Abstract
Objectives
Spinal cord is known to be innervated with dopaminergic cells with catecholaminergic projections arising from the medulla and pons and dopaminergic transmission in the spinal cord is vital for sensory and motor function. Our goal was to evaluate and compare the imaging capability of dopamine D2/D3 receptors in the rat spinal cord using PET ligands 18F-fallypride and 11C-fallypride.
Methods
Male Sprague-Dawley rats were used in all in vitro and in vivo studies. Spinal cord and brain sections were used for in vitro autoradiography and ex vivo autoradiography. For in vivo studies animals received a 18F-fallypride scan or a 11C-fallypride PET scan. The spinal cord and the brain were then harvested, flash-frozen and imaged ex vivo. For in vivo analysis Logan plots with cerebellum as a reference was used to evaluate binding potentials (BP). Tissue ratios were used for ex vivo analysis. Drug effects were evaluated using clozapine, haloperidol and dopamine were evaluated on spinal cord sections in vitro.
Results
In vitro studies showed 18F-fallypride binding to superficial dorsal horn (SDH), dorsal horn (DH), ventral horn (VH) and the pars centralis (PC). In the cervical section, the greatest amount of binding appeared to be in the SDH. Ex vivo studies showed approximately 6% of 18F-fallypride in SDH compared to that observed in the striatum. In vivo analysis of both 18F-fallypride and 11C-fallypride in the spinal cord were comparable to that in the extrastriatal regions. Haloperidol and clozapine displaced more than 75% of the 18F-fallypride in spinal cord sections.
Conclusions
Our studies showed 18F-fallypride and 11C-fallypride binding in the spinal cord in vitro and in vivo. The binding pattern correlates well with the known distribution of dopamine D2/D3 receptors in the spinal cord.
Keywords: Spinal cord, Dopamine receptors, PET, 18F-fallypride, 11C-fallypride
1. Introduction
Spinal cord has been known to be innervated with catecholaminergic projections. Autoradiographic and immunohistochemical studies in rodent spinal cord sections show the presence of dopamine D2-like receptors (Levant and McCarson, 2001; Holstege et al., 1998). Apomorphine induced hypotension in rats was blocked by the D2 receptor antagonist, domperidone which is not able to cross the blood brain barrier has shown presence of D2 receptors in the spinal cord (Lahlou, 2000; Lahlou, 2003). Immunocytochemical studies have shown a distribution of D2 receptors throughout the spinal cord (van Dijken et al., 1996), and immunochemistry studies of the central nervous system indicate that D2 receptors in the spinal cord may be involved in regulation of bladder control (Ishizuka et.al., 2002). Dopamine D3 receptors were found in lower abundance compared to the D2 receptors (Levant and McCarson, 2001).
No published report is available on non-invasive imaging of these receptors in the spinal cord. Dopaminergic system in the spinal cord may be involved as targets for 1) treatment of restless leg syndrome (e.g., using the dopamine agonist pramipexole); 2. chronic pain (nociception; Keeler et.al., 2012); 3. antipsychotic drugs, thus causing side-effects (e.g., akathesia); 4. anitparkinsonian drugs; 5. abnormal movements from long-term L-DOPA treatment, and 6. various sensorimotor functions (Barriere et.al., 2004; Cavalloti et. al., 2004).
Unlike the spinal cord, imaging studies of brain dopamine D2/D3 receptors using positron emission tomography (PET) are being carried out using various imaging agents including fallypride in various neurological and psychiatric disorders (e.g., Buchsbaum et al., 2006; Fisher 2013). More recently, dopamine receptors in the pancreas have been studied as potential surrogate markers for tracking insulin-secreting islet cells in the pancreas or in transplanted islet cells (Garcia et al., 2011, 2014). The presence of D2-like receptors in the intestines has been reported to affect motility in mice (Liz et al., 2006). Using 124I-epidepride, a dopamine D2/D3 receptor antagonist for extended time imaging, we have reported activity in the intestines at late times (Pandey et al., 2014). It remains to be shown if this intestinal activity is dopamine D2/D3 receptor bound.
Our goal in this work is to evaluate the feasibility of imaging spinal cord dopamine D2/D3 receptors using PET radiotracers, 18F-fallypride and 11C-fallypride. 18F-fallypride is an established high affinity D2/D3 radioligand and has been used in numerous human and primates studies (e.g., Mukherjee et al., 1999, Mukherjee et al., 2002). Its high affinity for the binding sites has allowed imaging of extrastriatal regions in the brain that have a lower density of receptors in the rat (Constantinescu et al., 2011). 18F-fallypride in rats has sufficient bone uptake in the vertebral column due to 18F-fluoride and can confound quantitation during in vivo imaging, we used comparative in vivo imaging with 11C-fallypride. 11C-fallypride has been shown to possess properties similar to 18F-fallypride in delineating extrastriatal regions in the brain (Mukherjee et al., 2004).
We first studied the possibility of visualizing dopamine receptors in vitro and ex vivo in rodent spinal cord using 18F-fallypride. The spinal cord was split into four sections cervical, thoracic, lumbar, and sacral regions. In vivo and ex vivo microPET studies using 18F-fallypride and 11C-fallypride in rodents were then performed to evaluate spinal cord binding, and this binding was compared to binding in the striatum within the brain where binding is known to be very strong (Mukherjee et al., 1999). 11C-fallypride was used in the in vivo and ex vivo microPET studies to account for bone uptake issues of 18F-fluoride and which is not an issue for autoradiographic studies. Drug effects in vitro were measured against 18F-fallypride using antagonists haloperidol and clozapine and agonist dopamine for dopamine D2/D3 receptors in autoradiographic studies.
2. Materials and Methods
2.1 General Methods
All chemicals and solvents were of analytical or HPLC grade from Aldrich Chemical Co. and Fisher Scientific. Electrospray mass spectra were obtained on a Model 7250 mass spectrometer (Micromass LCT). Proton NMR spectra were recorded on a Bruker OMEGA 500 MHz spectrometer. Analytical thin layer chromatography (TLC) was carried out on silica coated plates (Baker-Flex, Phillipsburg, NJ). Chromatographic separations were carried out on preparative TLC (silica gel GF 20×20 cm 2000 micron thick; Alltech Assoc. Inc., Deerfield, IL) or silica gel flash column or semi-preparative reverse-phase columns using the Gilson high performance liquid chromatography (HPLC) systems. High specific activity 18F-fluoride was produced in the MC-17 cyclotron using oxygen-18 enriched water (18O to 18F using p, n reaction) and 11C-carbondioxide was produced using a nitrogen gas target (14N to 11C using p, α reaction). The high specific activity 18F-fluoride and 11C-carbondioxide were used in subsequent reactions which were carried out in automated radiosynthesis units (chemistry processing control unit (CPCU) for 18F-fallypride and GE Tracer Lab FXC Pro for 11C-fallypride). Fluorine-18 and carbon-11 radioactivity were counted in a Capintec CRC-15R dose calibrator while low level counting was carried out in a Capintec Caprac-R well-counter. Radioactive thin layer chromatographs were obtained by scanning in a Bioscan system 200 Imaging scanner (Bioscan, Inc., Washington, DC). Rat brain and spinal cord slices were prepared at 20 μm thick using a Leica 1850 cryotome. Fluorine-18 autoradiographic studies were carried out by exposing tissue samples on storage phosphor screens (Perkin Elmer Multisensitive, Medium MS). The apposed phosphor screens were read and analyzed by OptiQuant acquisition and analysis program of the Cyclone Storage Phosphor System (Packard Instruments Co., Boston, MA). A preclinical Inveon dedicated PET scanner (Siemens Medical Solutions, Knoxville, TN) with a transaxial FWHM of 1.46 mm, and axial FWHM of 1.15 mm (Constantinescu and Mukherjee, 2009) was used for the PET studies. Both in vivo and ex vivo PET images of rat brains were obtained and analyzed using ASIProVM software. All animal studies were approved by the Institutional Animal Care and Use Committee of University of California-Irvine.
2.2 Radiopharmaceutical
2.2.1 18F-Fallypride
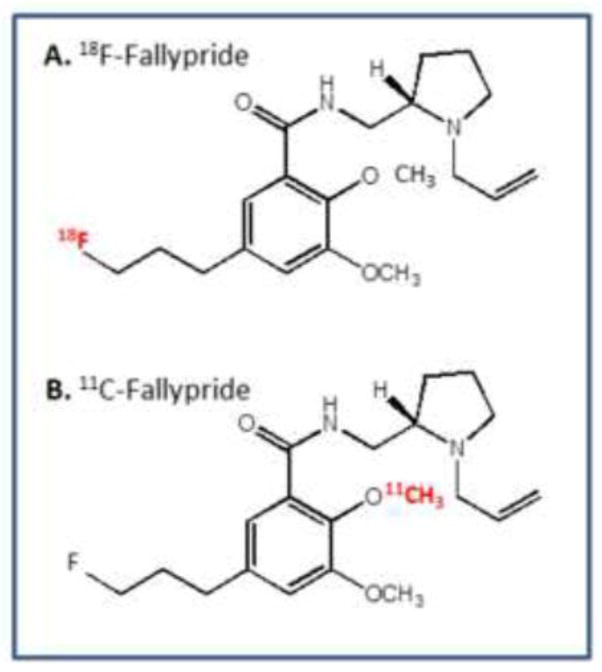
The synthesis of 18F-fallypride (Fig-1) was carried out in the chemical process control unit (CPCU) using modifications of previously reported methods (Mukherjee et al., 1995, 2002). Purification of 18F-fallypride was carried out by high performance liquid chromatography (HPLC) separation on an Econosil reverse-phase C-18 semi-prep column 250 × 10 mm; 10μm particle size (Alltech Assoc., Deerfield, IL). Eluents used were 60% acetonitrile and triethylamine (0.1%) in water at a flow rate of 2.5 ml/min in a Gilson Gradient System consisting of one UV detector with wavelength fixed at 280 nm and a radiation flow detector. The radioactive fraction appearing at 20 min was collected, solvents removed and the residue was taken up in sterile saline and passed through a Whatman 0.22-μm filter and dispensed for in vitro and in vivo studies in specific activity was 74 GBq/μmol with a radiochemical purity of >98%.
Figure 1. Chemical Structures of the PET radiotracers.
(A). 18F-fallypride (B). 11C-fallypride.
2.2.2 11C-Fallypride
The synthesis of 11C-fallypride (Fig-1) was carried out using modifications of our previously described method (Mukherjee et al., 2004). The two major differences were the use of 11C-methyl triflate instead of 11C-methyl iodide for 11C-methylation and the automated unit synthesis unit, GE Tracerlab was a single combined unit, which synthesized the 11C-methyl triflate from 11C-carbondioxide and reacted it with the phenolic precursor to provide 11C-fallypride. Chromatographic separation was carried out as described above. The radiochemical yield of 11C-fallypride was in the range of 30 to 40% decay-corrected from 11C-methyl triflate and specific activity was 37 GBq/μmol with a radiochemical purity of >98%.
2.3 In Vitro Studies
Adult, Sprague-Dawley rats (250–300 g, from Charles River) were housed with free access to laboratory chow and water. The temperature and humidity-controlled animal facility had a 12 hrs dark-light cycle. Rats were obtained at least 7 days before the experiment. Rats were anesthetized and decapitated and the different regions of the spinal cord were rapidly dissected and removed in cold condition and frozen in cold isopentane solution. The cervical, thoracic, lumber and sacral portion of the spinal cord were embedded separately with freezing medium and stored in −20 °C freezer. Spinal cord coronal sections (20μm) of the cervical, thoracic, lumbar and sacral regions were obtained. The brain of these rats were also excised and coronal slices contained regions known to have dopamine receptors which include the striata and cortex.
Slides containing the brain and spinal cord sections were preincubated in Tris buffer (50 mM Tris HCl, 2.5 mM CaCl2, 125 mM NaCl, 1 mM MgCl, 5 mM KCl, 0.l mM sodium ascorbate, pH 7.4) at room temperature for 10 mins. Sections were then incubated with 18F-fallypride (37–93 kBq/cc; fallypride concentration <1 nM) for a period of 1 hr at 37 °C in the above Tris buffer in a total buffer volume of 60 mL. The nonspecific binding was measured in the presence of 10 μM sulpiride. After incubation, slides were washed twice (each wash lasting one minute) with ice-cold buffer. Slides were then quickly dipped in cold deionized water, air dried, and exposed to a phosphor screen for 24 hours. The autoradiographs were generated using the Phosphor Cyclone Imager. The amount of binding was evaluated in digital light units (DLU/mm2) using OptiQuant acquisition and analysis program (Packard Instruments Co.).
Drug competition of 18F-fallypride was evaluated against the drugs clozapine, haloperidol and dopamine for dopamine D2/D3 receptors and SCH 23390 for dopamine D1 receptors. Aliquots of the different drugs at defined final concentrations (dopamine 1 μM and 20 μM, clozapine 1 μM and 20 μM, haloperidol 1 μM and 20 μM and SCH 23390 1 μM) were added to the respective incubation chambers. Slides were incubated with 70–100 kBq/cc of 18F-fallypride in buffer in the presence of the various drugs and control (no drug added) and incubated for 1 hour at 37°C. Effects of various drugs were tested on the spinal cord and brain sections. After 60 minute incubation, the slides were washed with cold Tris buffer (2 × 3 min) and cold deionised water (1 × 3 min). The slides were dried with a gentle stream of air and left apposed to phosphor screens overnight. The binding of 18F-fallypride in spinal cord and brain were read with the Cyclone Phosphor Imager as described above (Packard Instruments Co.)
2.4 Ex Vivo Studies
Rats received 18F-fallypride (16–37 MBq) through tail i.v. injection, and nonspecific binding group of rat received 10 μM sulpiride 5 minutes prior to administration of 18F-fallypride. Rats were decapitated after 3 hrs receiving the i.v. injection of 18F-fallypride and the coronal sections of brain and spinal cord was isolated. The spinal cords were rapidly dissected and removed in cold condition and frozen with cold isopentane. The cervical, thoracic, lumber and sacral portion of spinal were embedding separately with freezing medium and stored at −20 °C. The different parts of spinal cord were cut at 20 μm on a cryostat, thaw-mounted onto gelatin coated slides and put into a plate for exposure overnight. The binding of 18F-fallypride in different regions of spinal cord was read with the cyclone.
2.5 MicroPET Imaging Studies
Male Sprague-Dawley rats (250–300 g) were single housed in a climate controlled room and had full access to food and water. The rats were fasted 24 hours prior to time of scan. On the day of the study, rats were anesthetized using 4.0% isoflurane. The rat was then positioned on the scanner bed by placing it on a warm-water circulating heating pad and kept anesthetized with 2.5% isoflurane anesthesia applied using a nose-cone. A transmission scan of 20 mins duration was subsequently acquired using the Co-57 source for attenuation correction. Preparation of dose injection was as follows: approximately 16 MBq of 18F-fallypride or approximately 150 MBq of 11C-fallypride was drawn into a 1 mL syringe with a 25 gauge needle and diluted with sterile saline to a final volume of 0.3 mL. The dose was injected intravenously into the tail vein of the rat. Isoflurane was reduced and maintained at 2.5% following injection. Scans were carried out for 90 minutes and acquired by the Inveon MicroPET in full list mode. All images were corrected for scatter, attenuation and radioactive decay. List-mode data from the in vivo scans were sorted dynamically into multiple frames for 18F-fallypride and 11C-fallypride. Image reconstruction was performed with an OSEM3D/fast MAP algorithm (16 OSEM 3D subsets, 2 iterations, 18 MAP iterations, 0 smoothing factor) resulting in 128 × 128 × 159 image array with a 0.79 mm pixel size. Images were visualized and analyzed with ASIPro (CTI Concorde Microsystems, LLC.) and PMOD (PMOD Technologies, Inc.) software. Volumes of interest (VOIs) were drawn on spinal cord and in the brain on D2/D3 receptor rich areas including striatum as well as cerebellum, a region devoid of D2/D3 receptors. For analysis of the brain regions from in vivo PET all images were first co-registered to an MR brain template (Schweinhardt et.al 2003) to allow the use of standard VOIs following a procedure described previously (Constantinescu et.al, 2011). Time-activity curves from all regions were extracted and used to compute binding potentials (BPND) using Logan graphical analysis (Logan et.al, 1996) with cerebellum as a reference region.
2.6 Ex vivo MicroPET
After completion of the in vivo MicroPET 18F-fallypride and 11C-fallypride scans, rats were sacrificed and the brain and spinal cord was extracted for ex vivo MicroPET imaging. The whole brain and spinal cord were placed in a hexagonal polystyrene weighing boat and covered with powdered dry ice. This boat was placed securely on the scanner bed and a transmission scan acquired. Both organs were placed side-by-side on the scanner bed and ex vivo scanning proceeded for 30 minutes. List mode was collected in a single frame and reconstruction of images was similar to the procedure previously described. Images were analyzed using ASIPRO VM software. The 30 min ex vivo scans were reconstructed with Fourier rebinning and 2D filtered back projection algorithm with a Hanning filter and cutoff at Nyquist frequency.
3. RESULTS
3.1 In Vitro Autoradiographic Studies
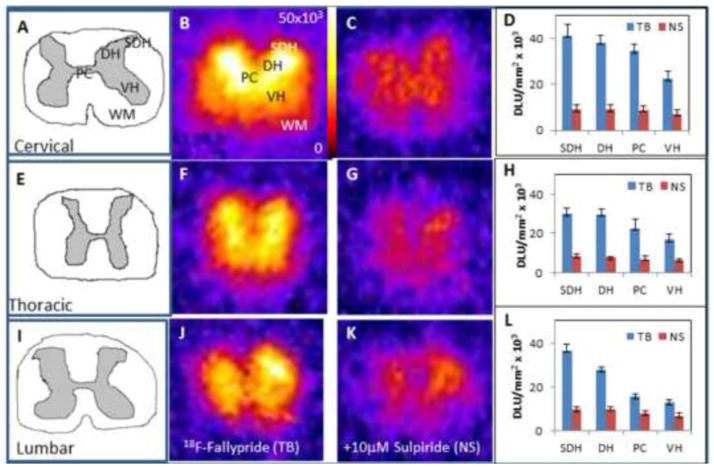
The gray matter in the spinal cord sections consisted of the superficial dorsal horn (SDH), dorsal horn (DH), ventral horn (VH) and the pars centralis (PC) as shown in Fig-2A. In the cervical section, the greatest amount of binding appeared to be in the SDH, followed by the DH and VH and lastly the PC (Fig-2B, D). In the presence of sulpiride, displacement of 18F-fallypride occurred from all the grey matter regions (Fig-2C) with the extent of reduction being 68% to 75% (Table-I). Similar extent of binding of 18F-fallypride was observed for the thoracic and lumbar regions.
Figure 2. In Vitro Studies on the Rat Spinal Cord with 18F-Fallypride.
20 μm slices of the cervical, thoracic and lumbar rat spinal cord sections from rats injected with 18F-fallypride (n=6). Figures A, E, I are drawings of the cervical, thoracic, lumbar sections respectively, which illustrate the gray matter and white matter of each section. Figures B, F, and J show 18F-fallypride binding in vitro in corresponding sections. Figures C, G, K show nonspecific binding with 10 μM of sulipride in corresponding adjacent sections. Figures D, H, L show total binding and nonspecific binding in the various spinal cord regions (SDH: Superficial dorsal horn; DH: Dorsal horn VH: Ventral horn; PC: Pars centralis; WM: White matter).
Table I.
In Vitro Studies with 18F-fallypride on Spinal Cord Sections
White matter has the potential to serve as a reference region due to the low binding of 18F-fallypride. The gray matter to white matter ratio of 18F-fallypride of the spinal cord section is listed in Table I. Binding generally appears to decrease as the spinal column descends with the cervical region displaying the highest amount of binding, followed by the thoracic and lumbar regions. Within each region, there was also decreased binding from the exterior to the interior regions of the spinal cord. For each region, the highest amount of binding occurred in the SDH, followed by the DH then by the PC and the VH. For the three spinal regions, displacement by sulpiride suggested that 18F-fallypride binding is to D2/D3 receptor sites.
3.2 Ex Vivo Study
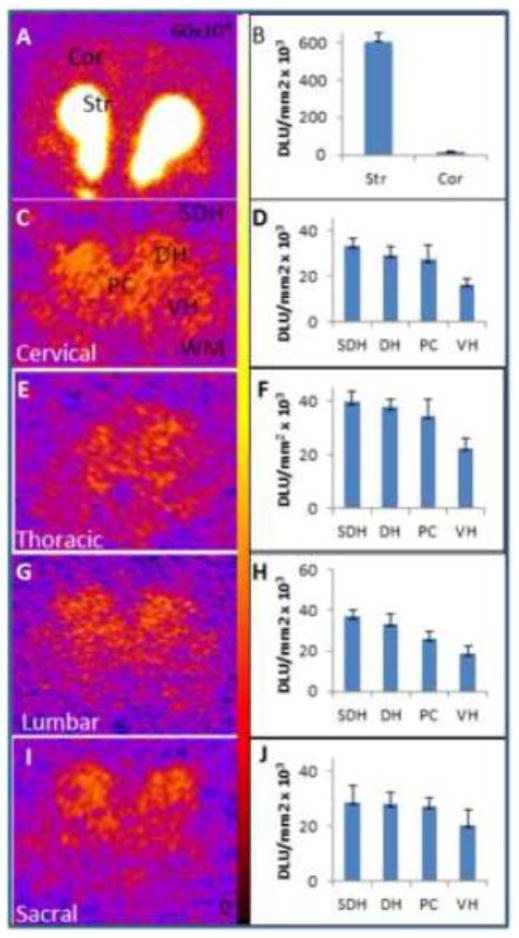
Coronal sections (20 μm) were cut from cervical, thoracic, lumbar and sacral areas of the spinal cord and cortical and striatal regions of the brain. Significant 18F-fallypride binding was observed in regions of the rat spinal cord. Region of interest (ROIs) were drawn on different regions of the spinal cord (SDH, DH, PC, VH, and white matter (WM)) and for brain, ROI was drawn on striatum and cortex. Binding in the various regions was clearly evident with SDH and DH showing approximately 6% of the binding compared to that observed in the striatum. The VH and PC were somewhat lower (approx. 3 to 4% of that found in the striatum). In the brain slice, striatum to cortex ratio was 39 while the SDH to WM was 3.60 for the cervical region.
The values for spinal cord binding as ratios versus white matter for the cervical, thoracic, lumbar, and sacral regions are listed in Table II. Binding appeared highest in the lumbar region followed by the thoracic, cervical and then the sacral region. For each region of the spinal cord, binding stayed the highest in the SDH followed by the DH, then the PC, and lastly the VH which is consistent with the in vitro studies. This level of 18F-fallypride binding is comparable to the extrastriatal dopamine receptor levels found in regions of the brain.
Table II.
Ex Vivo Autoradiography with 18F-fallypride on spinal cord and brain
| Section | Brain (striatum) | Cervical | Thoracic | Lumbar | Sacral |
|---|---|---|---|---|---|
| Str/Cor | 39 | -- | -- | -- | -- |
| SDH/WM | -- | 3.60 | 3.69 | 3.90 | 2.40 |
| DH/WM | -- | 3.15 | 3.50 | 3.55 | 2.20 |
| PC/WM | -- | 2.94 | 3.20 | 2.72 | 1.70 |
| VH/WM | -- | 1.75 | 2.09 | 1.98 | 1.30 |
SDH = superficial dorsal horn; DH = dorsal horn; PC = pars centralis; VH = ventral horn; WM = white matter; Str = striatum; Cor = cortex (n=3).
3.3 MicroPET Studies
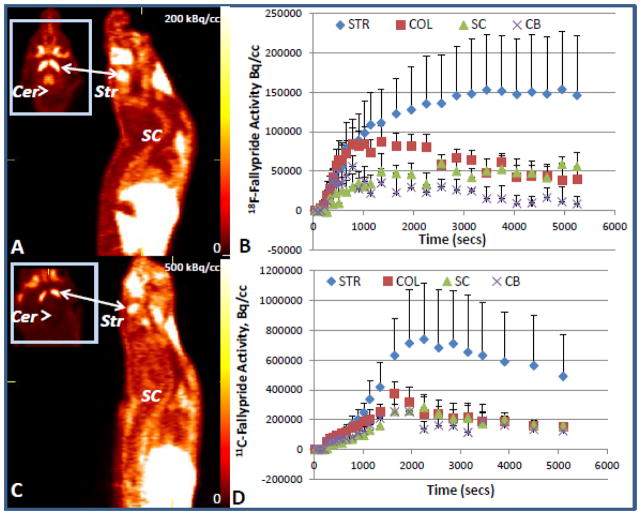
In vivo binding of the 18F-fallypride and the 11C-fallypride were evaluated in the PET scan in the striatum and the cerebellum of the brain and overall binding in the spinal cord. In vivo binding potentials are presented in Table III for the striatum (STR), superior and inferior colliculi (COL) and the spinal cord (SC). Binding potential of both 18F-fallypride and 11C-fallypride in the spinal cord is comparable to those in the extrastriatal regions. The binding activity curves for both 18F- and 11C-fallypride exhibited similar profiles. Binding was the highest in the striatum followed by decreasing binding as the CNS descends from the striatum to the spinal cord which is similar to the in vitro findings. As expected there was no binding found in the cerebellum. Binding in the spinal cord was relatively stable throughout the time period shown for 18F-fallypride. There were some minor fluctuations shown in the 11C-fallypride binding in the spinal cord, after reaching a peak around 30 min, the binding decreases slightly and remains at the same activity level from 35 to 55 min. Around 60 min the binding decreases again and remains consistent throughout the last 20 min of the experiment. Binding in the superior and inferior colliculi was comparable to binding in the spinal cord for both radiotracers which can be interpreted as the concentration of dopamine receptors being similar in the spinal cord and the colliculi. The in vivo BPND values relative to striatum were similar to those measured in the ex vivo scans.
Table III.
In vivo and Ex vivo PET measures
3.4 Ex Vivo MicroPET Studies
Ex Vivo PET images in (a) the striatum, (b) coronal view and (c) sagital view of 18F-fallypride and the 11C-fallypride binding in the brain and the spinal cord. The relative specific binding ratios with respect to cerebellum are shown in Table III; all values were scaled with respect to striatum, the region with highest density of D2/D3 receptors taken as 100% binding. Binding was similar in the lower and upper spinal cord. 18F-Fallypride binding was the greatest in the striatum of the brain followed by a gradual decrease in the spinal cord, with binding nonetheless indicating the presence of D2/D3 receptors. Binding is similar to in vitro and in vivo findings with binding decreasing as the spinal cord descends.
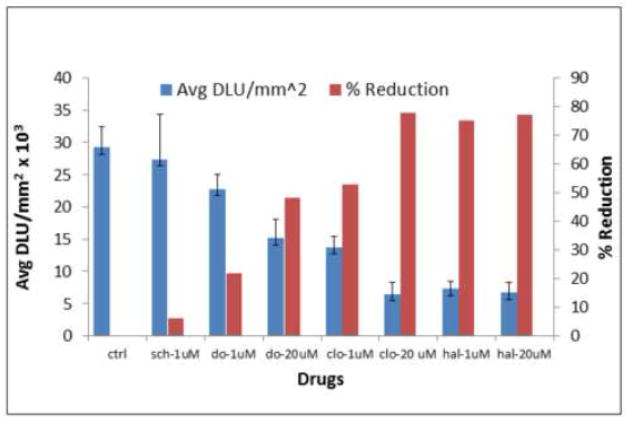
3.5 Drug Effects
Drug challenges were evaluated in the spinal cord sections and results are shown in Figure-6. Dopamine at concentrations of 1 μM and 20 μM reduced 22% and 48% of 18F-fallypride binding. Clozapine, which is known to bind to dopamine D2/D3 receptors at similar concentration displaced 53% and 78% of 18F-fallypride respectively. Haloperidol, a potent D2/D3 receptor antagonist displaced approx. 75% and 77% respectively at the same concentrations. The dopamine D1 receptor antagonist, SCH 23390 had little effect on the binding of 18F-fallypride.
Figure 6. Drug Effects using two different agonists.
In vitro binding of 18F-fallypride in the spinal cord sections in control (ctrl) and effect of 1μM and 20μM of dopamine (do), clozapine (clo) and haloperidol (hal) 1μM and 20μM each, and with 1μM of SCH 23390 (sch). The graph shows individual drug effects with different concentrations, as the concentration increased there was a reduction in the D2/D3 receptor binding in the spinal cord.
4. Discussion
Our in vitro findings show that dopamine D2/D3 receptors are present throughout the spinal cord and can be measured by using the PET imaging agent 18F-fallypride. This is consistent with previous autoradiographic and immunoreactivity studies on the varying distribution of dopamine receptors throughout the spinal cord (Holstege et al., 1998). The D2/D3 receptors are more concentrated in the SDH and least in the VH and is similar to that previously observed autoradiographically. This concentration gradient between SDH to VH is retained throughout the length of the spinal cord, from the cervical to the sacral region. Little binding of 18F-fallypride was seen in the white matter, which is known not to have any significant amount of dopamine receptors. Reversibility of binding of 18F-fallypride was ascertained in the presence of sulpiride and is consistent with brain findings, thus suggesting that 18F-fallypride is specifically bound to dopamine D2/D3 receptors.
Ex vivo study in the rat confirmed the findings seen in the in vitro studies. All regions of the spinal cord exhibited 18F-fallypride retention and the levels of binding were in the same order as in the in vitro studies, SDH>DH>PC>VH. As expected, the sriatum had the maximum binding (Fig-3). Rat cortical regions are known to have small levels of D2/D3 receptors (Mukherjee et al., 1999). Levels of 18F-fallypride in the spinal cord were higher than in the cortex and were approximately 5% of that found in the striatum. These findings suggested that it may be possible to visualize spinal cord D2/D3 receptors in vivo using 18F-fallypride PET.
Figure 3. Rat Ex Vivo Studies with 18F-Fallypride of the Brain and Spinal Cord Regions.
Autoradiographic studies of ex vivo binding of 18F-fallypride in rat brain coronal sections and spinal cord sections 3 hrs after intravenous administration of 37 MBq 18F-fallypride (n=3). (A&B) Ex vivo binding of 18F-fallypride in the brain comparing the cortical and striatum regions and the corresponding plot for 18F-fallypride binding. Spinal cord sections and corresponding plots showing 18F-fallypride binding (C–J): (C and D) cervical, (E and F) thoracic, (G and H) lumbar, and (I and J) sacral.
Uptake of 18F-fallypride in the rat brain was consistent with our previous findings as seen in Fig-4A (Constantinescu et al., 2011). Visualizing and establishing accurate region of interests for the spinal cord proved to be difficult using 18F-fallypride because of bone uptake of 18F-fluoride resulting from defluorination of 18F-fallypride. The time-activity curve in Fig-4B shows close parallel to the levels of 18F-fallypride in the colliculi within the brain, and distinctly higher than the reference region, cerebellum. Table-III shows approximately 9% of binding in the spinal cord compared to the striatum, which may be a slight overestimation compared to the ex vivo findings shown in Fig-3 due to 18F-fluoride spillover effects in vivo. Additionally, it should be noted that the cerebellum was used as a reference region for the spinal cord since distinguishing white matter in the spinal cord in vivo is not possible due to the small size of the entire spinal column.
Figure 4. Rat In Vivo PET studies with 18F-Fallypride and 11C-Fallypride.
PET images whole body sagittal and head horizontal sections of a rat brain and spinal cord showing 18F-fallypride (panel A) and 11C-fallypride (panel C) after iv injection (n=2). 18F-Fallypride (B) and 11C-fallypride (D) time activity curves are shown for striatum (Str), spinal cord (SC), colliculi (Col) and cerebellum (Cer).
In order to avoid 18F-fluoride uptake in the vertebral column resulting from 18F-fallypride, 11C-fallypride was considered for imaging the dopamine receptors in the spinal cord (Mukherjee et al., 2004). Imaging with 11C-fallypride eliminated bone uptake of free 18F-fluoride while maintaining the benefit of the high affinity of fallypride for visualizing extrastriatal dopamine D2/D3 receptors. On the other hand, this eliminated the use of the spinal column as a useful landmark for establishing the exact location of the spinal cord. But sufficient uptake of 11C-fallypride in the spinal cord was still visible as seen in Fig-4C. Approximately 6% of 11C-fallypride was bound to the spinal cord compared to the stratum and is more consistent with the ex vivo results.
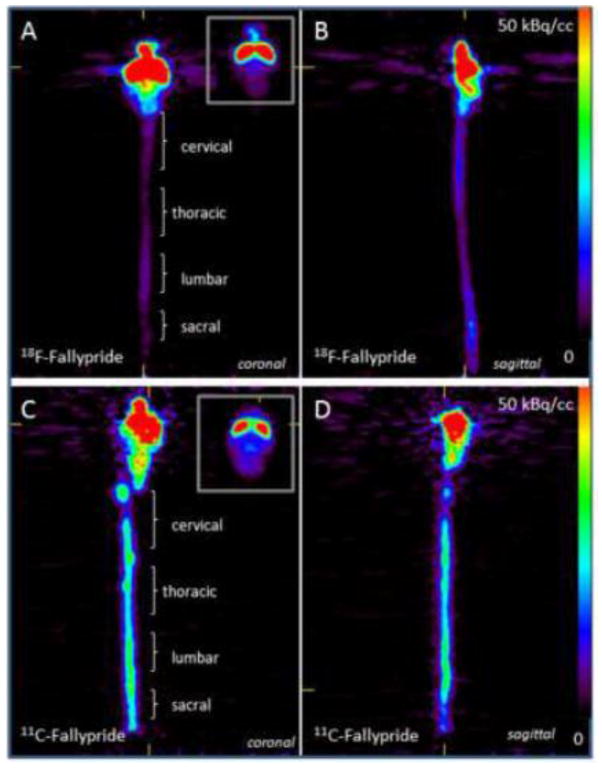
In order to ascertain that the 18F-fallypride and 11C-fallypride spinal cord binding seen in the in vivo PET studies (Fig-4) was bound to the spinal cord and not entirely to the vertebral column, ex vivo PET studies of the brain and the entire length of the spinal cord were performed after the in vivo PET study. The spinal cord showed considerable binding in the ex vivo PET scans with both 18F-fallypride (Fig-5A, B) and 11C-fallypride (Fig-5C, D). Binding was seen in the entire length of the spinal cord- however, the cervical and lumbar/lumbosacral regions of the spinal cord showed increased binding, consistent with our in vitro and ex vivo findings for 18F-fallypride (Fig-2,3). This may be consistent with the greater amount of anatomical locations of the cervical and lumbar plexuses in the rat spinal cord. The relative amounts of 18F-fallypride and 11C-fallypride measured in the striatum are different (Table-III), since measurements of 11C-fallypride are not at equilibrium.
Figure 5. Rat Ex Vivo PET studies with 18F-Fallypride and 11C-Fallypride of the brain and spinal cord.
Ex vivo PET image of a rat sacrificed after initial PET scan (Figure 4) and the brain and spinal cord excised for ex vivo PET imaging (n=2). Ex vivo 18F-fallypride (A and B) and 11C-fallypride (C and D) images of brain and spinal cord in the coronal (A and C) and sagittal view (B and D) are shown. Binding of 18F-fallypride and 11C-fallypride is evident in the brain (striatum, at the top right corner of A and C) and in the various regions of the spinal cord as shown in C (cervical, thoracic, lumbar, sacral).
Antagonists clozapine and haloperidol are well-known neuroleptic drugs and a significant amount of effort has gone into receptor occupancy studies in the brain (Mukherjee et al., 2001). Our in vitro findings with the spinal cord suggest that both of these drugs are able to compete with spinal cord-bound 18F-fallypride. This may be of significance either from a therapeutic standpoint or be causing adverse effects due to neurlopetic treatment. As expected, dopamine an agonist also displaced 18F-fallypride, although to a lesser extent than the antagonists. It may be postulated that pramipexole, a dopamine agonist, used for restless leg syndrome, may have an affect on the spinal cord dopamine receptors (Comella 2014).
In summation, significant amounts of dopamine D2/D3 receptors in the rat spinal cord can be visualized using in vivo PET. Challenges include issues of defluorination of fluorine-18 radiotracers that may confound accurate quantification of the receptors in the spinal cord. This may be overcome by the use of carbon-11 analogs. The size of the regions within the spinal cord may be an issue when measuring smaller sections of the entire spinal column—however, the length of the spinal cord offers the possibility that breaks in the spinal column may be measureable by comparing adjacent regions. Translation of these rodent model findings to human studies remains to be demonstrated.
Conclusions
Binding of 18F-fallypride in rat spinal cord sections confirms dopamine D2/D3 receptor binding. Our results indicate that 5% of 18F-fallypride binding was observed in the rat spinal cord compared to striatum suggesting presence of D2/D3 receptors in the spinal cord. Dopamine, clozapine and haloperidol reduce binding of 18F-fallypride in the spinal cord. This binding pattern also shows possible involvement of the D2/D3 receptor in restless leg syndrome as it correlates well with lower extremity nerves. While viewing and evaluating the spinal cord in the ex vivo studies proved to be much simpler than the in vivo studies, strides should be made to eliminate bone absorption in order to have better in vivo evaluation techniques.
Acknowledgments
This research was supported by NIH Grants RC1 DK087352 (JM) and R21 DK092917 (JM). We like to thank Dr. Baogang Xue and Christopher Liang for technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barriere G, Mellen N, Cazalets J-R. Neuromodulation of the locomotor network by dopamine in the isolated spinal cord of newborn rat. Eur J Neurosci. 2004;19(5):1325–35. doi: 10.1111/j.1460-9568.2004.03210.x. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Christian BT, Lehrer DS, Narayanan TK, Shi B, Mantil J, Kemether E, Oakes TR, Mukherjee J. D2/D3 Dopamine receptor binding with [F-18]fallypride in thalamus and cortex of patients with schizophrenia. Schizophrenia Research. 2006;85:232–244. doi: 10.1016/j.schres.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Cavallotti C, Frati A, Cavallotti D, Leali FMT. Dopaminergic receptors in rat dura mater: pharmacological characteristics. Clin Exptl Pharm Physiol. 2004;31(3):190–4. doi: 10.1111/j.1440-1681.2004.03972.x. [DOI] [PubMed] [Google Scholar]
- Comella CL. Treatment of restless legs syndrome. Neurotherapeutics. 2014;11:177–187. doi: 10.1007/s13311-013-0247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinescu CC, Coleman RA, Pan ML, Mukherjee J. Striatal and extrastriatal microPET imaging of D2/D3 dopamine receptors in rat brain with 18F-fallypride and 18F-desmethoxyfallypride. Synapse. 2011;65:778–787. doi: 10.1002/syn.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinescu CC, Mukherjee J. Performance evaluation of an Inveon PET preclinical scanner. Phys Med Biol. 2009;54:2885–2899. doi: 10.1088/0031-9155/54/9/020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher B, Li Q, Nacca A, Salem GJ, Song J, Yip J, et al. Treadmill exercise elevates striatal dopamine D2 receptor binding potential in patients with early Parkinson’s disease. Neuroreport. 2013;24(10):509–514. doi: 10.1097/WNR.0b013e328361dc13. [DOI] [PubMed] [Google Scholar]
- Garcia A, Mirlobooki MR, Constantinescu C, Pan ML, Sevrioukov E, Milne N, Wang PH, Lakey JRT, Chandy KG, Mukherjee J. 18F-Fallypride PET imaging of pancreatic islets cells: In vitro and in vivo rodent studies. J Nucl Med. 2011;52:1125–1132. doi: 10.2967/jnumed.111.088583. [DOI] [PubMed] [Google Scholar]
- Garcia A, Venugopal A, Pan M-L, Mukherjee J. Imaging pancreas in healthy and diabetic rodent model using 18F-fallypride PET/CT. Diabetes Tech & Ther Dev. 2014;16 doi: 10.1089/dia.2014.0041. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege JC, van Dijken H, Buijs RM, Goedknegt H, Gosens’and T, Bongers CMH. Distribution of dopamine immunoreactivity in the rat, cat, and monkey spinal cord. J Comp Neurol. 1998;376(4):631–652. doi: 10.1002/(SICI)1096-9861(19961223)376:4<631::AID-CNE10>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Ishizuka O, Mizusawa H, Nishizawa O. Roles of dopaminergic receptors in bladder and erectile function at the spinal level. Asian J Androl. 2002;4(4):287–290. [PubMed] [Google Scholar]
- Keeler B, Baran C, Brewer K, Clemens K. Increased excitability of spinal pain reflexes and altered frequency-dependent modulation in the dopamine D3-receptor knockout mouse. Exp Neurol. 2012;2:273–283. doi: 10.1016/j.expneurol.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Lahlou S. Blunted pressor responsiveness to intravenous quinpirole in conscious, chronic spinal cord-transected rats: peripheral vs. spinal mechanisms. Eur J Pharmacol. 2000;408:51–62. doi: 10.1016/s0014-2999(00)00692-0. [DOI] [PubMed] [Google Scholar]
- Lahlou S. Enhanced hypotensive response to intravenous apomorphine in chronic spinalized, conscious rats: role of dopamine D1 and D2 receptors. Neurosci Lett. 2003;349:115–119. doi: 10.1016/s0304-3940(03)00793-6. [DOI] [PubMed] [Google Scholar]
- Levant B, McCarson KE. D3 dopamine receptors in rat spinal cord: implications for sensory and motor function. Neurosci Lett. 2001;303:9–12. doi: 10.1016/s0304-3940(01)01692-5. [DOI] [PubMed] [Google Scholar]
- Liz ZS, Schmauss C, Cuenca A, Ratcliffe E, Gershon MD. Physiological modulation of intestinal motility by enteric dopaminergic neurons and the D2 receptor: analysis of dopamine receptor expression, location, development, and function in wild-type and knockout mice. J Neurosci. 2006;26(10):2798–2807. doi: 10.1523/JNEUROSCI.4720-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Yang ZY, Brown T, Lew R, Wernick M, Ouyang X, et al. Preliminary assessment of extrastriatal dopamine D-2 receptor binding in the rodent and nonhuman primate brains using the high affinity radioligand, 18F-fallypride. Nucl Med Biol. 1999;26:519–527. doi: 10.1016/s0969-8051(99)00012-8. [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Christian BT, Dunigan KA, Shi B, Narayanan TK, Satter M, et al. Brain imaging of 18F-fallypride in normal volunteers: blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse. 2002;46:170–188. doi: 10.1002/syn.10128. [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Christian BT, Narayanan TK, Shi B, Mantil J. Evaluation of dopamine D-2 receptor occupancy in vivo by clozapine, risperidone and haloperidol in rodents and non-human primates using 18F-fallypride. Neuropsychopharmacology. 2001;25:476–488. doi: 10.1016/S0893-133X(01)00251-2. [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Shi B, Christian BT, Chattopadhyay S, Narayanan TK. 11C-Fallypride: radiosynthesis and preliminary evaluation of a novel dopamine D2/D3 receptor PET radiotracer in non-human primate brain. Bioorg Med Chem. 2004;12:95–102. doi: 10.1016/j.bmc.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Pandey SK, Venugopal A, Kant R, Coleman RA, Mukherjee J. 124I-Epidepride: A high affinity and selective PET radiotracer with potential for extended imaging of dopamine D2/D3 receptors. Nucl Med Biol. 2014;41:426–431. doi: 10.1016/j.nucmedbio.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinhardt P, Fransson P, Olson L, Spenger C, Andersson JL. A template for spatial normalisation of MR images of the rat brain. J Neurosci Methods. 2003;129:105–113. doi: 10.1016/s0165-0270(03)00192-4. [DOI] [PubMed] [Google Scholar]
- van Dijken H, Dijk J, Voom P, Holstege JC. Localization of dopamine D2 receptor in rat spinal cord identified with immunocytochemistry and in situ hybridization. Eur J Neurosci. 1996;8:621–628. doi: 10.1111/j.1460-9568.1996.tb01247.x. [DOI] [PubMed] [Google Scholar]