Abstract
Group B streptococcus (GBS) can cause severe disease in susceptible hosts, including newborns, pregnant women, and the elderly. GBS serine-rich repeat (Srr) surface glycoproteins are important adhesins/invasins in multiple host tissues, including the vagina. However, exact molecular mechanisms contributing to their importance in colonization are unknown. We have recently determined that Srr proteins contain a fibrinogen-binding region (BR) and hypothesize that Srr-mediated fibrinogen binding may contribute to GBS cervicovaginal colonization. In this study, we observed that fibrinogen enhanced wild-type GBS attachment to cervical and vaginal epithelium, and that this was dependent on Srr1. Moreover, purified Srr1-BR peptide bound directly to host cells, and peptide administration in vivo reduced GBS recovery from the vaginal tract. Furthermore, a GBS mutant strain lacking only the Srr1 “latching” domain exhibited decreased adherence in vitro and decreased persistence in a mouse model of GBS vaginal colonization, suggesting the importance of Srr–fibrinogen interactions in the female reproductive tract.
Keywords: fibrinogen, GBS, Srr1, vaginal colonization
Streptococcus agalactiae, or Group B streptococcus (GBS), is a Gram-positive bacterium frequently isolated from the gastrointestinal tract of healthy adults [1], which likely fosters GBS vaginal colonization [2]. Yet during pregnancy, GBS can disseminate to the fetus by crossing placental barriers to cause chorioamnionitis, funisitis, and pneumonitis [3], or can spread to the newborn during vaginal birth through aspiration of infected fluids [4]. Consequently, GBS is currently a leading cause of neonatal infectious morbidity and mortality, annually impacting over 2000 live births in the United States [5, 6]. Additionally, although mucosal colonization rates are similar between elderly populations and younger individuals, elderly adults account for >40% of GBS invasive disease and >50% of GBS-related deaths [7]. Initial exposure to GBS begins with asymptomatic colonization of mucosal tissues such as the vaginal tract. To persist in the vagina, a volatile environment constantly modulated by factors including commensal flora, pH, and steroid hormones, GBS must elaborate determinants that adhere to host cells and extracellular proteins. However, little is known about elements that promote GBS persistence in the female reproductive tract.
GBS surface-associated organelles, such as pilus tip adhesin (PilA) and serine-rich repeat (Srr) proteins, can instigate initial contact with host epithelium and promote cervical and vaginal attachment [8]. Srr glycoproteins are a large family of surface organelles found in numerous Gram-positive organisms that contribute to host-cell adhesion in various model systems [9–11]. Based on structural modeling, Srr proteins possess several conserved domains, including a specialized signal sequence, 2 extensive glycosylated Srr regions, and an LPXTG cell wall anchoring motif [12]. In GBS, there are 2 recognized srr gene alleles (srr1 and srr2) that are architecturally similar, but share <20% identity [13]. Thus far, Srr1 appears highly conserved (>85% nucleotide identity) and is found in the majority of GBS clinical isolates, including serotypes Ia, III, and V, while Srr2 is currently restricted to serotype III, multilocus sequencing type (ST)–17 strains [13, 14]. These alleles likely both play important roles in host–pathogen contact, since epidemiological surveillance of maternal GBS isolates has determined that serotypes Ia, Ib, II, III, and V are readily isolated from cervicovaginal tract and account for 96% of neonatal cases [15]. GBS Srr-deficient strains exhibit reduced persistence in murine vaginal colonization [8] and are impaired in the development of sepsis [13] and meningitis [14]. However, the overall contribution of Srr proteins, and governing molecular mechanisms, to GBS colonization and pathogenesis has not fully been recognized.
We recently identified that Srr1 and Srr2 both contain a fibrinogen-binding region that secures fibrinogen through a “dock, lock, and latch” (DLL) mechanism, similar to staphylococcal adhesins SdrG and ClfA [16, 17]. The Srr binding region (Srr-BR) performs an analogous function to staphylococcal N2 and N3 domains, which dock fibrinogen into the binding cleft between N2 and N3 [16, 18]. Upon ligand binding, the N3 C-terminal extension (the “latch”) alters its conformation to lock the ligand in place by forming an intramolecular β sheet complementation with N2 [18]. Interestingly, although structurally similar, these staphylococcal and streptococcal adhesins interact with different fibrinogen regions; Srr1-BR and Srr2-BR both bind the Aα chain [16, 17]. Previously, we have demonstrated that Srr1-BR and Srr2-BR, including the C-terminal latch domain, promote GBS adherence to human brain microvascular endothelial cells through interaction with fibrinogen [16, 17]. Therefore, we hypothesize that the DLL mechanism of Srr proteins establishes and stabilizes fibrinogen binding to aid GBS interaction with reproductive tract epithelium and prolong GBS colonization.
MATERIALS AND METHODS
Bacterial Strains
S. agalactiae (GBS) wild-type (WT) clinical isolates used in this study, all originally isolated from human blood or cerebral spinal fluid, were NCTC 10/84 (serotype V) [19], COH1 (serotype III) [20], H36B (serotype Ib) [21], 515 (serotype Ia) [22], and A909 (serotype Ia) [23]. All isogenic srr mutants have been described previously [8, 14]. Briefly, the NCTC 10/84 Δsrr1 strain was constructed by allelic exchange mutagenesis (as described in [24]) where polymerase chain reaction (PCR) was used to generate an in-frame substitution of srr with the chloramphenicol acetyltransferase (cat) gene [14]. Additional srr1/2 mutants in COH1, H36B, 515, and A909 were generated by gene disruption using plasmid insertional mutagenesis as described elsewhere [25]. The isogenic variant of NCTC 10/84 in which the latch-like domain of the Srr1 binding region (BR) has been deleted, NCTC 10/84 Δlatch, has also been described previously [16]. GBS isolates from vaginal tracts of pregnant women were obtained from the Detroit Medical Center, Detroit, Michigan. GBS isolates were confirmed by Gram stain, colony morphology, ability to grow as pink colonies on CHROMagar StrepB (DRG International), and rapid agglutination using Streptex Latex Group B test (Remel). GBS strains were grown in Todd–Hewitt Broth (THB) at 37°C. THB was supplemented with chloramphenicol (2 µg/mL) for Δsrr mutants, and erythromycin (5 µg/mL) for Δlatch.
PCR to Determine srr Alleles
Genomic DNA was isolated from A909 (srr1-positive control), COH1 (srr2-positive control), and clinical vaginal isolates using a standard phenol:chloroform extraction and ethanol precipitation. PCR was performed with primers (Srr1 forward, 5′AGTGTCTGATACTGAAATGTTAGG TA3′ and Srr1 reverse, 5′TCGCATCAGGACTTGGGAATCTA3′; Srr2 forward, 5′TCACGCAAAGTTCGAGTTAAAA3′ and Srr2 reverse, 5′AGATTTAGTAGCTCCTAA3′) to amplify srr1 and srr2 genes. Allele status was determined by visualization of products using gel electrophoresis.
Cell Lines
Immortalized human vaginal (VK2/E6E7), ectocervical (Ect1/E6E7), and endocervical (End1/E6E7) epithelial cell lines were obtained from American Type Culture Collection (ATCC CRL-2616, ATCC CRL-2614, and ATCC CRL-2615, respectively) [26]. Cell lines (passage 5–20) were maintained in keratinocyte serum-free medium (KSFM) with 0.5 ng/mL human recombinant epidermal growth factor and 0.05 mg/mL bovine pituitary extract at 37°C with 5% CO2.
In Vitro Adherence Assays
Cells were grown to confluency (monolayer) in 24-well plates, and incubated for 30 minutes with mid-log phase GBS at a multiplicity of infection (MOI) of 1. Cells were washed 6× with phosphate buffered saline (PBS), and 0.25% trypsin–ethylenediaminetetraacetic acid (EDTA) and 0.025% Triton X-100 added to lyse cells. Lysate was plated on THB agar to enumerate bacterial colony-forming units (CFUs). Total adherent CFU percentage was calculated as (recovered CFU/original inoculum CFU) × 100%. For exogenous fibrinogen assays, human fibrinogen (Haematologic Technologies) was either added to cells, or incubated with mid-log phase GBS for 30 minutes prior to infection. For inhibition assays, 10 µg/mL of rabbit anti-(h)-fibrinogen antibody (Aniara) was added to media 30 minutes before performing adherence assays.
GBS Adherence to Immobilized Fibrinogen
Purified fibrinogen (0.4 µM) was immobilized on 96-well microtiter plates, incubated overnight at 4°C, and washed 3× with PBS. Overnight GBS cultures were adjusted to 106 CFU/mL in PBS, and 100 µL incubated on fibrinogen-coated plates for 30 minutes at 37°C. Wells were washed with PBS, and attached bacteria released with 100 µL of 0.25% trypsin-EDTA for 10 minutes at 37°C, and quantified on THB agar.
Light Microscopy
Cells were cultured on glass coverslips to confluency. After an adherence assay (MOI of 100), cells were washed 6× with PBS. Coverslips were air-dried, heat-fixed, Gram-stained, and mounted on slides. Images were acquired using a Zeiss upright microscope with attached Axiocam Icc3 camera.
Immunofluorescence Microscopy
Cells were propagated on glass coverslips to 70%–90% confluency. After mounting coverslips on slides using Vectashield with 4’,6-diamidino-2-phenylindole (DAPI; Vector Laboratories), proteins were detected under laser scanning microscope (Leica Microsystems). For protein binding assays, Srr1-BR and Srr1-BRΔlatch peptides were purified using methods described previously [16]. Cells were washed 3× with PBS and incubated in KSFM with 50 µg/mL of protein for 1 hour at 37°C with 5% CO2. Cells were fixed with 2% paraformaldehyde and incubated with primary antibody (mouse anti-FLAG antibody; 1:1000, Sigma Aldrich) at 4°C overnight. After washing, secondary antibody (Alexa Fluor 594-conjugated rabbit antimouse antibody; 1:1000, Life Technologies) was added for 1 hour. For endogenous fibrinogen detection, vaginal and cervical cells grown to approximately 90% confluency were fixed with 2% paraformaldehyde and primary (rabbit antihuman fibrinogen, CalBioChem) and secondary (Alexa Fluor 594-conjugated donkey antirabbit, Life Technologies) antibodies were applied to cells as described above.
Mouse Model of GBS Vaginal Colonization
All mouse work was approved by the Office of Lab Animal Care at San Diego State University and carried out using accepted veterinary standards. Eight- to twelve-week-old female CD1 mice (Charles River Laboratories) were injected intraperitoneally with 0.5 mg β-estradiol in 100 µL sesame oil on day -1 [8, 27]. On day 0, 1 hour prior to bacterial inoculation, 14–26 µg of Srr1-BR or Srr2-BR in a 10 µL suspension, or 10 µL of PBS, was administered into the vaginal lumen. All mice were vaginally inoculated with 1 × 107 NCTC 10/84, and 1 hour later, peptides (or PBS) were dosed as described above. Five hours postinoculation with GBS, each vaginal lumen was swabbed with an ultrafine swab, and recovered bacteria were enumerated on CHROMagar StrepB agar [28]. For competition assays, mice were synchronized with β-estradiol on day -1. On day 0, mice were vaginally inoculated with a 1:1 ratio of NCTC 10/84 and Δlatch (a total of 1 × 107 CFU suspended in 10 µL PBS). On successive days (days 1–5), vaginal lumens were swabbed as described above. The Δlatch mutant strain was distinguished from WT by plating on CHROMagar plates with 5 µg/mL erythromycin.
Statistical Analysis
GraphPad Prism version 5.04 was used for statistical analyses. Differences in recovered bacteria from in vitro assays were evaluated using Student t test. In vivo results for peptide treatment assays or competition experiments were analyzed by Mann–Whitney and repeated measures 2-way ANOVA, respectively. Statistical significance was accepted at P < .05.
RESULTS
GBS Srr Contribution to Fibrinogen Binding and Reproductive Tract Adherence
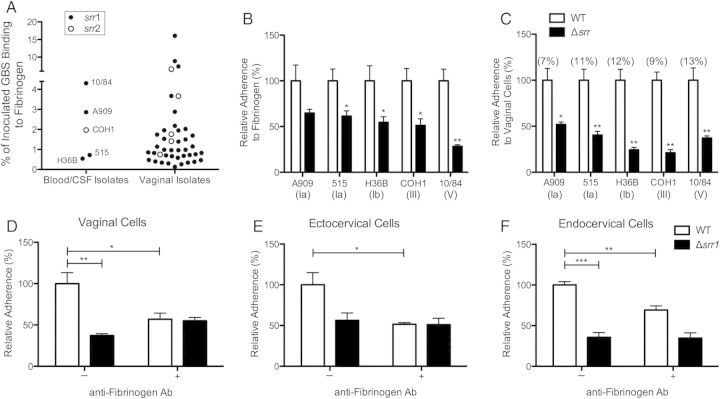
Numerous Gram-positive pathogens express fibrinogen-binding proteins, and interaction with this and other extracellular matrix proteins may be vital to the early stages of pathogenesis and survival within the host [29]. Recent evidence suggests fibrinogen binding may be a widespread characteristic of invasive neonatal clinical GBS isolates [16, 30]. However, it is not known whether this is also a common feature of colonizing GBS. We compared fibrinogen-binding ability of 40 GBS vaginal isolates from pregnant women to 5 well-studied laboratory GBS strains originally isolated from human blood or cerebral spinal fluid. Our results indicate that GBS isolated from either invasive or colonizing niches displayed varying abilities to interact with fibrinogen, with some vaginal isolates exhibiting extremely high affinity (Figure 1A). We recently identified that GBS Srr proteins contain a fibrinogen-binding region, and that Srr-deficient GBS display impaired capacity to bind fibrinogen [16, 17]. Using PCR, we observed an Srr1 allele frequency of >85% in the vaginal isolates (Figure 1A). Next, we compared fibrinogen binding and vaginal cell attachment of 5 GBS strains representing different serotypes and sequence types and their isogenic Δsrr mutants. In all strains tested, Δsrr mutants exhibited decreased ability to bind fibrinogen compared to WT parental strains, indicating that Srr significantly enables GBS interaction with fibrinogen (Figure 1B). Further, cellular adherence of GBS strains lacking srr were decreased by 50% or more compared to WT, establishing the importance of Srr in GBS adhesion to vaginal epithelium (Figure 1C). To confirm that Srr-mediated fibrinogen binding accounts for decreased adherence of Δsrr mutants, an antifibrinogen antibody was added to cell cultures prior to infection to interrupt GBS binding with host cell–surface fibrinogen. We observed that WT attachment to vaginal and cervical cells was significantly inhibited after fibrinogen blockage, yet in control studies, there was no impact on Srr1- or Srr2-deficient GBS, (Figure 1D–F, Supplementary Figure 1A–C). This further validates that fibrinogen is a key mediator of GBS Srr-conferred attachment to host cells.
Figure 1.
GBS interaction with fibrinogen and contribution of Srr to reproductive tract adherence. A, GBS clinical isolates (n = 40) from vaginal tract of pregnant women and invasive isolates A909, 515, H36B, COH1, and NCTC 10/84 (n = 5) were incubated on 96-well microtiter plates coated with immobilized fibrinogen (0.4 µM). Srr allele status was determined by PCR as described in Materials and Methods. B, Representative GBS serotypes Ia, Ib, III, and V and isogenic Δsrr mutants were incubated with immobilized fibrinogen as in (A). Statistical comparisons were made between mutant and parental strains. C, Adherence of WT GBS and isogenic Δsrr mutants to hVECs at an MOI of 1. Total adherence values, percentage, of parental strains compared to original inoculum are indicated in parentheses. Statistical comparisons were made between mutant and parental strains. D–F, Adherence of WT NCTC 10/84 or isogenic Δsrr1 to hVEC, ecto-, or endocervical cells (MOI of 1) with or without blocking by antifibrinogen antibody (10 µg/mL). Values are expressed as the relative total of cell-associated CFUs recovered compared to original inoculum. Experiments were repeated at least twice with 4 replicates, and data from a representative experiment are shown. Data were analyzed by Student t test. *P < .05; **P < .005; ***P < .001. Abbreviations: CFUs, colony-forming units; CSF, cerebral spinal fluid; GBS, group B streptococcus; hVECs, human vaginal epithelial cells, MOI, multiplicity of infection; PCR, polymerase chain reaction; srr, serine-rich repeat gene; WT, wild type.
We next examined whether this phenotype could be reproduced in vivo. We previously found that Δsrr1 displays reduced murine vaginal persistence over time [8]. To investigate the importance of Srr–fibrinogen interaction to initial GBS establishment within the vagina, we performed a competitive binding experiment by administering purified peptides of Srr1- or Srr2-binding regions (Srr1-BR, Srr2-BR) prior to and during vaginal inoculation with WT GBS. We detected a significant reduction of GBS load 5 hours postinoculation in mice treated with either Srr1-BR (Figure 2A) or Srr2-BR (Figure 2B) compared to PBS-treated controls. This reduction in bacterial load was still present 24 hours postinoculation (data not shown). Our data suggest that Srr-mediated fibrinogen binding aids GBS in vaginal epithelial attachment early on during colonization, and supports our previous observation of reduced Δsrr1 vaginal persistence [8].
Figure 2.
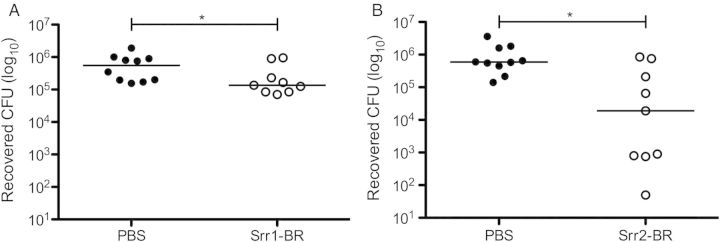
Srr-BR peptides compete with WT GBS to reduce vaginal bacterial load in vivo. Purified Srr1-BR (A) or Srr2-BR (B) was introduced to the murine vaginal lumen 1 hour before and 1 hour after inoculation with 1 × 107 CFUs of WT NCTC 10/84. Five hours postinoculation, vaginal tracts were swabbed, and recovered GBS quantified as described in Materials and Methods. Dots represent values for individual mice and lines represent median values. Experiments were conducted independently at least twice, and data from 1 representative experiment are shown. Data were analyzed by Mann–Whitney test. *P < .05. Abbreviations: BR, binding region; CFUs, colony-forming units; GBS, group B streptococcus; PBS, phosphate buffered saline; Srr, serine-rich repeat; WT, wild type.
Fibrinogen Enhances GBS Attachment to Vaginal and Cervical Epithelial Cells
Fibrinogen is an abundant, soluble blood protein synthesized mainly by the liver, and although well known for its involvement in blood clotting, fibrinogen is also incorporated into extracellular matrix of multiple tissues [31]. Because we observed that GBS–fibrinogen interactions contribute to cervicovaginal adherence (Figure 1), we examined the hypothesis that fibrinogen enhances GBS–host cell attachment. Exogenous fibrinogen was added to GBS cultures prior to vaginal and cervical cell inoculation, which significantly enhanced WT adherence to epithelium, but did not alter adherence of the Δsrr1 mutant (Figure 3A). Although the liver is the primary source of fibrinogen, extrahepatic synthesis has been described in mucosal epithelial cells from the cervix, intestine, and lung [32–34]. Additionally, fibrinogen has been identified in human vaginal lavage fluid [35, 36]. To confirm fibrinogen production in our immortalized cell lines, we performed immunofluorescent microscopy and detected fibrinogen in all 3 cell lines, although to a lesser extent in ectocervical epithelium (Figure 3B). Moreover, reverse-transcription PCR and Western blot analysis of cell lysates revealed that hVEC, ecto-, and endocervical cells produce fibrinogen (data not shown).
Figure 3.
Expression of fibrinogen and its contribution to Srr1-mediated GBS attachment to cervicovaginal epithelia in vitro. A, Adherence of WT NCTC 10/84 or isogenic Δsrr1 to epithelial cells (MOI of 1) with or without the addition of exogenous fibrinogen (20 µg/mL). Data were analyzed by Student t test. *P < .05; **P < .005; ***P < .001. B, Immunofluorescence staining of endogenous fibrinogen (red) on all 3 cell types, and nuclei were visualized with DAPI (blue). (Magnification = 630×, scale bar = 20 µm). Experiments were repeated at least twice with 4 replicates, and data from a representative experiment are shown. Abbreviations: DAPI, 4’,6-diamidino-2-phenylindole; GBS, group B streptococcus; MOI, multiplicity of infection; Srr, serine-rich repeat; srr, serine-rich repeat gene; WT, wild type.
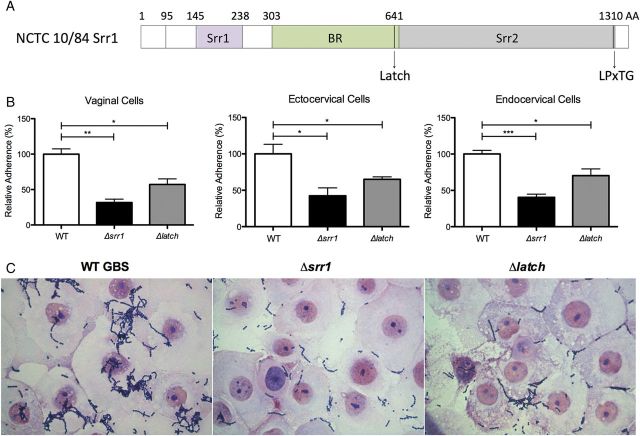
Srr1 Latch Domain Promotes Fibrinogen Binding and Cervicovaginal Adherence
In our collection of vaginal GBS isolates, we observed that the majority of vaginal isolates possessed srr1 (Figure 1A), and therefore examined the molecular mechanisms of Srr1 in greater detail. A 13-amino acid sequence (NISGDSDANAEIK) within the C-terminal domain of Srr1 fibrinogen-binding region of strain NCTC 10/84, designated the “latch” domain (Figure 4A), was recently identified and shown to mediate GBS-fibrinogen interaction, promoting the development of GBS meningitis [16]. To examine the role of the latch domain in GBS vaginal colonization, we measured adherence of a Δlatch mutant in vitro. GBS lacking the latch domain exhibited a significantly decreased ability to attach to vaginal and cervical cells compared to WT, although this phenotype was not as dramatic as the entire srr1 deletion (Figure 4A). Micrographs revealed fewer adherent Srr1 or Srr1-latch deficient GBS on vaginal cells compared to WT (Figure 4B).
Figure 4.
Srr1 Latch domain contributes to GBS cervicovaginal adherence. A, Schematic diagram of the structure of Srr1 protein, including the latch domain. B, Adherence of WT NCTC 10/84, Δsrr1 or Δlatch to epithelial cell types (MOI of 1). Data were analyzed by Student t test. *P < .05; **P < .005; ***P < .001. C, Gram stain of human vaginal epithelial cells infected with WT NCTC 10/84, Δsrr1 or Δlatch at an MOI of 100 (magnification = 1000×). Experiments were repeated at least twice with 4 replicates, and data from a representative experiment are shown. Abbreviations: AA, amino acids; BR, binding region; GBS, group B streptococcus; LPxTG, cell wall anchoring motif; MOI, multiplicity of infection; Srr, serine-rich repeat; srr, serine-rich repeat gene; WT, wild type.
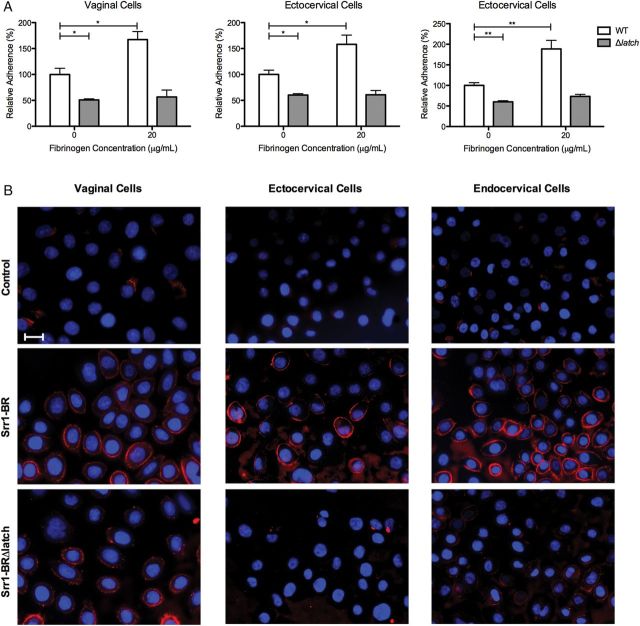
We next sought to confirm the importance of Srr–latch–fibrinogen interactions to GBS-host adherence. The WT and Δlatch strains were cultured with exogenous fibrinogen, and then incubated with host-cell monolayers. As we observed previously, WT adherence to vaginal and cervical epithelium was enhanced by adding fibrinogen, while Δlatch binding was not affected (Figure 5A). These results indicate that the latch domain plays a crucial role in GBS interaction with fibrinogen, which in turn enhances host–bacteria association.
Figure 5.
Contribution of Srr1 latch domain to GBS–fibrinogen interaction cellular attachment. A, Adherence of WT NCTC 10/84 or isogenic Δlatch to epithelial cells (MOI of 1) with or without the addition of exogenous fibrinogen (20 µg/mL). Data were analyzed by Student t test. *P < .05; **P < .005. B, Immunofluorescence staining of epithelial cells incubated with FLAG-tagged purified peptides (Srr1-BR and Srr1-BRΔlatch) probed with anti-FLAG antibody (red). Nuclei were visualized with DAPI (blue). (Magnification = 630×, scale bar = 20 µm.) Experiments were repeated at least twice with 4 replicates, and data from a representative experiment are shown. Abbreviations: BR, binding region; DAPI, 4’,6-diamidino-2-phenylindole; GBS, group B streptococcus; MOI, multiplicity of infection; Srr, serine-rich repeat; WT, wild type.
Because we observed here that the Srr-BR was sufficient to limit GBS colonization in vivo (Figure 2), we examined cellular binding of purified Srr1-BR and Srr1-BRΔlatch peptides. Vaginal and cervical cells were incubated with FLAG-tagged Srr1-BR peptides [16] and protein binding was evaluated using fluorescence-conjugated antibodies against FLAG. As predicted, Srr1-BR strongly attached to cell surfaces, but attachment to cervical cells was dramatically reduced when the latch region was deleted. This same effect was observed in vaginal cells, but to a lesser extent (Figure 5B). These results provide additional evidence that interaction between GBS-Srr1 and multiple host-cell types is largely dependent on the presence of the latch domain.
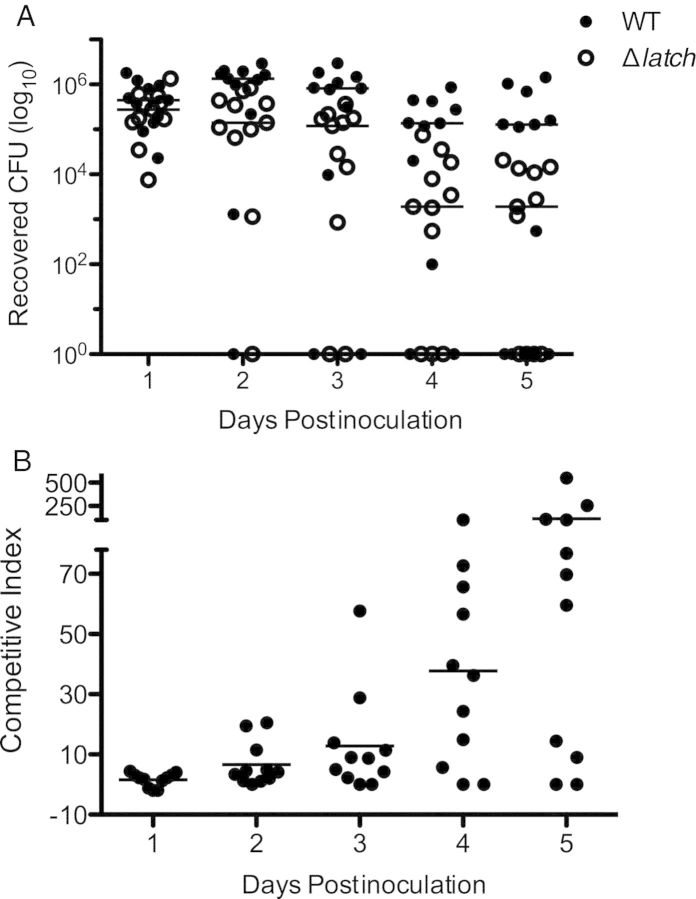
Srr1 Latch Domain Contributes to Vaginal Colonization In Vivo
Our in vitro data indicated that Srr1 latch domain contributes to GBS cervical and vaginal adherence. To verify this contribution in vivo, we utilized a mouse model of GBS vaginal colonization that has been described previously by our lab [8, 27]. Briefly, β-estradiol was first injected into the peritoneal cavity to synchronize estrus, and equal amounts of WT NCTC 10/84 and Δlatch GBS were simultaneously inoculated into the vaginal lumen to assess the competitive index for vaginal persistence. Bacterial load was monitored by swabbing mice on successive days, and plating recovered bacteria on CHROMagar StrepB, with antibiotic selection to distinguish between the 2 strains. Consistently, more WT GBS was recovered from the vagina than the Δlatch mutant over time (P = .0003)(Figure 6A), and the mean ratio of WT CFU to Δlatch CFU exceeded 100 at day 5 postinoculation (Figure 6B). These results substantiate the importance of Srr1 latch domain to GBS vaginal colonization in vivo and indicate that latch domain deficiency results in reduced fitness in the vaginal tract.
Figure 6.
Contribution of Srr1 latch domain to GBS vaginal colonization in vivo. Equal amounts of WT NCTC 10/84 and Δlatch (0.5 × 107 CFUs each) were introduced into the vaginal vault of mice (n = 11) in a competition assay. A, Persisting GBS were enumerated by plating on CHROMagar StrepB plates with and without 5 µg/mL erythromycin on successive days after infection. B, The ratio of WT GBS CFU to Δlatch CFUs over time. An overall ratio of 1 indicates equal numbers of WT and Δlatch CFUs. Dots represent values for individual mice and lines represent median values. Experiments were conducted independently at least twice, and data from 1 representative experiment are shown. Data were analyzed by repeated measures 2-way ANOVA. Abbreviations: CFUs, colony-forming units; GBS, group B streptococcus; Srr, serine-rich repeat; WT, wild type.
DISCUSSION
Although GBS is an opportunistic microbe that rarely causes diseases in healthy individuals, it remains a significant neonatal pathogen despite prenatal screening and antibiotics prophylaxis. The female reproductive tract is a major reservoir for GBS, and GBS vaginal colonization has been reported in women from all ethnic backgrounds and stages of life [37–39]. Bacterial surface–associated organelles facilitate initial contact with host cells, and GBS Srr1 contributes to cervicovaginal attachment and vaginal persistence [8]; however, the precise mechanism of this host–pathogen interaction remains elusive. In this study, we focused on the contribution of Srr1 and its fibrinogen-binding capability to promote GBS cervicovaginal adherence and colonization.
Repetitive DNA sequences such as srr1 are common among microbial genomes and display size variability likely due to high recombination rates between repeats [40]. Intraspecies size divergence of srr1 appears in GBS isolates; however, the 5′ end (bp 1-1928) exhibits higher conservation in length [11], suggesting preserved function of the Srr1 5′ region across GBS strains. Human keratin 4 (K4) was previously determined to bind amino acids (aa) 485–642 of Srr1 promoting GBS adherence to laryngeal epithelium [11]. Our past results also indicated K4 contributes to Srr1-mediated GBS vaginal adherence; however, blocking with anti-K4 antibody only resulted in a 30% decrease in GBS adherence [8] compared to a ≥50% decrease in Δsrr1 adherence (Figure 1), suggesting other host factors also engage Srr1. We recently demonstrated that human fibrinogen is another Srr1 ligand, and interestingly, the fibrinogen-binding site is located between aa 303–641 [16], overlapping the K4-binding site. To confirm fibrinogen ligand specificity of the latch domain (aa 629–641) in Srr1-mediated GBS adherence, we compared WT and Δlatch attachment to vaginal epithelial cells pretreated with or without anti-K4 antibody. Our results show decreased Δlatch binding when cell-surface K4 is blocked (data not shown) suggesting that Srr1 latch domain absence does not affect GBS interaction with keratin 4. The fact that Srr proteins may interact with multiple host proteins, including K4, may explain why treatment with an antifibrinogen antibody did not always reduce WT adherence to the level observed with Srr-deficient GBS (Figure 1D-F, Supplmentary Figure 1).
Fibrinogen synthesis is dramatically upregulated during inflammation or under stress exposure [41, 42], and blood fibrinogen levels increase 50% in pregnant women [43]. Based on this, and the presence of fibrinogen in vaginal lavage fluid [35, 36], it is highly likely that fibrinogen is available during GBS vaginal colonization. Our data indicate that 3 cell types from the female genital tract produce fibrinogen and immobilize fibrinogen on the cell surface. We have determined that vaginal cells express the fibrinogen-binding integrin αVβ1 (data not shown); however, further investigation to identify specific fibrinogen receptors on cervical and vaginal epithelial cells is still needed.
Although Δsrr1 mutants exhibit reduced adherence to immobilized fibrinogen compared to WT, its binding is still detectable. Likewise, Δsrr1 mutants still adhere to cervicovaginal epithelium, although to a lesser extent than WT, suggesting that additional bacterial factors promote this host–microbe interaction. Other GBS fibrinogen-binding proteins have been characterized, although specific binding sites and overall contribution to pathogenesis are not well understood. Multiple studies have established that GBS fibrinogen-binding protein from S. agalactiae (Fbs)–A and FbsB are important for cellular adherence or invasion [44–46], and recently, FbsA was suggested to have immunogenic potential [47]. Thus, it is likely that Srr is not the only factor interacting with fibrinogen during GBS vaginal colonization. Our data clearly establish that colonizing GBS isolates are capable of fibrinogen binding and genome sequence analysis is underway to completely profile fibrinogen-binding proteins. We have recently shown that Srr2 possesses a higher affinity for fibrinogen, and although Srr2-expressing strains are thought to be more virulent [13], it is currently unknown whether these 2 characteristics are directly related. Data presented here suggest that the binding region of both Srr1 and Srr2 can competitively inhibit GBS colonization in vivo; however, whether Srr2 is promotes GBS vaginal persistence during colonization remains to be determined.
In summary, our results demonstrate that fibrinogen plays an important role for Srr1-mediated GBS adherence within in the female reproductive tract, although the degree of fibrinogen binding varies across clinical isolates. This interaction is promoted by the 13-aa latch domain, which likely stabilizes fibrinogen binding via the DLL mechanism discovered previously. These findings, combined with further investigation of potential fibrinogen receptors on cervicovaginal epithelium, may provide novel targets for therapeutic development in preventing GBS vaginal colonization.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We would like to thank the vivarium manager and staff at San Diego State University for support with animal husbandry. We are also grateful to Donna Runft and Lauryn Keeler for technical assistance with GBS vaginal isolates.
Financial support. This work was supported by grants from the California State University Program for Education and Research in Biotechnology (CSUPERB) and National Institutes of Health (NS051247 to K.S.D. and AI41513 and AI057433 to P.M.S.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Dillon HC, Jr, Gray E, Pass MA, Gray BM. Anorectal and vaginal carriage of group B streptococci during pregnancy. J Infect Dis. 1982;145:794–9. doi: 10.1093/infdis/145.6.794. [DOI] [PubMed] [Google Scholar]
- 2.Meyn LA, Krohn MA, Hillier SL. Rectal colonization by group B Streptococcus as a predictor of vaginal colonization. Am J Obstet Gynecol. 2009;201:76 e1–7. doi: 10.1016/j.ajog.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desa DJ, Trevenen CL. Intrauterine infections with group B beta-haemolytic streptococci. Br J Obstet Gynaecol. 1984;91:237–9. doi: 10.1111/j.1471-0528.1984.tb04759.x. [DOI] [PubMed] [Google Scholar]
- 4.Maisey HC, Quach D, Hensler ME, et al. A group B streptococcal pilus protein promotes phagocyte resistance and systemic virulence. FASEB J. 2008;22:1715–24. doi: 10.1096/fj.07-093963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59:1–36. [PubMed] [Google Scholar]
- 6.Prevention CfDCa. Active Bacterial Core Surveillance Report, Emerging Infections Program Network. 2009. Group B Streptococcus, 2008.
- 7.Edwards MS, Baker CJ. Group B streptococcal infections in elderly adults. Clin Infect Dis. 2005;41:839–47. doi: 10.1086/432804. [DOI] [PubMed] [Google Scholar]
- 8.Sheen TR, Jimenez A, Wang NY, Banerjee A, van Sorge NM, Doran KS. Serine-rich repeat proteins and pili promote Streptococcus agalactiae colonization of the vaginal tract. J Bacteriol. 2011;193:6834–42. doi: 10.1128/JB.00094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stinson MW, Alder S, Kumar S. Invasion and killing of human endothelial cells by viridans group streptococci. Infect Immun. 2003;71:2365–72. doi: 10.1128/IAI.71.5.2365-2372.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siboo IR, Chambers HF, Sullam PM. Role of SraP, a serine-rich surface protein of Staphylococcus aureus, in binding to human platelets. Infect Immun. 2005;73:2273–80. doi: 10.1128/IAI.73.4.2273-2280.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samen U, Eikmanns BJ, Reinscheid DJ, Borges F. The surface protein Srr-1 of Streptococcus agalactiae binds human keratin 4 and promotes adherence to epithelial HEp-2 cells. Infect Immun. 2007;75:5405–14. doi: 10.1128/IAI.00717-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pyburn TM, Bensing BA, Xiong YQ, et al. A structural model for binding of the serine-rich repeat adhesin GspB to host carbohydrate receptors. PLoS Pathog. 2011;7:e1002112. doi: 10.1371/journal.ppat.1002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seifert KN, Adderson EE, Whiting AA, Bohnsack JF, Crowley PJ, Brady LJ. A unique serine-rich repeat protein (Srr-2) and novel surface antigen (epsilon) associated with a virulent lineage of serotype III Streptococcus agalactiae. Microbiology. 2006;152:1029–40. doi: 10.1099/mic.0.28516-0. [DOI] [PubMed] [Google Scholar]
- 14.van Sorge NM, Quach D, Gurney MA, Sullam PM, Nizet V, Doran KS. The group B streptococcal serine-rich repeat 1 glycoprotein mediates penetration of the blood-brain barrier. J Infect Dis. 2009;199:1479–87. doi: 10.1086/598217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phares CR, Lynfield R, Farley MM, et al. Epidemiology of invasive group B streptococcal disease in the United States, 1999–2005. JAMA. 2008;299:2056–65. doi: 10.1001/jama.299.17.2056. [DOI] [PubMed] [Google Scholar]
- 16.Seo HS, Mu R, Kim BJ, Doran KS, Sullam PM. Binding of glycoprotein Srr1 of Streptococcus agalactiae to fibrinogen promotes attachment to brain endothelium and the development of meningitis. PLoS Pathog. 2012;8:e1002947. doi: 10.1371/journal.ppat.1002947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seo HS, Misanov G, Seepersaud R, et al. Characterization of fibrinogen binding by glycoproteins Srr1 and Srr2 of Streptococcus agalactiae. J Biol Chem. 2013;288:35982–96. doi: 10.1074/jbc.M113.513358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponnuraj K, Bowden MG, Davis S, et al. A “dock, lock, and latch” structural model for a staphylococcal adhesin binding to fibrinogen. Cell. 2003;115:217–28. doi: 10.1016/s0092-8674(03)00809-2. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson HW. Nontypable group B streptococci isolated from human sources. J Clin Microbiol. 1977;6:183–4. doi: 10.1128/jcm.6.2.183-184.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson CB, Weaver WM. Comparative susceptibility of group B streptococci and Staphylococcus aureus to killing by oxygen metabolites. J Infect Dis. 1985;152:323–9. doi: 10.1093/infdis/152.2.323. [DOI] [PubMed] [Google Scholar]
- 21.Lancefield RC, McCarty M, Everly WN. Multiple mouse-protective antibodies directed against group B streptococci. Special reference to antibodies effective against protein antigens. J Exp Med. 1975;142:165–79. doi: 10.1084/jem.142.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wessels MR, Paoletti LC, Rodewald AK, et al. Stimulation of protective antibodies against type Ia and Ib group B streptococci by a type Ia polysaccharide-tetanus toxoid conjugate vaccine. Infect Immun. 1993;61:4760–6. doi: 10.1128/iai.61.11.4760-4766.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madoff LC, Michel JL, Kasper DL. A monoclonal antibody identifies a protective C-protein alpha-antigen epitope in group B streptococci. Infect Immun. 1991;59:204–10. doi: 10.1128/iai.59.1.204-210.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doran KS, Engelson EJ, Khosravi A, et al. Blood-brain barrier invasion by group B Streptococcus depends upon proper cell-surface anchoring of lipoteichoic acid. J Clin Invest. 2005;115:2499–507. doi: 10.1172/JCI23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pritzlaff CA, Chang JC, Kuo SP, Tamura GS, Rubens CE, Nizet V. Genetic basis for the beta-haemolytic/cytolytic activity of group B Streptococcus. Mole Microbiol. 2001;39:236–47. doi: 10.1046/j.1365-2958.2001.02211.x. [DOI] [PubMed] [Google Scholar]
- 26.Fichorova RN, Rheinwald JG, Anderson DJ. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol Reprod. 1997;57:847–55. doi: 10.1095/biolreprod57.4.847. [DOI] [PubMed] [Google Scholar]
- 27.Patras KA, Wang NY, Fletcher EM, et al. Group B Streptococcus CovR regulation modulates host immune signaling pathways to promote vaginal colonization. Cell Microbiol. 2013;288:35982–96. doi: 10.1111/cmi.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poisson DM, Evrard ML, Freneaux C, Vives MI, Mesnard L. Evaluation of CHROMagar StrepB agar, an aerobic chromogenic medium for prepartum vaginal/rectal Group B Streptococcus screening. J Microbiol Methods. 2011;84:490–1. doi: 10.1016/j.mimet.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Rivera J, Vannakambadi G, Hook M, Speziale P. Fibrinogen-binding proteins of Gram-positive bacteria. Thromb Haemost. 2007;98:503–11. [PubMed] [Google Scholar]
- 30.Dramsi S, Caliot E, Bonne I, et al. Assembly and role of pili in group B streptococci. Mole Microbiol. 2006;60:1401–13. doi: 10.1111/j.1365-2958.2006.05190.x. [DOI] [PubMed] [Google Scholar]
- 31.Pereira M, Rybarczyk BJ, Odrljin TM, Hocking DC, Sottile J, Simpson-Haidaris PJ. The incorporation of fibrinogen into extracellular matrix is dependent on active assembly of a fibronectin matrix. J Cell Sci. 2002;115:609–17. doi: 10.1242/jcs.115.3.609. [DOI] [PubMed] [Google Scholar]
- 32.Lee SY, Lee KP, Lim JW. Identification and biosynthesis of fibrinogen in human uterine cervix carcinoma cells. Thromb Haemost. 1996;75:466–70. [PubMed] [Google Scholar]
- 33.Molmenti EP, Ziambaras T, Perlmutter DH. Evidence for an acute phase response in human intestinal epithelial cells. J Biolog Chem. 1993;268:14116–24. [PubMed] [Google Scholar]
- 34.Haidaris PJ. Induction of fibrinogen biosynthesis and secretion from cultured pulmonary epithelial cells. Blood. 1997;89:873–82. [PubMed] [Google Scholar]
- 35.Tang LJ, De Seta F, Odreman F, et al. Proteomic analysis of human cervical-vaginal fluids. J Proteome Res. 2007;6:2874–83. doi: 10.1021/pr0700899. [DOI] [PubMed] [Google Scholar]
- 36.Dasari S, Pereira L, Reddy AP, et al. Comprehensive proteomic analysis of human cervical-vaginal fluid. J Proteome Res. 2007;6:1258–68. doi: 10.1021/pr0605419. [DOI] [PubMed] [Google Scholar]
- 37.Whitney CG, Daly S, Limpongsanurak S, et al. The international infections in pregnancy study: group B streptococcal colonization in pregnant women. J Matern Fetal Neonatal Med. 2004;15:267–74. doi: 10.1080/14767050410001668617. [DOI] [PubMed] [Google Scholar]
- 38.Tsui MH, Ip M, Ng PC, Sahota DS, Leung TN, Lau TK. Change in prevalence of group B Streptococcus maternal colonisation in Hong Kong. Hong Kong Med J. 2009;15:414–9. [PubMed] [Google Scholar]
- 39.Lin FY, Weisman LE, Azimi P, et al. Assessment of intrapartum antibiotic prophylaxis for the prevention of early-onset group B Streptococcal disease. Pediatr Infect Dis J. 2011;30:759–63. doi: 10.1097/INF.0b013e31821dc76f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Belkum A. Short sequence repeats in microbial pathogenesis and evolution. Cell Mol Life Sci. 1999;56:729–34. doi: 10.1007/s000180050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simpson-Haidaris PJ, Courtney MA, Wright TW, Goss R, Harmsen A, Gigliotti F. Induction of fibrinogen expression in the lung epithelium during Pneumocystis carinii pneumonia. Infect Immun. 1998;66:4431–9. doi: 10.1128/iai.66.9.4431-4439.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lawrence SO, Simpson-Haidaris PJ. Regulated de novo biosynthesis of fibrinogen in extrahepatic epithelial cells in response to inflammation. Thromb Haemost. 2004;92:234–43. doi: 10.1160/TH04-01-0024. [DOI] [PubMed] [Google Scholar]
- 43.Hemangi C. Chapter 1. In: Vinita S, editor. Medical and Surgical Diagnostic Disorders in Pregnancy. 1st ed. London: JP Brothers Medical Publishers; 2003. p. 5. [Google Scholar]
- 44.Schubert A, Zakikhany K, Schreiner M, et al. A fibrinogen receptor from group B Streptococcus interacts with fibrinogen by repetitive units with novel ligand binding sites. Mole Microbiol. 2002;46:557–69. doi: 10.1046/j.1365-2958.2002.03177.x. [DOI] [PubMed] [Google Scholar]
- 45.Schubert A, Zakikhany K, Pietrocola G, et al. The fibrinogen receptor FbsA promotes adherence of Streptococcus agalactiae to human epithelial cells. Infect Immun. 2004;72:6197–205. doi: 10.1128/IAI.72.11.6197-6205.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gutekunst H, Eikmanns BJ, Reinscheid DJ. The novel fibrinogen-binding protein FbsB promotes Streptococcus agalactiae invasion into epithelial cells. Infect Immun. 2004;72:3495–504. doi: 10.1128/IAI.72.6.3495-3504.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papasergi S, Lanza Cariccio V, Pietrocola G, et al. Immunogenic Properties of Streptococcus agalactiae FbsA Fragments. PloS One. 2013;8:e75266. doi: 10.1371/journal.pone.0075266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.