10.1016/j.neuroimage.2009.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, et al. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Argyelan M, Szabo Z, Kanyo B, Tanacs A, Kovacs Z, Janka Z, et al. Dopamine transporter availability in medication free and in bupropion treated depression: a 99mTc-TRODAT-1 SPECT study. J Affect Disord. 2005;89:115–123. doi: 10.1016/j.jad.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Meyer JH, Goulding VS, Wilson AA, Hussey D, Christensen BK, Houle S. Bupropion occupancy of the dopamine transporter is low during clinical treatment. Psychopharmacology (Berl) 2002;163:102–105. doi: 10.1007/s00213-002-1166-3. [DOI] [PubMed] [Google Scholar]
- 17.Mansvelder HD, Fagen ZM, Chang B, Mitchum R, Mcgehee DS. Bupropion inhibits the cellular effects of nicotine in the ventral tegmental area. Biochem Pharmacol. 2007;74:1283–1291. doi: 10.1016/j.bcp.2007.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glick SD, Sell EM, Maisonneuve IM. Brain regions mediating alpha3beta4 nicotinic antagonist effects of 18-MC on methamphetamine and sucrose self-administration. Eur J Pharmacol. 2008;599:91–95. doi: 10.1016/j.ejphar.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dwoskin LP, Crooks PA. A novel mechanism of action and potential use for lobeline as a treatment for psychostimulant abuse. Biochem Pharmacol. 2002;63:89–98. doi: 10.1016/s0006-2952(01)00899-1. [DOI] [PubMed] [Google Scholar]
- 20.Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33:1162–1170. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- 21.Shoptaw S, Heinzerling KG, Rotheram-Fuller E, Steward T, Wang J, Swanson AN, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96:222–232. doi: 10.1016/j.drugalcdep.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mccann DJ, Li SH. A novel, nonbinary evaluation of success and failure reveals bupropion efficacy versus methamphetamine dependence: reanalysis of a multisite trial. CNS neuroscience & therapeutics. 2012;18:414–418. doi: 10.1111/j.1755-5949.2011.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson AL, Li SH, Biswas K, Mcsherry F, Holmes T, Iturriaga E, et al. Modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2012;120:135–141. doi: 10.1016/j.drugalcdep.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Somoza EC, Winship D, Gorodetzky CW, Lewis D, Ciraulo DA, Galloway GP, et al. A multisite, double-blind, placebo-controlled clinical trial to evaluate the safety and efficacy of vigabatrin for treating cocaine dependence. JAMA psychiatry. 2013;70:630–637. doi: 10.1001/jamapsychiatry.2013.872. [DOI] [PubMed] [Google Scholar]
- 25.Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. Journal of studies on alcohol. 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- 26.Kessler RC, Green JG, Adler LA, Barkley RA, Chatterji S, Faraone SV, et al. Structure and diagnosis of adult attention-deficit/hyperactivity disorder: analysis of expanded symptom criteria from the Adult ADHD Clinical Diagnostic Scale. Arch Gen Psychiatry. 2010;67:1168–1178. doi: 10.1001/archgenpsychiatry.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinzerling KG, Swanson AN, Kim S, Cederblom L, Moe A, Ling W, et al. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2010;109:20–29. doi: 10.1016/j.drugalcdep.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spitzer R, Williams J, Gibbbon M, First M. The Structured Clinical Interview for DSM-IV. Washington, D.C: American Psychiatric Press; 1995. [Google Scholar]
- 29.Mclellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 30.Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E. The reliability of a timeline method for assessing normal drinker college students' recent drinking history: utility for alcohol research. Addictive behaviors. 1986;11:149–161. doi: 10.1016/0306-4603(86)90040-7. [DOI] [PubMed] [Google Scholar]
- 31.Mcgregor C, Srisurapanont M, Mitchell A, Longo MC, Cahill S, White JM. Psychometric evaluation of the Amphetamine Cessation Symptom Assessment. J Subst Abuse Treat. 2008;34:443–449. doi: 10.1016/j.jsat.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- 33.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 34.Carroll KM, Kiluk BD, Nich C, Devito EE, Decker S, Lapaglia D, et al. Toward empirical identification of a clinically meaningful indicator of treatment outcome: Features of candidate indicators and evaluation of sensitivity to treatment effects and relationship to one year follow up cocaine use outcomes. Drug Alcohol Depend. 2014;137c:3–19. doi: 10.1016/j.drugalcdep.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubin DB. Multiple imputation for nonresponse in surveys. Hoboken, N.J: Wiley-Interscience; 2004. [Google Scholar]
- 36.Abraham AJ, Roman PM. Early adoption of injectable naltrexone for alcohol-use disorders: findings in the private-treatment sector. Journal of studies on alcohol and drugs. 2010;71:460–466. doi: 10.15288/jsad.2010.71.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pettinati HM, Weiss RD, Dundon W, Miller WR, Donovan D, Ernst DB, et al. A structured approach to medical management: a psychosocial intervention to support pharmacotherapy in the treatment of alcohol dependence. Journal of studies on alcohol. 2005:170–178. doi: 10.15288/jsas.2005.s15.170. discussion 168–179. [DOI] [PubMed] [Google Scholar]
- 38.Herron AJ, Mariani JJ, Pavlicova M, Parrinello CM, Bold KW, Levin FR, et al. Assessment of riboflavin as a tracer substance: Comparison of a qualitative to a quantitative method of riboflavin measurement. Drug Alcohol Depend. 2013;128:77–82. doi: 10.1016/j.drugalcdep.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finitsis DJ, Pellowski JA, Johnson BT. Text message intervention designs to promote adherence to antiretroviral therapy (ART): a meta-analysis of randomized controlled trials. PloS one. 2014;9:e88166. doi: 10.1371/journal.pone.0088166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galloway GP, Coyle JR, Guillén JE, Flower K, Mendelson JE. A Simple, Novel Method for Assessing Medication Adherence: Capsule Photographs Taken With Cellular Telephones. Journal of addiction medicine. 2011;5:170–174. doi: 10.1097/ADM.1090b1013e3181fcb1095fd. [DOI] [PubMed] [Google Scholar]
- 41.Brensilver M, Heinzerling KG, Swanson AN, Shoptaw SJ. A retrospective analysis of two randomized trials of bupropion for methamphetamine dependence: suggested guidelines for treatment discontinuation/augmentation. Drug Alcohol Depend. 2012;125:169–172. doi: 10.1016/j.drugalcdep.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perkins KA, Lerman C. An efficient early phase 2 procedure to screen medications for efficacy in smoking cessation. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-013-3364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]