Abstract
We identified a standard core set of patient-reported symptoms and health-related quality-of-life (HRQOL) domains to be assessed in head and neck (H&N) cancer clinical trials. The core symptom and HRQOL domain scores were used to guide recommendations by a working group of experts as part of a National Cancer Institute Symptom Management and HRQOL Clinical Trials Planning Meeting. A PubMed search was conducted using the search terms of “health-related quality of life” and “head & neck cancer,” limited to publications from January 1, 2000, to December 31, 2010. Fifty-four articles were used to guide the choice of recommendations. Twenty-nine symptoms and nine domains were identified, from which 12 H&N-specific core symptoms and HRQOL domains were recommended: swallowing, oral pain, skin changes, dry mouth, dental health, opening mouth/trismus, taste, excess/thick mucous/saliva, shoulder disability/motion, voice/hoarseness, social domain, and functional domain. This core set of 12 H&N-specific, patient-reported symptoms and HRQOL domains should be assessed in future H&N cancer clinical trials.
Head and neck (H&N) cancers are in close proximity to vital anatomic structures that are involved in important physiological (eg, swallowing, eating) and social functions (eg, communication). H&N cancer patients often receive multimodality treatment resulting in acute and chronic toxicity rates of up to 40% to 50% (1,2). The National Cancer Institute Symptom Management and Quality of Life Steering Committee conducted a clinical trials planning meeting with the goal of identifying a standard core set of patient-reported outcomes (PROs) symptoms and/or health-related quality-of-life (HRQOL) domains to be assed in clinical trials with cancer patients (3). In this article, we report the recommended core set of symptoms and HRQOL domains agreed upon by the H&N working group subcommittee that should be assessed in future clinical trials for H&N cancer patients.
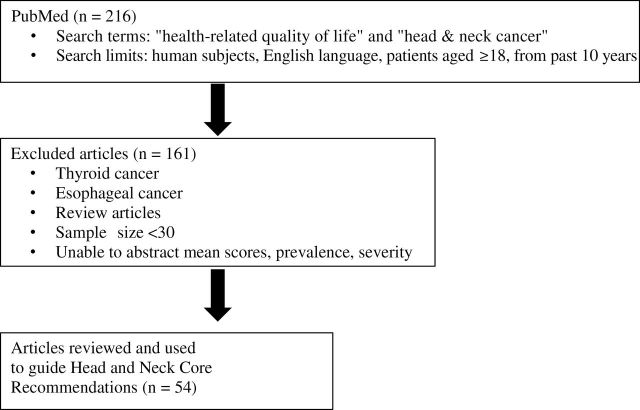
A literature review was conducted to guide the committee’s discussion (Figure 1). A PubMed (4) search was performed with the following search terms: “head & neck cancer” and “health-related quality of life.” The following search limitations were used (by all working groups) to narrow down the number of articles: 1) published in the period from January 1, 2000, to December 31, 2010; 2) published in English; 3) human subjects; and 4) patients aged 18 years or older. Two hundred sixteen articles were found, and 161 were excluded for the following reasons: sample size less than 30, primary cancer site was not squamous cell carcinoma of the H&N, review article, or mean/severity/prevalence scores were not reported. Thus, 54 articles (Figure 1; Supplementary Table 1, available online) were reviewed and used to guide the choice of recommended core symptoms and HRQOL domains. Mean symptom and HRQOL domain scores were abstracted from each study. Psychometric properties of the PRO measures used in the study and variability of clinical factors (eg, cancer site, treatment type) were not evaluated.
Figure 1.
Flowchart of the selection process of articles for the literature review. Fifty-four articles were selected for evaluation and review.
The H&N working group subcommittee members included experts in the fields of H&N radiation oncology (B. Movsas, A. Eisbruch, and B. S. Chera), H&N surgery (D. Ridge), medical oncology (B. Murphy), and HRQOL (B. Movsas and B. A. Murphy) and a patient advocate who is an H&N cancer survivor (P. Gavin). B. S. Chera was responsible for the literature search, providing the committee with a synopsis of the literature review and authorship of session summaries and manuscript materials. The expert panel had monthly teleconferences between February 2011 and August 2012. A list of symptoms and HRQOL domains based on the literature and expert review was created, and all six committee members completed a Web-based survey ranking each as high, medium, or low priority. Symptoms/HRQOL domains with more than five or six high-priority votes were considered first tier, those with three or four high-priority votes were considered second tier, and those with two or less high-priority votes were discarded. A core set of symptoms and HRQOL domains were selected from the first- and second-tier symptom/domain list. Consensus was reached through internal committee discussions. Symptoms that were independently identified by the cross-cutting working group were labeled as cross-cutting (3).
Fifty-four articles guided the committee’s recommendations (Figure 1; Supplementary Table 1, available online). The study design was prospective in 15 studies, and a majority of the remaining studies were cross-sectional. The most frequently used instrument (Table 1) was the European Organization for Research and Treatment of Cancer Quality of Life Questionniare-30 (EORTC QLQ-C30) and its companion H&N module (H&N-35). Other common instruments included the University of Washington Quality of Life Questionnaire and the Functional Assessment of Cancer Therapy–Head and Neck (FACT–HN). For the vast majority of the studies, pre- and post-treatment scores for symptoms and HRQOL domains returned to baseline values. Also, HRQOL was equivalent between H&N cancer survivors and normative cohorts. Only responses to questions for dry mouth and swallowing were consistently higher after treatment across studies. There were 29 symptoms and seven HRQOL domains that were commonly assessed in the literature and through the voting process and discussion at the subcommittee level (expert panel) and at the National Cancer Institute clinical trials planning meeting; 12 H&N-specific core symptoms and HRQOL domains were selected: 1) swallowing, 2) pain/oral, 3) skin changes, 4) dry mouth, 5) dental health, 6) opening mouth/trismus, 7) taste, 8) excess/thick mucous/saliva, 9) shoulder disability/motion, 10) voice/hoarseness, 11) social domain, and 12) functional domain (Supplementary Table 2, available online). An additional seven cancer cross-cutting symptoms were identified: 1) anorexia, 2) pain/general, 3) nausea/vomiting, 4) anxiety, 5) dyspnea, 6) fatigue, and 7) depression (Supplementary Table 2, available online). Of note, the symptom of skin changes was not found in the literature review but was selected as a core symptom based on expert opinion.
Table 1.
Health-related quality-of-life instruments used in the literature*
| Instruments | No. of Studies (n = 54) |
|---|---|
| EORTC QLQ-30/H&N-35 | 26 |
| UW-QOL | 11 |
| SF-36 | 7 |
| HADS | 5 |
| FACT-G & HN | 3 |
| Other | 17 |
* These are the most common health-related quality-of-life instruments used in studies obtained from the literature review. EORTC QLQ-30/H&N-35 = European Organization for Research and Treatment of Cancer Quality of Life Questionnaire 30/Head & Neck 35; FACT-G & HN = Functional Assessment of Cancer Therapy General and Head and Neck; HADS = Hospital Anxiety and Depression Scale; SF-36 = Short Form 36; UW-QOL= University of Washington Quality of Life Questionnaire.
The H&N working group identified 12 H&N cancer–specific symptoms and HRQOL domains that are most relevant for assessment in H&N clinical trials. In addition, we identified seven cross-cutting symptoms that should also be evaluated. These more general symptoms were also independently recommended by the cancer cross-cutting working group (3). The importance of incorporating PROs in clinical trial design is being recognized in the H&N oncology research community. Cooperative groups are now designing clinical studies with PROs as primary and secondary objectives/endpoints. However, several issues may need to be resolved before PROs are effectively used in clinical trials to yield practice-changing results.
Since the early 1990s, the major focus in H&N cancer has been the intensification of nonsurgical treatment with the addition of chemotherapy neoadjuvantly and concomitantly with radiotherapy. This has led to a dramatic escalation of the prevalence/severity/duration of acute and chronic toxicities with each successive clinical trial but with relatively small incremental improvements in outcomes (5). In our quest for finding a cure, toxicity has, at times, taken a back seat. Patient perspectives (ie, PROs) should be incorporated into H&N cancer clinical trials with focused hypotheses.
Recently it has been observed that more than 50% of oropharyngeal squamous cell carcinomas are associated with the human papilloma virus, and this patient cohort has been observed to be a unique clinical/biological/epidemiological entity that is associated with a markedly better prognosis (6–9). There are several initiatives underway with nonsurgical and surgical treatments to deintensify therapy with the purpose of reducing the toxicity burden on patients. This favorable cohort of patients may provide an excellent opportunity for incorporating PROs into clinical study designs.
The ongoing RTOG 1016 phase III study is an example of a combined endpoint analysis trial that both evaluates survival and PROs (10). As compared with radiation therapy (RT) alone, cetuximab in conjunction with RT has been shown to improve survival in locally advanced H&N cancer while not increasing the rate of grade 3 or greater mucositis or dysphagia, late effects, or worsening HRQOL (11,12). RTOG 1016 is evaluating the noninferiority and the acute and chronic toxicity burden of cetuximab vs cisplatin in conjunction with RT. In addition to clinician assessments of toxicity, RTOG 1016 incorporates relevant PRO measures (eg, Patient-Reported Outcomes version of the CTCAE, EORTC QLQ-C30/H&N-35).
Another approach for deintensification of treatment is to primarily treat with transoral laser microsurgery and transoral robotic surgery. The potential benefits of primary transoral laser microsurgery/transoral robotic surgery are subsequent reduction in the intensity of chemotherapy and RT without compromising oncologic outcomes. The RTOG and ECOG are currently conducting phase II trials that have incorporated PROs (RTOG 1221 and ECOG 3311) (13,14).
Our clinical knowledge regarding the trajectory of toxicities is limited. Occurrences of toxicities are traditionally divided into several time periods as they relate to treatment: acute, subacute, and chronic. It is likely that acute/subacute/chronic is a spectrum, and thus this arbitrary categorization may limit our understanding of treatment-related toxicity. Chronic toxicities (ie, xerostomia, dysphagia, dental health) likely begin during treatment, although their impact on patient HRQOL during the acute treatment phase may be overshadowed by other toxicities (ie, oral/pain). Furthermore, the impact of a particular toxicity for a patient may differ depending upon expectations, goals of treatment, and personal values. Furthermore, the interdependence of symptoms and HRQOL domains has not been well studied. For example, swallowing is affected by xerostomia, excess/thick mucous/saliva, and direct damage to the swallowing muscles.
In our literature review, we noted that PRO assessments were not performed during treatment. Thus, the validity of established PRO instruments during the acute treatment phase should be studied. Table 2 lists several commonly used validated instruments and denotes whether the recommended core symptoms are assessed. Of note, the validity of PRO instruments is already known to be affected by certain phenomena: response shift, floor/ceiling effect, and so on (15,16). The impact of these issues with repeated assessment of acute toxicities is unknown. In addition, most H&N cancer patients experience grade 3 or greater toxicity during treatment. A possible issue is the potential difficulty of collecting PRO data from patients who are acutely sick from their cancer treatments.
Table 2.
Selected health-related quality-of-life instruments for assessment of head and neck cancer core symptoms in clinical trials*
| Core symptom | EORTC QLQ-H&N-35 | UW-QOL | FACT-HN |
|---|---|---|---|
| Swallowing | Have you had problems swallowing liquids? Have you had problems swallowing pureed food? Have you had problems swallowing solid food? Have you choked when swallowing? |
I can swallow as well as ever. I cannot swallow certain solid foods. I can only swallow liquid food. I cannot swallow because it “goes down the wrong way” and chokes me. |
I can swallow naturally and easily. |
| Pain/oral | Have you had pain in your mouth? | I have no pain. There is mild pain not needing medication. I have moderate pain–requires regular medication (codeine or nonnarcotic). I have severe pain controlled only by narcotics. I have severe pain, not controlled by medication. |
I have pain in my mouth, throat, or neck. |
| Skin changes | NA | NA | NA |
| Dry mouth | Have you had a dry mouth? | My saliva is of normal consistency. I have less saliva than normal, but it is enough. I have too little saliva. I have no saliva. |
My mouth is dry. |
| Dental health | Have you had problems with your teeth? | NA | NA |
| Opening mouth/ trismus | Have you had problems opening your mouth wide? | NA | NA |
| Taste | Have you had problems with your sense of taste? | I can taste food normally. I can taste most foods normally. I can taste some foods. I cannot taste any foods. |
NA |
| Excess/thick mucous/ saliva | Have you had sticky saliva? | NA | NA |
| Shoulder disability/ motion | NA | I have no problem with my shoulder. My shoulder is stiff, but it has not affected my activity or strength. Pain or weakness in my shoulder has caused me to change my work. I cannot work due to problems with my shoulder. |
NA |
* Recommended 10 core head and neck cancer–specific symptoms that are assessed by selected health-related quality-of-life instruments. Functional and social domains are assessed in the European Organization for Research and Treatment of Cancer Quality of Life Questionniare-30 (EORTC QLQ-C30) and its companion Head & Neck module (H&N-35) and Functional Assessment of Cancer Therapy–Head and Neck (FACT-HN) but not the University of Washington Quality of Life Questionnaire (UW-QOL). There are many other validated instruments that may be appropriate. The most frequently used instruments in our literature are presented. NA = not assessed.
The recommended core set of symptoms/HRQOL domains (Supplementary Table 2, available online) is not meant to be all-encompassing. These recommendations are limited by what could be gleaned from the literature. Certainly there could be other symptoms and HRQOL domains that are important but were not reported/studied in the literature. Nevertheless, the experts on this panel agreed that these symptoms and HRQOL domains are relevant to most H&N cancer patients and should be evaluated in clinical trials. Depending on the clinical trial hypothesis, assessment of all of the recommended core symptoms and HRQOL domains may not be necessary, and other relevant symptoms/HRQOL domains may need to be included. Selection of which PROs to assess will also depend upon the specific questions being asked in the study. This recommended core set of PROs will provide a standardized framework to allow for uniformity of comparison and should serve as a foundation or starting point for incorporation of PROs as part of routine patient-reported assessment in H&N clinical trials.
In addition to the recommended symptoms from the cancer cross-cutting group (3), these symptoms and HRQOL domains should be assessed in H&N cancer clinical trials: swallowing, pain/oral, skin changes, dry mouth, dental health, opening mouth/trismus, taste, excess/thick mucous/saliva, shoulder disability/motion, voice/hoarseness, social domain, and functional domain.
The authors gratefully acknowledge Lori Stravers, MPH, for her help in the editing and formating of this manuscript.
References
- 1. Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26(21):3582–3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357(17):1705–1715 [DOI] [PubMed] [Google Scholar]
- 3. Reeve B. Recommended patient-reported core set of symptoms to measure in adult cancer treatment trials. J Natl Cancer Inst. 2014;XX(XX):XXX–XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. PubMed. http://www.ncbi.nlm.nih.gov/pubmed Accessed April 28, 2014
- 5. Trotti A, Pajak TF, Gwede CK, et al. TAME: development of a new method for summarising adverse events of cancer treatment by the Radiation Therapy Oncology Group. Lancet Oncol. 2007;8(7):613–624 [DOI] [PubMed] [Google Scholar]
- 6. Chaturvedi AK, Engels EA, Anderson WF, et al. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–619 [DOI] [PubMed] [Google Scholar]
- 7. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–269 [DOI] [PubMed] [Google Scholar]
- 9. Licitra L, Perrone F, Bossi P, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006;24(4):5630–5636 [DOI] [PubMed] [Google Scholar]
- 10.RTOG 1016 Protocol Information: Phase III Trial of Radiotherapy Plus Cetuximab Versus Chemoradiotherapy in HPV-Associated Oropharynx Cancer, Radiation Therapy Oncology Group, 2012. www.rtog.org Accessed April 28, 2014.
- 11. Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–578 [DOI] [PubMed] [Google Scholar]
- 12. Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11(1):21–28 [DOI] [PubMed] [Google Scholar]
- 13. RTOG. Disease Sites Table. http://www.rtog.org/ClinicalTrials/ProtocolTable.aspx Accessed April 28, 2014
- 14. ECOG-ACRIN. Head and Neck Disease Site Table. http://ecog-acrin.org/trials/head-and-neck#title Accessed April 28, 2014
- 15. Murphy BA, Ridner S, Wells N, Dietrich M. Quality of life research in head and neck cancer: a review of the current state of the science. Crit Rev Oncol Hematol. 2007;62(3):251–267 [DOI] [PubMed] [Google Scholar]
- 16. Breetvelt IS, Van Dam FS. Underreporting by cancer patients: the case of response-shift. Soc Sci Med. 1991;32(9):981–987 [DOI] [PubMed] [Google Scholar]