Abstract
Objective This meta-analysis examined the effectiveness of recent adherence-promoting interventions for youth with chronic health conditions. Methods Peer-reviewed randomized controlled trials of adherence-promoting interventions for youth with a chronic illness published between 2007 and 2013 (n = 23) were reviewed. Intervention delivery (in-person vs. technology-based) and outcome measurement (e.g., self-report) were examined as potential moderators of treatment effects. Results Mean effect sizes were small at posttreatment (d = 0.20, 95% confidence interval (CI): 0.08, 0.31, n = 23) and follow-up (d = 0.29, 95% CI: 0.15, 0.43, n = 9). Intervention delivery and outcome measurement did not account for variation in treatment effects (p > .05). Conclusions The small treatment effects of recent adherence-promoting intervention (APIs) reflect the methodological limitations of the included studies and the need to reexamine the delivery and mechanisms of adherence-promoting interventions.
Keywords: adherence, chronic illness, computer applications/eHealth, effect size, meta-analysis
An increasing number of children and adolescents are diagnosed with a chronic condition (Perrin, Bloom, & Gortmaker, 2007; Van Cleave, Gortmaker, & Perrin, 2010; van Dyck, Kogan, McPherson, Weissman, & Newacheck, 2004). A recent study examining a national sample of children showed the prevalence of at least one chronic condition over a 6-year period was >50% (Van Cleave et al., 2010). The majority of these chronic conditions require daily self-management including medication administration and dietary and physical activity requirements. Adherence, defined here as “the extent to which a person’s behavior coincides with medical or health advice” (Haynes, 1979; Modi et al., 2012) to these self-managed treatment regimens is essential for attaining adequate treatment exposure and achieving optimal health outcomes. However, treatment adherence is difficult, regardless of the severity of the condition. On average, rates of treatment regimen nonadherence are ∼50% and have been documented to be as high as 75% for adolescents and young adults (Rapoff, 2010). Pervasive nonadherence has substantial individual and societal implications, placing children and adolescents at an increased risk for morbidity and mortality and accounting for up to $300 billion in health-care costs (DiMatteo, 2004).
Given the profound and far reaching impact of nonadherence on individual health outcomes and overall public health as well as the modifiable nature of self-management behaviors, numerous psychological interventions targeting adherence have been developed. To date, three systematic reviews have been conducted on studies of adherence-promoting interventions for youth with chronic health conditions (Dean, Walters, & Hall, 2010; Graves, Roberts, Rapoff, & Boyer, 2010; Kahana, Drotar, & Frazier, 2008). These reviews show that adherence-promoting interventions can effectively increase adherence and lead to improved health outcomes (Dean et al., 2010; Graves etal., 2010; Kahana et al., 2008). The review by Kahana et al. (2008) also examined intervention characteristics and showed that behavioral (mean d = 0.54) and multicomponent (mean d = 0.51) adherence-promoting interventions demonstrate medium effect sizes. However, only a portion of these studies (n = 42, 60%) were randomized controlled trials, which yielded a much smaller effect size (mean d = 0.23).
Since the publication of these reviews, the field of adherence research has seen tremendous growth. The scientific maturation of the field has been accompanied by an increased rigor in scientific methods. Advances in measurement have facilitated the use of electronic monitoring devices to assess patient adherence, providing a more detailed and objective outcome variable (Quittner, Modi, Lemanek, Ievers-Landis, & Rapoff, 2008). The efficacy of adherence-promotion interventions is also being increasingly examined using randomized controlled trials. Previous reviews include many studies published before the development and implementation of electronic monitoring devices and include both randomized controlled trials and pre–post designs (Graves et al., 2010; Kahana et al., 2008). To accurately reflect the efficacy of adherence-promotion interventions as examined by these rigorous methods, it is imperative to conduct a systematic review of recently published studies.
In recent years, the nature of adherence-promotion interventions has also been significantly impacted by advances in technology and changes in the health-care system. Specifically, alternative methods of adherence-promoting intervention delivery (e.g., video conferencing) are increasingly being developed and tested. These methods have the potential to expand the reach of adherence-promoting interventions to those who would otherwise have difficulty accessing these interventions and may impact intervention efficacy. A review of recent literature is necessary to describe these innovative methods of delivery and examine their efficacy relative to traditional “in-person” interventions.
The purpose of the current review is to address the aforementioned gaps in the current state of science and assess the overall impact of adherence-promotion interventions for children, adolescents, and young adults with chronic health conditions published in the last 7 years on adherence and disease outcomes. The proposed review is particularly timely given the ongoing significant changes to the U.S. health-care system, including those outlined in the Patient Protection and Affordable Health Care Act (Public law 111–148). The Patient Protection and Affordable Health Care Act (Public law 111–148) includes provisions for medication management and the assessment of related outcomes, prompting health-care systems to prioritize patient adherence. By documenting the effectiveness of recent adherence-promotion interventions, this review will provide the information necessary for shaping health-care programs and policy.
In addition to assessing the overall effectiveness of adherence-promotion interventions, this review will explore the potential impact of advances in technology-based intervention delivery and adherence assessment methodology. Specifically, the secondary aims of this review are to (1) explore differences in effect sizes between adherence-promoting interventions that include defined technology-based treatments (e.g., delivered via tele-health) versus those that are not technology-based, and (2) examine differences in effect sizes based on the adherence assessment methodology used (e.g., adherence assessed via electronic monitors vs. self-report vs. biological assays). In contrast to previous meta-analyses examining adherence-promoting interventions, the current study will only include studies that report randomized controlled trials. This study also includes samples of adherence-promoting interventions with adolescents and young adults, an understudied population at high risk for poor adherence (Rapoff, 2010).
Based on previous adherence meta-analyses in pediatric and adult populations (Kahana et al., 2008), we predict that technology-based treatments will have smaller adherence effect sizes than in-person interventions, and adherence outcomes assessed via electronic monitors or bioassays will have smaller effect sizes than adherence outcomes assessed via self-report or parent report (Modi, Guilfoyle, Morita, & Glauser, 2011; Wu, Pai, Gray, Denson, & Hommel, 2013).
Methods
The systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines (Moher, Liberati, Tetzlaff, & Altman, 2009). The PRISMA Statement guidelines include a checklist of 27 items essential for transparent reporting and a four-phase flow diagram used to illustrate identified, screened, eligible, and included studies.
Literature Search
MEDLINE, EMBASE, PsychINFO, SCOPUS, EBMR, and CINAHL were searched in September 2013 for peer-reviewed original research articles. The search strategy included all possible combinations of medical subject headings terms from each of the following three categories: (1) infant, child, adolescent; (2) chronic disease, asthma, cystic fibrosis, sickle cell, obesity, transplant, hematology, gastrointestinal diseases, chronic pain, arthritis, communicable diseases, cancer, human immunodeficiency virus, inflammatory bowel disease, and juvenile rheumatoid arthritis; and (3) patient compliance, medication adherence. Search criteria were modified to include only articles published originally in English peer-reviewed journals between January 2007 and September 2013. To ensure all studies were subjected to a peer-review process required for publication in a journal, gray material was not included (Schmucker et al., 2013). The reference sections of articles and books/book chapters and reviews identified by the searches were also reviewed to capture additional citations not identified by the electronic searches.
Study Selection
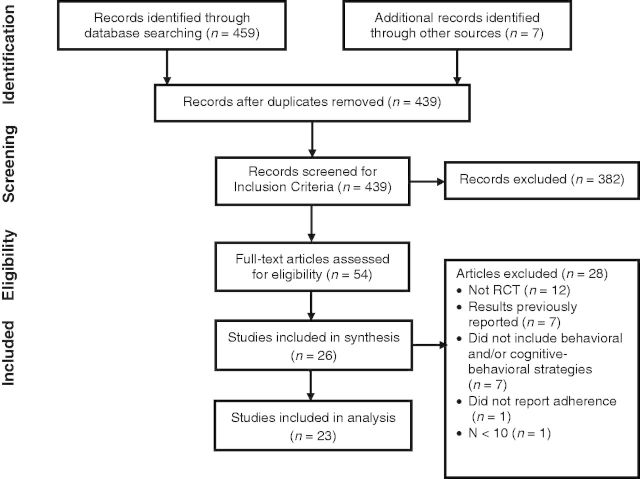
The PRISMA four-phase flow diagram detailing study selection is depicted in Figure 1 (Moher et al., 2009). The initial search resulted in 459 records (439 after removing duplicates). We screened 439 records and assessed 54 relevant manuscripts for eligibility. In all, 26 met the following inclusion criteria: (1) the sample comprised youth with a chronic health condition (and/or their parents); (2) the study was a randomized controlled trial targeting adherence via cognitive and/or behavioral interventions; (3) included an assessment of adherence; (4) n ≥ 10 in both arms; (5) mean age of participants for a given study was ≤19 years of age to reflect that the article had a pediatric focus; and (6) the upper age of the young adult period for a given study was ≤35 years. Thirty-five years age requirement was selected to exclude predominately adult adherence articles and is consistent with the upper age limit of adolescent and young adulthood as defined by the Patient Protection and Affordable Health Care Act (Public law 111–148).
Figure 1.
The PRISMA four-phase flow diagram detailing study selection.
Missing Data
In all cases where additional unreported data were needed to calculate an effect size, substantial and repeated attempts were made to contact the authors of the articles in question (n = 10 authors contacted, n = 7 authors provided data). Three studies were excluded from the review after all possible avenues for obtaining the necessary data were exhausted yielding a final sample size of 23.
Data Extraction
Data extraction was completed by one of the authors (M.McG.) and one research assistant using a standardized data collection form. Data retrieved from the articles included: (1) study design; (2) intervention characteristics (length, format, mode, interventionists); (3) control group description; (4) participant demographic and clinical characteristics; (5) measures of adherence; and (6) outcomes for adherence measures. Interrater agreement for data extraction was calculated on a random 50% of articles (n = 12) and found to be acceptable (κ = 0.74). All discrepancies were resolved via discussion and subsequent consensus of the authors (A.L.H.P. and M.McG.).
Definitions of Variables
The primary outcome measure was youth adherence, defined as “the extent to which a person’s behavior coincides with medical or health advice” (Haynes, 1979). Adherence outcome measures varied across studies and included bioassays, self-report questionnaires, parent-report questionnaires, semistructured interviews, prescription refill histories, daily diaries, and electronic monitoring devices. When multiple measures of adherence were available, the results as demonstrated by the most well-established instrument were included (e.g., electronic monitoring selected over self-report measures; Quittner et al., 2008).
Risk of Bias Assessment
Risk of bias was assessed using the Cochrane Collaboration’s recommended tool (Higgins & Green, 2008). This tool addresses six domains that impact risk of bias: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and “other issues.” Consistent with published guidelines (Guyatt, Oxman, Vist, et al., 2011), one author (M.McG.) categorized the risk of bias of each study on each domain as “low,” “unclear,” or “high” risk of bias. The proportion of studies with each judgment (“low,” “unclear,” or “high”) was calculated by compiling ratings for each domain across all studies. As a measure of reliability, a postdoctoral fellow completed risk of bias ratings on a random 50% of articles (n = 12). Interrater agreement was acceptable (κ = 0.70), and all discrepancies were resolved via discussion.
Quality of the Evidence
The overall quality of evidence for each outcome was rated using the GRADE approach (Balshem et al., 2011; Guyatt, Oxman, Kunz, Brozek, et al., 2011; Guyatt, Oxman, Kunz, Woodcock, Brozek, Helfand, Alonso-Coello, Falck-Ytter, etal., 2011; Guyatt, Oxman, Kunz, Woodcock, Brozek, Helfand, Alonso-Coello, Glasziou, et al., 2011; Guyatt, Oxman, Montori, et al., 2011; Guyatt, Oxman, Sultan, etal., 2011; Guyatt, Oxman, Vist, et al., 2011). The GRADE approach is a method for rating the quality of evidence and grading the strength of recommendations for a particular outcome. One author (M.McG.) and one postdoctoral fellow examined all studies that included the outcomes of interest (adherence, n = 23; adherence at follow-up, n = 9; disease outcome, n = 10; disease outcome at follow-up, n = 5) and rated limitations on design and implementation, inconsistency, indirectness, imprecision, and publication bias for that group of studies. Results of rating comparisons indicated that raters were in complete agreement (κ = 1.00). GRADEpro software (GRADEpro, 2008) was then used to synthesize these ratings and determine the final quality of evidence. Outcomes are rated as being supported by high-quality evidence when there is a low likelihood of bias and there are no concerns regarding the aforementioned categories. Concerns regarding consistency, directness, precision, and publication bias result in downgrades to moderate, low, or very low.
Analytic Plan
Study procedures, including effect size calculation, were based on guidelines outlined by the Cochrane Collaboration (Higgins & Green, 2008) and Lipsey and Wilson (2001). Comprehensive meta-analysis (Biostat, 2005) was used to calculate Cohen’s d, the primary summary effect size measure used in this study. Effect sizes for the adherence-promoting interventions were examined in the following ways: (1) the overall effectiveness of adherence-promoting interventions were examined across studies at posttreatment and at follow-up, (2) adherence-promoting intervention effectiveness by the type of measure used to assess adherence, and (3) adherence-promoting intervention effectiveness was compared between interventions delivered via technology and those delivered in person. Random effects models were used to calculate all mean effect sizes to include study-level as well as subject-level sampling error and yield a more conservative estimate of the mean effect size. This approach is recommended when analyzing a small number of studies that have small sample sizes (Lipsey & Wilson, 2001).
Quantification of Heterogeneity. To describe the degree of inconsistency across studies, we calculated the I2 statistic (Higgins, Thompson, Deeks, & Altman, 2003). I2 refers to the proportion of heterogeneity across effect sizes that is unlikely to be due to chance and is typically expressed as a percentage. It can be used to describe heterogeneity related to the choice of measure and intervention subgroups (Higgins et al., 2003). An I2 of <30% is considered mild heterogeneity, and an I2 of ≥50% is considered substantial (Higgins & Thompson, 2002) This method was chosen over alternative methods (e.g., Cochran’s Q-test [Cochran, 1954; Whitehead & Whitehead, 1991]) for indicating the extent of heterogeneity because of the poor power the Q-test has in meta-analyses that include relatively few studies.
Results
Participant and Study Design
A total of 23 reports representing an equal number of randomized controlled studies and 3898 participants (Msample size = 169, SD = 149) were included in the current meta-analysis (Table 1). The majority of adherence-promoting interventions (n = 14, 61%) included both youth and their families. Four adherence-promoting interventions (17%) targeted youth only, two (9%) targeted caregivers only, and three (13%) were multisystemic. Across studies, 55% of patients were male. Patients included were between 2 and 29 years of age. Three studies included young children under the age of 11 years, six included patients between 12 and 29 years of age, and 14 studies included children spanning childhood and adolescence (1–18 years of age). The most common chronic condition represented was asthma (n = 10, 43%) followed by diabetes (type 1 or type 2, n = 6, 26%), and other chronic conditions (n = 7, 30%; cancer, human immunodeficiency virus, inflammatory bowel disease, and juvenile rheumatoid arthritis). Four studies (17%) specifically targeted youth with adherence concerns or poor disease control. Control groups included usual care conditions (n = 11, 48%), education groups (n = 5, 22%), and other (e.g., multiple control groups, n = 7, 30%).
Table I.
Characteristics of Included Studies
| Author (year) | n | Medical Diagnosis | Patient ages (range) | Intervention components* | Interventionist | Adherence behavior | Adherence measure |
|---|---|---|---|---|---|---|---|
| Burgess (2010) | 26 | Asthma | 6–14 years | B, E, T | Health-care provider | Controller medication | Electronic monitor |
| Burkhart (2007) | 77 | Asthma | 7–11 years | B, E | Health-care provider | Daily peak expiratory flow monitoring | Electronic monitor |
| Christakis (2012) | 603 | Asthma | 2–10 years | B, T, C | Web-based | Controller medication | Parent report |
| Ducharme (2011) | 219 | Asthma | 1–17 years | B, E | Health-care provider | Controller medication | Electronic monitor |
| Duncan (2013) | 48 | Asthma | 9–15 years | B, E, H, S, F | Psychology trainee | Controller medication | Electronic monitor |
| Ellis (2007) | 117 | Type 1 diabetes | 10–17 years | B, O, E, H, C, S, F | Psychologist/social worker | Blood glucose monitoring frequency | Blood glucose meter download |
| Ellis (2012) | 120 | Type 1 or 2 diabetes | 10–18 years | B, O, E, H, C, S, F | Psychologist/social worker | Diabetes regimen adherence | Parent report |
| Hederos (2009) | 54 | Asthma | 0–6 years | E, C, S | Health-care providers and psychologists | Adherence to asthma-related recommendations | Parent report |
| Hommel (2012) | 40 | Inflammatory bowel disease | 11–17 years | B, O, E, F | Psychologist/psychology trainee | 6-MP/azathioprine and/or Mesalamine | Electronic monitor |
| Joseph (2007) | 314 | Asthma | 15–19 years | B, E, T | Web-based | Controller medication | Self-report |
| Kato (2008) | 371 | Cancer | 13–29 years | B, E, T, C | Web-based | Antimicrobial prophylaxis | Electronic monitor |
| Krieger (2009) | 271 | Asthma | 3–13 years | B, E, H, C, S | Health-care provider and community health worker | Controller medication | Parent report |
| Letourneau (2012) | 34 | HIV/AIDS | 9–17 years | B, O, E, H, C, S, F | Psychologist/psychology trainee | Medication | Patient report |
| Mulvaney (2010) | 52 | Type 1 diabetes | 13–17 years | B, T, C, S | Web-based | Diabetes self-management | Patient report |
| Naar-King (2013) | 76 | HIV | 16–24 years | B, E, T, C | Web-based | Medication | Patient report |
| Nansel (2007) | 81 | Type 1 diabetes | 11–16 years | B, E, C | “Health advisor” | Diabetes self-management | Patient report |
| Nansel (2012) | 390 | Type 1 diabetes | 9–15 years | B, C, F | “Personal trainer” | Blood glucose monitoring frequency | Blood glucose meter download |
| Otsuki (2009) | 250 | Asthma | 2–12 years | B, E | Health-care provider | Controller medication | Pharmacy refills |
| Scholten (2013) | 194 | Chronic illness | 8–18 years | E, C, F, S | Psychologist | Medical regimen adherence | Patient report |
| Stinson (2010) | 46 | Juvenile idiopathic arthritis (JIA) | 12–18 years | B, E, T, C, S | “Coach” | JIA regimen adherence | Patient report |
| Wamalwa (2009) | 99 | HIV | 15 months–12 years | O | Health-care provider | Antiretrovial adherence | Parent report |
| Wysocki (2007) | 104 | Type 1 diabetes | 12–17 years | B, E, C, F | Psychologist | Diabetes self-management | Parent- and patient report |
| Zivkovic (2008) | 302 | Asthma | 5–18 years | B, E | Health-care provider | Controller medication | Parent report |
Note. *B = behavioral; O = organizational; E = education; H = health-care system; T = technology-based; C = cognitive-behavioral; S = social support; F = family therapy.
Measures
Adherence outcomes were most frequently assessed via electronic monitoring (n = 8, 35%), followed by youth report (n = 7, 30%), parent report (n = 5, 22%). Other outcomes included pharmacy records and combined youth and parent report. Adherence and health outcomes were assessed at posttreatment (i.e., first reported assessment after the completion of the intervention; range: 0–180 days) and at follow-up (range: 1–72 months).
Intervention Characteristics
Eleven (48%) of the adherence-promoting interventions were explicitly grounded in a theoretical framework (e.g., Social Cognitive Theory, n = 6; Self-Regulation Model, n = 1). The majority of adherence-promotion interventions were delivered by a psychologist (n = 7, 30%). The remaining adherence-promoting interventions were delivered by a health-care provider (n = 6, 26%), multiple or other providers [n = 5, 22%; diabetes personal trainer (n = 2), bachelor’s level “coach” (n = 1), health-care provider and psychologist (n = 1), health-care provider, and community interventionist (n = 1)], or via automated Web-based content (n = 5, 22%). The interventions were delivered in outpatient medical clinics (n = 4, 17%), multiple settings (e.g., home and school or clinic and home, n = 4, 17%), a setting of the family’s choice (e.g., home or public location, n = 2, 9%), a psychology clinic (n = 1, 4%), a research space next to clinic (n = 1, 4%), the family’s home (n = 1, 4%), the emergency department (n = 1, 4%), a school (n = 1, 4%), or an undefined “clinic” (n = 1, 4%). The remaining trials used Web-based delivery of intervention content (n = 6, 26%) or did not specify the intervention location (n = 1, 4%). Almost all of the interventions were multicomponent interventions (n = 22, 96%). The only single-component intervention was retained in the study because it met all a priori criteria for study inclusion (Wamalwa et al., 2009). The most commonly used intervention techniques were behavioral (n = 20, 87%), educational (n = 17, 74%), and cognitive behavioral (n = 14, 61%). Thirteen studies (57%) included a set number of intervention sessions in which all youth or families participated [M number of sessions (SD) = 4.31(2.87), range = 1–12]. In four studies (13%), the number of intervention sessions was dependent on the needs of the participants (n = 3, 13%, range of M sessions=46–53) or the frequency of clinic appointments (n = 1, 4%). Web-based interventions reporting on session content (n = 5, n = 1 missing) consisted of 4–12 modules. Only two studies reported completion rates, and three additional studies reported the average number of hours spent in the intervention or the average number of intervention sessions the patients were exposed to. All studies targeted “adherence” or “self-management,” only 13 (57%) reported specific behaviors targeted as part of the intervention. These included the following: medication taking (n = 8, 35%), disease-related coping skills (n = 1, 4%), self-management and problem-solving skills (n = 1, 4%), adherence to daily peek expiratory flow monitoring (n = 1, 4%), medication availability, use, and other health behaviors (n = 1, 4%), and medication taking and adherence to clinic visits (n = 1, 4%).
Overall Adherence-Promoting Intervention Effect Sizes
Effect sizes for all analyses are listed in Table II. Across adherence-promoting interventions, a small effect was observed at posttreatment where those receiving the adherence-promoting intervention had higher adherence than those in the control condition (d = 0.20, 95% confidence interval (CI): 0.08, 0.31, n = 23 trials). Only nine studies had more than one follow-up time point allowing for the examination of the maintenance of treatment effects. The effect size reflecting maintenance of treatment effects was medium (d = 0.29, 95% CI: 0.15, 0.43, n = 9 trials). The effect size for disease outcomes posttreatment was d = 0.35 (95% CI: 0.22, 0.46, n = 10 trials) and included measures of pulmonary functioning [forced expiratory volume (FEV1), n = 3], glycemic control [hemoglobin A1c (HbA1c), n = 6], and immune functioning (CD4 count, n = 1). The effect size for disease outcomes at follow-up was d = 0.30 (95% CI: 0.11, 0.49, n = 5) and was assessed using similar measures (FEV1, n = 1; HbA1c, n = 3; CD4 count, n = 1)
Table II.
Summary of Findings
|
Patient or Population: Children and adolescents with a chronic medical condition | |||||
|---|---|---|---|---|---|
|
Settings: Medical clinic, community, or home | |||||
|
Intervention: Psychological intervention (i.e., behavioral, organizational, cognitive-behavioral) | |||||
| Outcome | Ratio of means (95% CI) | Change in intervention group | Participants (studies) N | Quality of the evidence (GRADE) | Standard mean difference (95% CI) |
| Adherence (all studies) | 3,070 (23) | ⊕⊕⊕⊝ Moderate1 | 0.20 (0.08, 0.31) | ||
| Adherence (studies with RoM data) | 1.06 (1.02, 1.09) | 6% change in the mean value of the intervention group relative to the control group | 1,195 (12) | ⊕⊕⊝⊝ Low2 | 0.24 (0.09, 0.39) |
| Adherence (studies with RoM data at follow-up) | 1.11 (1.03, 1.21) | 11% change in the mean value of the intervention group relative to the control group | 831 (9) | ⊕⊕⊝⊝ Low2 | 0.29 (0.15, 0.43) |
| Disease outcomes | 1,021 (10) | ⊕⊕⊝⊝ Low2 | 0.34 (0.22, 0.46) | ||
| Disease outcomes (at follow-up) | 432 (5) | ⊕⊕⊝⊝ Low2 | 0.30 (0.11, 0.49) | ||
Note. *CI = Confidence interval; RoM = ratio of means; 1Indirectness: small sample sizes; 2Indirectness: small sample sizes; Imprecision in results: wide confidence intervals.
GRADE Working Group grades of evidence.
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Adherence-Promoting Intervention Effect Sizes by Adherence Measures and Delivery Modality
We then examined whether the types of adherence outcomes used to test the adherence-promoting interventions and whether the adherence-promoting intervention was delivered via technology or not influenced the effect sizes (see Tables III and IV). Significant overall effects were observed for adherence outcomes when electronic monitors, patient and parent report, and physician reports were used (p's < .05). The overall effects of both in-person and technology-based interventions were significant (p's < .02).
Table III.
Forest Plot of Effects by Adherence Measurement
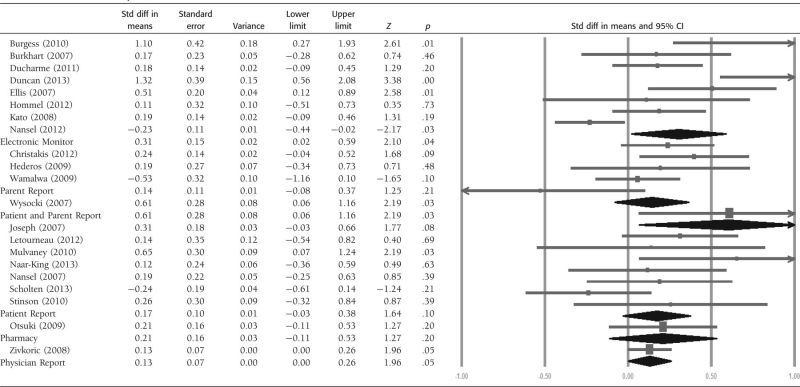 |
Table IV.
Forest Plot of Effect Sizes by Treatment Delivery Type
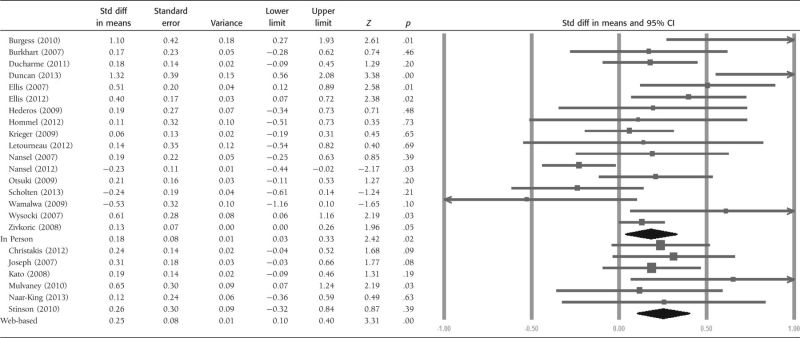 |
Description of Heterogeneity
Substantial heterogeneity (I2 = 76.75%) was observed across studies that used electronic monitoring (n = 8), but only moderate to low heterogeneity was observed for studies that used parent- or patient-reported adherence outcomes (I2 < 47%; n = 12). Substantial heterogeneity (I2 = 63.66%; n = 17) was observed for interventions that were delivered in person versus those delivered via technology.
Risk of Bias Analyses
The risk of bias ratings showed that two studies (9%) demonstrated high risk of bias and four (17%) demonstrated unclear risk of bias related to sequence generation (see Figure 2 for a summary of the risk of bias analysis and Supplementary Figure 1 Online Material for individual risk of bias ratings for each study). Studies demonstrating high risk of bias related to sequence generation were limited by logistical difficulties (i.e., interventionists not available at all study sites). All studies (n = 23) demonstrated low or unclear risk of bias regarding allocation concealment (e.g., sealed, opaque envelopes), accounting of patients and outcome events (e.g., describing all patients lost to follow-up), and selective reporting (e.g., reporting all results regardless of findings). Of note, 3 studies (13%) demonstrated high risk of bias and 18 (78%) demonstrated unclear risk of bias related to blinding. In all studies, the high or unclear risk of bias was due to difficulties in blinding participants involved in psychosocial interventions.
Figure 2.
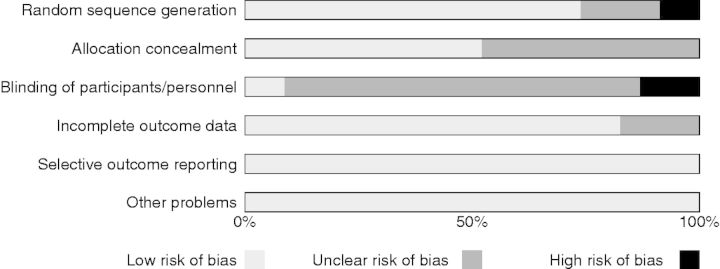
Risk of Bias summary.
GRADE Summary of the Evidence
The summary of findings for this study is presented in Table II. For the articles included in this study, the quality of the evidence base for postintervention adherence was moderate as a result of downgrading for imprecision in results. Moderate quality of an evidence base indicates that additional research will likely impact our confidence in the effect and may change the estimate itself. The quality of the evidence base for follow-up adherence and postintervention and follow-up disease outcomes was downgraded to low because of imprecision in results and indirectness. Low quality of an evidence base suggests that future research is very likely to significantly impact our confidence in the estimate of the effect and is likely to change the estimate.
Discussion
This meta-analysis examined the most recent body of literature of randomized clinical trials examining the efficacy of adherence-promoting interventions for children, adolescents, and young adults. An updated meta-analysis summarizing the effectiveness of adherence-promoting interventions was warranted, as technological advances have influenced both how we deliver and assess the outcomes of adherence interventions. This study also provides updated benchmarking on adherence-promoting intervention assessment and methodology since the previously published reviews (Dean et al., 2010; Graves et al., 2010; Kahana et al., 2008) as well as critical next steps for the field.
Overall, both adherence and disease outcome effect sizes for the included studies were small at postintervention and at follow-up. In this sample of adherence-promoting interventions, effect sizes differed modestly based on the type of measure used to assess adherence but not based on whether the intervention included technological components. The effect sizes observed in this meta-analysis were smaller than those in which researchers included all adherence-promotion interventions regardless of study design (Graves et al., 2010; Kahana et al., 2008). Results, however, are consistent with previous secondary analyses including only studies comparing experimental and control groups (d = 0.23, 95% CI: 0.17, 0.29, n = 54). Thus, it is likely that the increased methodological rigor of studies included in this review accounts for the smaller effect sizes observed in this meta-analyses compared with previous studies (Kahana et al., 2008).
The small effect sizes in this study may also be partially attributable to the inclusion of one single-component intervention. The effect size of this intervention was in an unexpected direction and likely attenuated the overall effect size reported in this study. Although the examination of treatment components as a moderating variable was planned, this analysis was not possible because only one single-component intervention met inclusion criteria. The increased number of multicomponent interventions may represent evidence-based intervention development consistent with findings by Kahana et al. (2008) illustrating that multicomponent interventions produce higher mean adherence effects than educational/instructional interventions. However, as this treatment component is a known predictor of intervention effectiveness, when sample sizes allow, future reviews should examine the potential moderating effect of single- versus multiple-component interventions.
Finally, the relatively small effects observed in this study may also reflect the need to test adherence-promoting interventions on those with identified adherence difficulties. Only four of the studies included patients and families with identified adherence problems. Recent longitudinal adherence studies show that >40% (42% Modi, Rausch, & Glauser, 2011; 43% Rohan et al., 2013) of youth with chronic illnesses may demonstrate “near-perfect adherence.” Therefore, the effects of studies that do not target patients with poor adherence patterns may be attenuated by ceiling effects.
Implications for Future Adherence-Promoting Intervention Research
The findings of this study highlight key methodological issues that should be considered when planning future adherence-promoting intervention research. Studies included in this review conducted posttreatment and follow-up assessments at a wide range of time points. To facilitate comparisons across studies, inform conclusions regarding intervention durability, and increase the likelihood of dissemination, the timing of assessments should be firmly anchored in theoretical or logistical rationale. For example, a study designed to include assessments at time points that map on to regular clinical care (e.g., corresponding to clinic follow-up visits that occur every 3 months) may facilitate the evaluation of specific treatments and subsequent dissemination efforts. In addition, studies designed to include assessments following transitions in the treatment regimen (e.g., transition from one medication to another) would provide increased information regarding transfer of skills to a new treatment regimen.
To date, the majority of adherence-promoting interventions have been designed for a specific chronic illness population. Although illness-specific tailoring allows interventions to specifically target the recommended medical regimen, additional attention to developmental level of the youth may offer an additional pathway to increasing adherence-promoting intervention effectiveness. The majority of the samples of the studies included in this meta-analysis (n = 14) included patients across childhood and adolescence. Regardless of the illness, adolescents consistently have more adherence difficulties than younger children (Rapoff, 2010). Therefore, it is possible that intervention effects may have been attenuated by samples including younger children with fewer adherence difficulties. Unfortunately, because of the high variability in the age ranges of the samples, we were unable to directly test whether developmentally specific interventions yielded larger effect sizes. Despite the logistical challenges that developmentally specific samples present, future adherence-promoting intervention research should strive to comprise relatively developmentally narrow samples to maximize the accuracy of adherence-promoting intervention effect estimates.
Limitations
The conclusions drawn from this meta-analysis must be considered in light of limitations inherent to meta-analytic methods and of the available existing literature. As the purpose of this study was to describe the efficacy of recent adherence-promotion interventions, only randomized controlled trials published since 2007 were included. This methodological decision resulted in a relatively small number of studies meeting inclusion criteria and excluded randomized controlled trials included in previous reviews (Graves et al., 2010; Kahana et al., 2008). Results should be interpreted in the context of this decision and may not be representative of the entire body of adherence-promotion intervention literature. Moreover, the quality of the evidence base, as evaluated using the GRADE approach, were low to moderate, suggesting that future research is likely to influence the estimates reported here. In short, additional research will be needed to say with confidence whether the effect sizes reported here are accurate representations of the treatment effects. Another limitation is the small number of studies coupled with the diversity of pediatric populations and adherence targets included in the study. Our small sample size inhibited the examination of several important treatment components (i.e., interventionist, guiding theoretical framework, pediatric population, single- vs. multi-component treatments) that may impact treatment effectiveness. Finally, as with all meta-analyses, this study is susceptible to the “file-drawer” problem and therefore may overrepresent studies that had statistically significant effects.
Conclusion
The current findings reflect the substantial challenges inherent in conducting adherence-promoting intervention research. Targeting subgroups of patients with adherence difficulties from already small pediatric populations makes sufficiently powering trials difficult. Going forward, an increased emphasis will be needed on collaborative and team research including multisite studies to obtain the sample sizes needed to effectively test adherence-promoting interventions. This will enable future meta-analyses to more thoroughly examine specific types of interventions, such as technology-based interventions, in the future. Finally, increasing the time to follow-up for adherence-promoting interventions could assist in determining the frequency of adherence-promoting intervention sessions are needed to maintain adherence over long periods. This is especially important in adherence-promoting intervention work, as the majority of these patients will be managing a treatment regimen for the rest of their lives.
Supplementary Data
Supplementary data can be found at: http://www.jpepsy.oxfordjournals.org/
Funding
The conduct of the study; collection, management, analysis, and interpretation of the data; and preparation of the manuscript were supported in part by grant T32HD068223 for M.E.M.
Conflicts of interest: None declared.
Supplementary Material
References
- *An asterisk in the list of references denotes studies included in the meta-analysis.
- Balshem H, Helfand M, Schunemann H J, Oxman A D, Kunz R, Brozek J, Vist G E, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt G H. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. doi:10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Biostat. Comprehensive meta-analysis. Englewood, NJ: 2005. [Google Scholar]
- *Burgess S W, Sly P D, Devadason S G. Providing feedback on adherence increases use of preventive medication by asthmatic children. Journal of Asthma. 2010;47:198–201. doi: 10.3109/02770900903483840. doi:10.3109/02770900903483840. [DOI] [PubMed] [Google Scholar]
- *Burkhart P V, Rayens M K, Oakley M G, Abshire D A, Zhang M. Testing an intervention to promote children's adherence to asthma self-management. Journal of Nursing Scholarship. 2007;39:133–140. doi: 10.1111/j.1547-5069.2007.00158.x. doi:10.1111/j.1547-5069.2007.00158.x. [DOI] [PubMed] [Google Scholar]
- *Christakis D A, Garrison M M, Lozano P, Meischke H, Zhou C, Zimmerman F J. Improving parental adherence with asthma treatment guidelines: A randomized controlled trial of an interactive website. Academic Pediatrics. 2012;12:302–311. doi: 10.1016/j.acap.2012.03.006. doi:10.1016/j.acap.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane W G. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. doi:10.2307/3001666. [Google Scholar]
- Dean A J, Walters J, Hall A. A systematic review of interventions to enhance medication adherence in children and adolescents with chronic illness. Archives of Diseases in Childhood. 2010;95:717–723. doi: 10.1136/adc.2009.175125. doi:10.1136/adc.2009.175125. [DOI] [PubMed] [Google Scholar]
- DiMatteo M R. Variations in patients’ adherence to medical recommendations: A quantitative review of 50 years of research. Medical Care. 2004;42:200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. doi:10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- *Ducharme F M, Zemek R L, Chalut D, McGillivray D, Noya F J, Resendes S, Khomenko L, Rouleau R, Zhang X. Written action plan in pediatric emergency room improves asthma prescribing, adherence, and control. American Journal Respiratory and Critical Care Medicine. 2011;183:195–203. doi: 10.1164/rccm.201001-0115OC. doi:10.1164/rccm.201001-0115OC. [DOI] [PubMed] [Google Scholar]
- *Duncan C L, Hogan M B, Tien K J, Graves M M, Chorney J M, Zettler M D, Koven L, Wilson N W, Dinakar C, Portnoy J. Efficacy of a parent–youth teamwork intervention to promote adherence in pediatric asthma. Journal of Pediatric Psychology. 2013;38:617–628. doi: 10.1093/jpepsy/jss123. doi:10.1093/jpepsy/jss123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Ellis D A, Naar-King S, Chen X, Moltz K, Cunningham P B, Idalski-Carcone A. Multisystemic therapy compared to telephone support for youth with poorly controlled diabetes: Findings from a randomized controlled trial. Annals of Behavioral Medicine. 2012;44:207–215. doi: 10.1007/s12160-012-9378-1. doi:10.1007/s12160-012-9378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Ellis D A, Templin T, Naar-King S, Frey M A, Cunningham P B, Podolski C L, Cakan N. Multisystemic therapy for adolescents with poorly controlled type I diabetes: Stability of treatment effects in a randomized controlled trial. Journal of Consulting and Clinical Psychology. 2007;75:168–174. doi: 10.1037/0022-006X.75.1.168. doi:10.1037/0022-006x.75.1.168. [DOI] [PubMed] [Google Scholar]
- GRADEpro. [Computer program] 2008. Version 3.2 for Windows. Jan Brozek, Andrew Oxman, Holger Schünemann. [Google Scholar]
- Graves M M, Roberts M C, Rapoff M, Boyer A. The efficacy of adherence interventions for chronically ill children: A meta-analytic review. Journal of Pediatric Psychology. 2010;35:368–382. doi: 10.1093/jpepsy/jsp072. doi:10.1093/jpepsy/jsp072. [DOI] [PubMed] [Google Scholar]
- Guyatt G H, Oxman A D, Kunz R, Brozek J, Alonso-Coello P, Rind D, Devereaux P J, Montori V M, Freyschuss B, Vist G, Jaeschke R, Williams J W, Jr, Murad M H, Sinclair D, Falck-Ytter Y, Meerpohl J, Whittington C, Thorlund K, Andrews J, Schunemann H J. GRADE guidelines 6. Rating the quality of evidence–imprecision. Journal of Clinical Epidemiology. 2011;64:1283–1293. doi: 10.1016/j.jclinepi.2011.01.012. doi:10.1016/j.jclinepi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Guyatt G H, Oxman A D, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Falck-Ytter Y, Jaeschke R, Vist G, Akl E A, Post P N, Norris S, Meerpohl J, Shukla V K, Nasser M, Schunemann H J, GRADE Working Group GRADE guidelines: 8. Rating the quality of evidence–indirectness. Journal of Clinical Epidemiology. 2011;64:1303–1310. doi: 10.1016/j.jclinepi.2011.04.014. doi:10.1016/j.jclinepi.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Guyatt G H, Oxman A D, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Glasziou P, Jaeschke R, Akl E A, Norris S, Vist G, Dahm P, Shukla V K, Higgins J, Falck-Ytter Y, Schünemann H J, GRADE Working Group GRADE guidelines: 7. Rating the quality of evidence—inconsistency. Journal of Clinical Epidemiology. 2011;64:1294–1302. doi: 10.1016/j.jclinepi.2011.03.017. doi:10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Guyatt G H, Oxman A D, Montori V, Vist G, Kunz R, Brozek J, Alonso-Coello P, Djulbegovic B, Atkins D, Falck-Ytter Y, Williams J W, Jr, Meerpohl J, Norris S L, Akl E A, Schunemann H J. GRADE guidelines: 5. Rating the quality of evidence–publication bias. Journal of Clinical Epidemiology. 2011;64(12):1277–1282. doi: 10.1016/j.jclinepi.2011.01.011. doi:10.1016/j.jclinepi.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Guyatt G H, Oxman A D, Sultan S, Glasziou P, Akl E A, Alonso-Coello P, Atkins D, Kunz R, Brozek J, Montori V, Jaeschke R, Rind D, Dahm P, Meerpohl J, Vist G, Berliner E, Norris S, Falck-Ytter Y, Murad M H, Schünemann H J, GRADE Working Group GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology. 2011;64:1311–1316. doi: 10.1016/j.jclinepi.2011.06.004. doi:10.1016/j.jclinepi.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Guyatt G H, Oxman A D, Vist G, Kunz R, Brozek J, Alonso-Coello P, Montori V, Akl E A, Djulbegovic B, Falck-Ytter Y, Norris S L, Williams J W, Jr, Atkins D, Meerpohl J, Schunemann H J. GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias) Journal of Clinical Epidemiology. 2011;64:407–415. doi: 10.1016/j.jclinepi.2010.07.017. doi:10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Haynes R B. Introduction. In: Haynes R B, Taylor D W, Sackett D L, editors. Compliance in health care. Baltimore, MD: Johns Hopkins University Press; 1979. [Google Scholar]
- *Hederos C A, Janson S, Hedlin G. Six-year follow-up of an intervention to improve the management of preschool children with asthma. Acta Paediatrics. 2009;98:1939–1944. doi: 10.1111/j.1651-2227.2009.01477.x. doi:10.1111/j.1651-2227.2009.01477.x. [DOI] [PubMed] [Google Scholar]
- Higgins J P, Green S. Cochrane handbook for systematic reviews of interventions. West Sussex: John Wiley & Sons; 2008. [Google Scholar]
- Higgins J P, Thompson S G. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21:1539–1558. doi: 10.1002/sim.1186. doi:10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Higgins J P, Thompson S G, Deeks J J, Altman D G. Measuring inconsistency in meta-analyses. British Medical Journal. 2003;237:557–560. doi: 10.1136/bmj.327.7414.557. doi: http://dx.doi.org/10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Hommel K A, Hente E A, Odell S, Herzer M, Ingerski L M, Guilfoyle S M, Denson L A. Evaluation of a group-based behavioral intervention to promote adherence in adolescents with inflammatory bowel disease. European Journal of Gastroenterology and Hepatology. 2012;24:64–69. doi: 10.1097/MEG.0b013e32834d09f1. doi:10.1097/MEG.0b013e32834d09f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Joseph C L, Peterson E, Havstad S, Johnson C C, Hoerauf S, Stringer S, Gibson-Scipio W, Ownby D R, Elston-Lafata J, Pallonen U, Strecher V, Asthma in Adolescents Research Team A web-based, tailored asthma management program for urban African-American high school students. American Journal of Respiratory and Critical Care Medicine. 2007;175:888–895. doi: 10.1164/rccm.200608-1244OC. doi:10.1164/rccm.200608-1244OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana S, Drotar D, Frazier T. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. Journal of Pediatric Psychology. 2008;33:590–611. doi: 10.1093/jpepsy/jsm128. doi:10.1093/jpepsy/jsm128. [DOI] [PubMed] [Google Scholar]
- *Kato P M, Cole S W, Bradlyn A S, Pollock B H. A video game improves behavioral outcomes in adolescents and young adults with cancer: A randomized trial. Pediatrics. 2008;122:e305–e317. doi: 10.1542/peds.2007-3134. doi:10.1542/peds.2007-3134. [DOI] [PubMed] [Google Scholar]
- *Krieger J, Takaro T K, Song L, Beaudet N, Edwards K. A randomized controlled trial of asthma self-management support comparing clinic-based nurses and in-home community health workers: The Seattle king county healthy homes II project. Archives of Pediatrics and Adolescent Medicine. 2009;163:141–149. doi: 10.1001/archpediatrics.2008.532. doi:10.1001/archpediatrics.2008.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Letourneau E J, Ellis D A, Naar-King S, Chapman J E, Cunningham P B, Fowler S. Multisystemic therapy for poorly adherent youth with HIV: Results from a pilot randomized controlled trial. AIDS Care. 2012;25:507–514. doi: 10.1080/09540121.2012.715134. doi:10.1080/09540121.2012.715134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsey M, Wilson D. Practical meta-analysis. Vol. 49. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- Modi A C, Guilfoyle S M, Morita D A, Glauser T A. Development and reliability of a correction factor for parent-reported adherence to pediatric antiepileptic drug therapy. Epilepsia. 2011;52:370–376. doi: 10.1111/j.1528-1167.2010.02789.x. doi:10.1111/j.1528-1167.2010.02789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi A C, Pai A L, Hommel K A, Hood K K, Cortina S, Hilliard M E, Guilfoyle S M, Gray W N, Drotar D. Pediatric self-management: A framework for research, practice, and policy. Pediatrics. 2012;129:e473–e485. doi: 10.1542/peds.2011-1635. doi:10.1542/peds.2011–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi A C, Rausch J R, Glauser T A. Patterns of nonadherence to antiepileptic drug therapy in children with newly diagnosed epilepsy. JAMA. 2011;27:1669–1976. doi: 10.1001/jama.2011.506. doi:10.1001/jama.2011.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman D G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Journal of Clinical Epidemiology. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. doi:10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- *Mulvaney S A, Rothman R L, Wallston K A, Lybarger C, Dietrich M S. An internet-based program to improve self-management in adolescents with type 1 diabetes. Diabetes Care. 2010;33:602–604. doi: 10.2337/dc09-1881. doi:10.2337/dc09-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Naar-King S, Outlaw A Y, Sarr M, Parsons J T, Belzer M, MacDonell K, Tanney M, Ondersma S J. Motivational Enhancement System for Adherence (MESA): Pilot randomized trial of a brief computer-delivered prevention intervention for youth initiating antiretroviral treatment. Journal of Pediatric Psychology. 2013;38:638–648. doi: 10.1093/jpepsy/jss132. doi:10.1093/jpepsy/jss132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Nansel T R, Iannotti R J, Liu A. Clinic-integrated behavioral intervention for families of youth with type 1 diabetes: Randomized clinical trial. Pediatrics. 2012;129:e866–e873. doi: 10.1542/peds.2011-2858. doi:10.1542/peds.2011-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Nansel T R, Iannotti R J, Simons-Morton B G, Cox C, Plotnick L P, Clark L M, Zeitzoff L. Diabetes Personal Trainer Outcomes: Short-term and 1-year outcomes of a diabetes personal trainer intervention among youth with type 1 diabetes. Diabetes Care. 2007;30:2471–2477. doi: 10.2337/dc06-2621. doi:10.2337/dc06-2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Otsuki M, Eakin M N, Rand C S, Butz A M, Hsu V D, Zuckerman I H, Ogborn J, Bilderback A, Riekert K A. Adherence feedback to improve asthma outcomes among inner-city children: A randomized trial. Pediatrics. 2009;124:1513–1521. doi: 10.1542/peds.2008-2961. doi:10.1542/peds.2008-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin J M, Bloom S R, Gortmaker S L. The increase of childhood chronic conditions in the United States. JAMA. 2007;297:2755–2759. doi: 10.1001/jama.297.24.2755. doi:10.1001/jama.297.24.2755. [DOI] [PubMed] [Google Scholar]
- Quittner A L, Modi A C, Lemanek K L, Ievers-Landis C E, Rapoff M A. Evidence-based assessment of adherence to medical treatments in pediatric psychology. Journal of Pediatric Psychology. 2008;33:916–936. doi: 10.1093/jpepsy/jsm064. doi:10.1093/jpepsy/jsm064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoff M. Adherence to pediatric medical regimens. New York, NY: Springer; 2010. [Google Scholar]
- Rohan J M, Rausch J R, Pendley J S, Delamater A M, Dolan L, Reeves G, Drotar D. Identification and prediction of group-based glycemic control trajectories during the transition to adolescence. Health Psychology. 2013 doi: 10.1037/hea0000025. 2013 Nov 25 epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Scholten L, Willemen A M, Last B F, Maurice-Stam H, Van Dijk E M, Ensink E, Zandbelt N, van der Hoop-Mooij A, Schuengel C, Grootenhuis M A. Efficacy of psychosocial group intervention for children with chronic illness and their parents. Pediatrics. 2013;131:e1196–e1203. doi: 10.1542/peds.2012-2222. doi:10.1542/peds.2012-2222. [DOI] [PubMed] [Google Scholar]
- Schmucker C, Bluemle A, Briel M, Portalupi S, Lang B, Motschall E, Schwarzer G, Bassler D, Mueller K F, von Elm E, Meerpohl J J. A protocol for a systematic review on the impact of unpublished studies and studies published in the grey literature in meta-analyses. The Cochrane Database of Systematic Reviews. 2013;2:24. doi: 10.1186/2046-4053-2-24. doi:10.1186/2046-4053-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Stinson J N, McGrath P J, Hodnett E D, Feldman B M, Duffy C M, Huber A M, Tucker L B, Hetherington C R, Tse S M, Spiegel L R, Campillo S, Gill N K, White M E. An internet-based self-management program with telephone support for adolescents with arthritis: A pilot randomized controlled trial. Journal of Rheumatology. 2010;37:1944–1952. doi: 10.3899/jrheum.091327. doi:10.3899/jrheum.091327. [DOI] [PubMed] [Google Scholar]
- Van Cleave J, Gortmaker S L, Perrin J M. Dynamics of obesity and chronic health conditions among children and youth. JAMA. 2010;303:623–630. doi: 10.1001/jama.2010.104. doi:10.1001/jama.2010.104. [DOI] [PubMed] [Google Scholar]
- van Dyck P C, Kogan M D, McPherson M G, Weissman G R, Newacheck P W. Prevalence and characteristics of children with special health care needs. Archives of Pediatric and Adolescent Medicine. 2004;158:884–890. doi: 10.1001/archpedi.158.9.884. doi:10.1001/archpedi.158.9.884. [DOI] [PubMed] [Google Scholar]
- *Wamalwa D C, Farquhar C, Obimbo E M, Selig S, Mbori-Ngacha D A, Richardson B A, Overbaugh J, Egondi T, Inwani I, John-Stewart G. Medication diaries do not improve outcomes with highly active antiretroviral therapy in Kenyan children: A randomized clinical trial. Journal of the International AIDS Society. 2009;12:8. doi: 10.1186/1758-2652-12-8. doi:10.1186/1758-2652-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead A, Whitehead J. A general parametric approach to the meta-analysis of randomized clinical trials. Statistics in Medicine. 1991;10:1665–1677. doi: 10.1002/sim.4780101105. [DOI] [PubMed] [Google Scholar]
- Wu Y P, Pai A L, Gray W N, Denson L A, Hommel K A. Development and reliability of a correction factor for family-reported medication adherence: Pediatric inflammatory bowel disease as an exemplar. Journal of Pediatric Psychology. 2013;38:893–901. doi: 10.1093/jpepsy/jst043. doi:10.1093/jpepsy/jst043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Wysocki T, Harris M A, Buckloh L M, Mertlich D, Lochrie A S, Mauras N, White N H. Randomized trial of behavioral family systems therapy for diabetes: Maintenance of effects on diabetes outcomes in adolescents. Diabetes Care. 2007;30:555–560. doi: 10.2337/dc06-1613. doi:10.2337/dc06-1613. [DOI] [PubMed] [Google Scholar]
- *Zivkovic Z, Radic S, Cerovic S, Vukasinovic Z. Asthma school program in children and their parents. World Journal of Pediatrics. 2008;4:267–273. doi: 10.1007/s12519-008-0049-z. doi:10.1007/s12519-008-0049-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.