Abstract
Background. We studied features that predict the presence of human papillomavirus (HPV) in a new sexual partnership.
Methods. We analyzed data from the “HPV Infection and Transmission Among Couples Through Heterosexual Activity” (HITCH) Cohort Study of recently formed partnerships (“dyads”). Women aged 18–24 and their male partners were recruited during 2005–2010 in Montreal, Canada. We tested genital swabs for detection of 36 HPV types. We defined HPV in a partnership as the presence of 1 or more HPV types in either or both partners. Using baseline data from 482 dyads, we calculated prevalence ratios to evaluate candidate risk factors.
Results. Most women (88%) were unvaccinated. Sixty-seven percent of dyads harbored HPV. For 49% of dyads, both partners were HPV+. HPV was least prevalent in dyads who were in their first vaginal sex relationship (17%) and was virtually ubiquitous in dyads for which both partners had concurrent partners (96%). Dyads that always used condoms with previous partner(s) were 27% (95% confidence interval, 9%–42%) less likely to have HPV.
Conclusions. The finding that condom use limited onward spread to future partners is in support of condom promotion to prevent sexually transmitted infections. Ongoing monitoring of HPV in sexual networks is needed, particularly in populations with suboptimal vaccine coverage.
Keywords: condoms, HPV vaccine, human papillomavirus, prevalence, sex partners, young adults
Human papillomavirus (HPV) is the most common sexually transmitted infection (STI), with an estimated prevalence of 27% among U.S. females aged 14–59 years, and 45% among women aged 20–24 [1], with similar high rates among men [2–4]. Persistent infections with high-risk HPV types (HR-HPV) are a necessary cause for cervical cancer and are further responsible for a substantial proportion of many other anogenital neoplasms and head and neck cancers [5, 6]. Infections with HPV types of low oncogenic risk (LR-HPV), such as HPV6 and 11, are associated with benign anogenital lesions, including condylomata acuminata (genital warts) [6].
Most HPV studies to date have focused on individuals, although transmission involves contact between sexual partners. Understanding patterns of HPV infection at the level of the partnerships (or “dyads”) is essential to characterize transmission of HPV in sexual networks. Some individuals within the network may contribute disproportionately to the spread of HPV by virtue of their greater number of partnerships and the timing of those partnerships [7]. At least 3 sexual network features are hypothesized to affect the spread of HPV between dyads: the number of sexual links between the 2 members of the dyad to other members of the larger sexual network, concurrent sexual partners external to the dyad, and short gap lengths between previous sexual partners and the formation of the new dyad. We are unaware of published HPV research that has examined these features at the dyadic level.
Intuitively, the more sexual connections there are between partnerships, either directly or indirectly (ie, one's partner's partners, their partner's partners, and so on), the greater the opportunity for the spread of an STI agent such as HPV [8]. Indeed, in individual-based studies, the number of sexual partners is consistently the strongest risk factor for infection in women and men [1, 9–14]. Aspects related to partnership timing have not been the focus of HPV research to date. A key concept in sexual network epidemiology is partner concurrency, or overlapping sexual partnerships, in which sexual intercourse with 1 partner occurs between 2 acts of intercourse with another partner [15]. Concurrency could result in rapid spread of HPV in a sexual network. If partnerships are not concurrent, then the duration of time serially monogamous individuals spend between partnerships, called the “gap length,” may determine whether HPV is transmitted from one partner to the next [16, 17]. Short gaps between partnerships would facilitate transmission if the gap length is less than the infectious period. HPV infections last, on average, 1 year for women [18] and may be as short as 6 months for men [12]. We hypothesize that short gaps of less than 1 year between partners would predict a greater likelihood of spread from one partnership to the next.
Our objective was to study the presence of HPV in a new sexual partnership among young adults, with a focus on sexual network features. We further explored the impact of 2 prevention approaches that should, in theory, limit entry of HPV into a partnership: frequent condom use with partners external to the dyad (concurrent or previous partners), and the woman's HPV vaccination status.
METHODS
The HITCH Study (HPV Infection and Transmission Among Couples Through Heterosexual Activity) was a longitudinal investigation with the overall aim to further our understanding of HPV transmission between heterosexual partnerships to better inform prevention strategies [19]. The study population consisted of young women attending a university or junior college in Montreal, Quebec, Canada, and their male partners. Eligible women had a current male sexual partner for which the relationship duration was no more than 6 months, had an intact uterus and no history of cervical lesions/cancer, and were not pregnant or planning to become pregnant in the next 24 months. From May 2005 to January 2010, volunteers were recruited on campuses and at venues frequented by students. Participants attended study visits at the student health services clinics at either McGill or Concordia Universities, and were compensated CDN $50 per visit. All provided written informed consent. Study procedures and documents were approved by the ethical review committees at McGill University, Concordia University, and Université de Montréal.
Data obtained at the baseline visit were the object of the present analysis. At their first visit, women self-collected vaginal swabs using validated methods [20, 21] and as previously described [19, 22]. For men, nurses collected specimens of epithelial cells from the penis (ie, the glans up to and including the external opening of the meatus, coronal sulcus, penile shaft, and foreskin in uncircumcised men) and scrotum using established methods [19, 22, 23].
Further, men and women self-completed separate online sexual history questionnaires, which included questions about the lifetime number of sex partners, sexual acts within the dyad (ie, with their “HITCH partner”), and sexual acts with concurrent partners or with the most recent partner prior to their HITCH partner, as appropriate. Participants reported condom use frequency using the following response categories: never (0% of the time), rarely (1%–25%), some of the time (26%–75%), most of the time (76%–99%), and always (100% of the time).
In Canada, the quadrivalent HPV vaccine was licensed in July 2006 for use among women and girls aged 9–26 years, 1 year after HITCH began recruitment. At that time, questions about HPV vaccination were added to the female questionnaire. We asked women if they had received the HPV vaccine, and if so, the number of injections received and date of last injection. HPV vaccination was not approved for men in Canada until February 2010.
The outcome of interest was the presence of HPV DNA in genital specimens within partnerships, defined as the presence of 1 or more HPV types in either or both partners. We tested genital specimens using the Linear Array HPV genotyping assay (LA-HPV) (Roche Molecular Systems), a polymerase chain reaction protocol based on the amplification of a 450-base-pair segment in the L1 HPV gene, which permits detecting 36 mucosal HPV genotypes [24]. Coamplification of a ß-globin DNA sequence permitted the determination of specimen adequacy. We used the definitions of the International Agency for Research on Cancer (IARC) to classify 13 genotypes as high-risk oncogenic types (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68; ie, IARC groups 1 and 2A) [25].
Statistical analysis was conducted using SAS version 9.3 (SAS Institute, Inc., Cary, NC). All P values were 2-sided. Statistical significance was based on P < .05. We calculated descriptive statistics for study participants and dyads. Dyad characteristics (ie, duration of the sexual partnership, frequency of sex per week) were based on the average of both partners' reports; we have previously reported good agreement between partner's reports [19]. We calculated HPV prevalence rates and 95% confidence intervals (CIs) for all types combined, high-risk oncogenic types, and the vaccine-preventable types HPV16, 18, 6, and 11. To assess risk factors for prevalent HPV in partnerships, we estimated prevalence ratios [26] rather than odds ratios because the latter can greatly overestimate the ratio of proportions for a common outcome. Potential risk factors were selected for consideration a priori and included: the sum total of extra dyadic partners (ie, the sum of the male's and female's reported lifetime number of partners), the minimum length of time since the most recent partner external to the dyad (classifying concurrent partners as an overlap), the dyad's minimum frequency of condom use with the most recent extra-dyadic partner, and the female participant's HPV vaccine status.
RESULTS
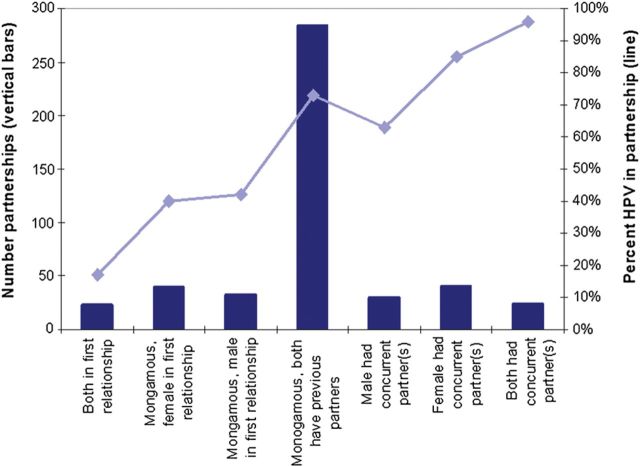
A total of 503 dyads were recruited. Of these, 482 had nonmissing HPV-DNA results and sexual histories and were the focus of analysis (Table 1). Most participants were sexually experienced, as few were in their first vaginal sex relationship, and most reported being monogamous since the start of their new relationship, on average 4 months prior to enrolment. For the majority of partnerships (60%, 284), both the male and female had had previous sexual partners but were monogamous for the current partnership (Figure 1). For between 5%–9% of partnerships, both partners were in their first vaginal sex relationship, it was the female's or male's first vaginal sex relationship, the male or female had concurrent partners, or both had concurrent partners (Figure 1). There was evidence of moderately assortative mixing according to Pearson's correlation coefficients between men's and women's lifetime log-transformed number of partners (ρ = 0.38, 95% CI, .29, .44) and between men's and women's gap length (ρ = 0.36, 95% CI, .27, .44, where overlaps were given a negative value and persons with no previous partner were assigned a value of 36 months).
Table 1.
Characteristics of 482 Recently Formed Heterosexual Partnerships in the HITCH Cohort Study, Montreal, Quebec, Canada, 2005–2010
| Characteristic | Women | Men | Partnership |
|---|---|---|---|
| Median age (range) | 21 (18–26) | 23 (18–46) | … |
| Born in Canada | 69% | 64% | … |
| Received HPV vaccine | 12% | N/A | … |
| Median lifetime no. of vaginal sex partners (range) | 6 (0–40) | 8 (0–56) | … |
| Median duration of sexual partnership, months (IQR) | … | … | 4.2 (2.8–5.3) |
| Median frequency of vaginal sex per week (IQR) | … | … | 4.0 (2.7–6.0) |
| Any HPV | 57.7% | 57.9% | 67.0% |
| Any high-risk oncogenic HPVa | 41.7% | 40.5% | 50.4% |
| Any HPV 6, 11, 16, or 18 | 22.0% | 22.0% | 28.6% |
Abbreviations: HPV, human papillomavirus; IARC, International Agency for Research on Cancer; IQR, interquartile range; N/A, not applicable.
a High-risk oncogenic HPV included IARC-classified group 1 carcinogens (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59) and group 2A carcinogens (type 68).
Figure 1.
Prevalence of HPV in recently formed partnerships, by men's and women's histories with partners external to the dyad. Abbreviation: HPV, human papillomavirus.
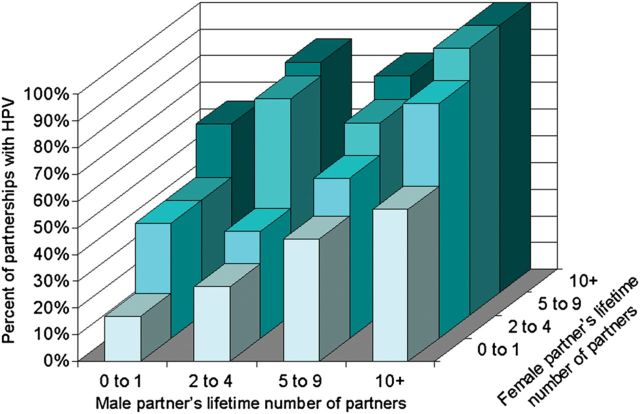
The prevalence of HPV in partnerships was strongly associated with the history of sexual activities with other partnerships (external to the dyads) (Figures 1 and 2). HPV was least prevalent in dyads who were in their first vaginal sex relationship (17%), and was virtually ubiquitous in dyads for which both partners had concurrent partners (96%). HPV was detected in all partnerships (44/44) for which both the male and female had had 10 or more lifetime partners. The proportion with HPV rose more steeply with the female partner's lifetime number of partners than for the male's, but the differences were not statistically significant (Figure 2).
Figure 2.
Prevalence of HPV in recently formed partnerships, by men's and women's lifetime number of vaginal sex partners. Abbreviation: HPV, human papillomavirus.
Concurrency and gap lengths in serially monogamous dyads did not remain independently associated with HPV in partnerships, upon adjustment for the sum total of the female and male partner's lifetime number of partners (Table 2), which was most strongly associated with HPV detection in partnerships. Dyads that had more than 20 lifetime sex partners between the male and female were over 3 times more likely to have detectable HPV, compared to dyads with 4 or fewer (Table 2). Men's and women's lifetime number of partners were both strongly associated with detectable HPV in partnerships (P < .0001; Figure 2, Table 2). However, there was a slight increased chance of HPV in partnerships for which the female had had more lifetime partners than the male partner, compared to partnerships where both the female and male had had approximately the same number of partners; however, this did not reach statistical significance (adjusted prevalence rate ratio [PRR] = 1.2, 95% CI, 1.0, 1.4).
Table 2.
Risk Factors for Presence of HPV in Recently Formed Partnerships
| Risk Factor | n | % HPV | Unadjusted Prevalence Rate Ratio (95% CI) | Adjusted Prevalence Rate Ratio (95% CI)a |
|---|---|---|---|---|
| Sum of female and male partner's lifetime number of partners | ||||
| ≤4 | 80 | 26.3 | Referent | Referent |
| 5–10 | 136 | 53.7 | 2.0 (1.4, 3.0) | 1.9 (1.2, 2.9) |
| 11–20 | 155 | 80.7 | 3.1 (2.1, 4.5) | 2.8 (1.8, 4.2) |
| >20 | 107 | 93.5 | 3.6 (2.5, 5.2) | 3.2 (2.0, 4.8) |
| Concordance on female's and male's lifetime number of partnersb | ||||
| Same number of partners | 190 | 61.1 | Referent | Referent |
| Female has had more partners than male | 127 | 71.7 | 1.2 (1.0, 1.4) | 1.2 (1.0, 1,4) |
| Male has had more partners than female | 161 | 69.6 | 1.1 (.98, 1.3) | 1.1 (.99, 1.1) |
| Minimum length of time since previous/concurrent partner | ||||
| Both virgins/>12 mo gap | 41 | 29.3 | Referent | Referent |
| Gap >6–12 mo | 37 | 54.1 | 1.8 (1.1, 3.2) | 1.2 (.68, 2.0) |
| Gap ≤6 mo | 233 | 70.0 | 2.4 (1.5, 3.9) | 1.4 (.83, 2.2) |
| Overlap/concurrent partner(s) | 74 | 83.8 | 2.8 (1.8, 4.7) | 1.4 (.86, 2.3) |
| Dyad's minimum condom use frequency with previous/concurrent partnersc | ||||
| Never | 95 | 80.0 | Referent | Referent |
| Rarely (1%–25%) | 83 | 79.5 | 0.99 (.86, 1.15) | 1.00 (.86, 1.17) |
| Sometimes (26%–75%) | 46 | 76.1 | 0.95 (.79, 1.15) | 1.04 (.87, 1.24) |
| Most of the time (76%–99%) | 46 | 60.9 | 0.76 (.59, .98) | 0.86 (.66, 1.12) |
| Always | 86 | 52.3 | 0.65 (.52, .82) | 0.73 (.58, .91) |
| Female's HPV vaccine statusd | ||||
| Unvaccinated | 413 | 68.0 | Referent | Referent |
| Vaccinated | 58 | 60.3 | 0.89 (.71, 1.10) | 0.88 (.68, 1.15) |
Abbreviations: CI, confidence interval; HPV, human papillomavirus.
a Adjusted for all variables shown.
b Concordance within the categories 0–1, 2–4, 5–9, and 10 or more lifetime partners.
c Limited to 356 dyads who reported vaginal sex with extra-dyadic partners.
d Missing for 11 dyads.
Dyads that always used condoms with previous partner(s) were 27% (95% CI, 9%–42%) less likely to have HPV after accounting for number of partners and gap length/concurrency (Table 2).
The female participant's HPV vaccine status had a modest and statistically insignificant protective effect (PRR = 0.88, 95% CI, .68–1.15). The magnitude of protection increased but did not reach statistical significance when the outcome was limited to HR-HPV (PRR = 0.85, 95% CI, .62–1.21) or to vaccine-preventable HPV types 6/11/16/18 (PRR = 0.74, 95% CI, .37–1.48). There were 12 dyads for whom the female reported vaccination, and a vaccine-preventable type was present; of these, 7 infections were present in the male only, 3 in both partners, and 2 in the female only.
We explored other attributes of partnerships. In unadjusted analysis, partnerships for which the male partner was at least 5 years older than the female were more likely to have prevalent HPV (crude PRR = 1.62, 95% CI, 1.34, 1.96) than partnerships for which both partners were the same age. Similarly, HPV was more prevalent in partnerships where the male was not a postsecondary student (unadjusted PRR = 1.16, 95% CI, 1.02, 1.32). However, both of these prevalence rate ratios were no longer statistically different from 1 upon adjustment for lifetime number of partners and gap lengths/concurrency (data not shown). We observed no effect of concordance/discordance on the male's and female's ethnicity or preferred language (English or French) in either unadjusted or adjusted analyses (data not shown).
DISCUSSION
In this sample of young adults in Montreal, Canada, HPV was present in two-thirds of recently formed partnerships. Prevalence is expected to be high in this population because acquisition of a new partner is an important risk factor for incident infection in women [11, 27, 28] and men [29]. The main driver of presence of HPV in partnerships was the number of lifetime partners reported by the male and female partner combined. The influence of the number of partners was expected, given the ubiquity of the observation that the number of sexual partners is a strong predictor of HPV infection and HPV-related cancers in women and men [1, 9–14] and further, that these outcomes tend to be observed more often in people whose partners have had multiple partners [27, 30, 31].
Dyads that had concurrent partners or short gaps since the most recent partner external to the partnership had high prevalence of HPV. This is consistent with findings among young women in Seattle for whom the rate of HPV acquisition was lower for those who waited at least 8 months to have sex with their new partner [27]. Contrary to expectation, we did not observe that partnership timing independently predicted partnership HPV prevalence after adjustment for the total number of partners, because dyads with short gaps or concurrency also had high numbers of lifetime partners. HPV infections last, on average, 1 year or less [12, 18]. One would expect that gaps longer than 1 year would predict a lower likelihood of HPV being present in a new partnership; however, we were unable to explore such effects because our study sample included few partnerships with long gaps since the most recent previous partner. To identify the meaningful gap length, it would be necessary to conduct a similar analysis in a population with less frequent partner turnover, with sufficient numbers of participants who have gap lengths at least as long as 2–3 years.
In our study sample, descriptive findings were suggestive that the female partner's behavior may be more influential on HPV entry into partnerships. HPV was more prevalent in partnerships where the female reported concurrent partners but the male was monogamous (81%) compared to partnerships where the male reported concurrent partners but the female was monogamous (63%). HPV was also more prevalent in partnerships where the female had 5 or more lifetime partners but the male did not (79%) compared to partnerships where the male had 5 or more lifetime partners but the female did not (69%). Nevertheless, these sex differences did not reach statistical significance and require confirmation in a larger sample. Such differences, if true, could be due to higher female-to-male transmission rates as some authors have observed [32, 33] (though we have not in HITCH [34]), or to a longer duration of infection in females compared to males [35].
The novel dyadic approach allowed for the identification of a previously undocumented protective effect of condoms on HPV transmission between partnerships. We observed that consistent condom use with previous partners predicted lower prevalence of HPV in current partnerships, suggesting that condoms may have some impact on limiting the spread of HPV, although protection was incomplete. Such a finding would not be possible to observe in individual-based studies. A protective effect of condoms on HPV infection has been reported by some but not all studies [36–40]. We have previously reported that, after accounting for the HPV infection status of the female partner, condom use is associated with a 36% reduction in HPV prevalence among men; among women, the effect did not reach statistical significance [22]. Lower transmission rates among initially HPV-discordant partnerships have been observed in HITCH, at least for male-to-female transmission [41], and among heterosexual couples in Hawaii [32]. The consistent use of condoms with past partners may have prevented HPV acquisition entirely, or it may have prompted more rapid clearance, such that people with HPV infection were less likely to have remained infected by the time they started a new partnership. In a randomized controlled trial among male partners of women with cervical intraepithelial neoplasia, condom users experienced faster regression of HPV-associated penile lesions [42].
This study was limited in its ability to identify HPV vaccine effects on preventing entry of infection into partnerships. First, despite some evidence for cross-protection against nonvaccine-preventable types [43], it would not be expected that receipt of quadrivalent vaccine would prevent infection with all types. Second, we had limited precision given the few women who received vaccine. During the recruitment period, the Quebec provincial vaccine initiative began but our target population was too old to receive vaccine via the publicly funded program. Among study participants, vaccination would have only occurred among women who sought it out with their own physician, and either paid out-of-pocket or had coverage via extended healthcare insurance. Third, female vaccination would not prevent men from having had HPV at partnership formation or women to be infected before vaccination; indeed, in dyads for which the female was vaccinated but HPV-preventable types were present, they were most likely to be detected in the male partner only. Fourth, all women were vaccinated after coitarche; therefore, they may have already been infected at time of vaccination. Moreover, it is possible that vaccinated women were those with greater motivation because of sexual behavior and exposure. In our study, we observed that female vaccinees reported more lifetime sex partners than nonvaccinees (data not shown); thus vaccinees may have been exposed before vaccination, resulting in vaccine inefficiency. HPV vaccines have no demonstrated therapeutic effect to clear existing infections [43]. In future, similar research would ideally recruit dyads for which vaccinees were immunized prior to sexual debut.
We focused on vaginal sex behaviors under the assumption that penile–vaginal exposures were most likely to result in transmission to the genitalia. Indeed, the lowest partnership prevalence (17%) was among the 23 dyads for which both partners were in their first vaginal sex relationship. Nevertheless, prevalence among such dyads was nonzero. It is possible that HPV may transmit via other sexual activities, including mutual masturbation and oral sex [27, 44–46].
To the best of our knowledge, our study is the first to explore the effects of partnership timing, condom use with previous partners, and female HPV vaccination status on the presence of HPV in newly formed heterosexual partnerships. The finding that consistent condom use limited onward spread to future partners is in support of broader sexual health programs that promote condom use to prevent infection with STIs. Ideally, high vaccine coverage will have the most potential to limit ongoing transmission in sexual networks, at least for vaccine-preventable types, given the high vaccine efficacy observed in trials [43]. We encourage ongoing epidemiologic monitoring of HPV in sexual networks, particularly in populations with suboptimal HPV vaccine coverage for which HPV will likely become concentrated in core groups [47].
Notes
Acknowledgments. The authors thank the collaborating student health services clinics at McGill and Concordia universities and current and past research staff (N. Slavtcheva, G. Kelsall, S. Dumais, N. Morykon, A. Rocamora, B. McCallum, E. Lyn, C. Pizzolongo, V. Legault, H. Voyer, J. Guenoun, J. Selinger, E. Lapointe, E. Montpetit Dubrule, V. D'Anjou Pomerleau, J. Bleecker, S. Rahayel, and J. Sammut). We also thank Ramandip Grewal at the Ontario HIV Treatment Network for administrative assistance preparing the manuscript.
Financial support. This work was supported by the Canadian Institutes for Health Research (operating grant 68893 and team grant 83320) and the US National Institutes of Health (grant AI073889). Molecular techniques were optimized with the support of the Reseau FRSQ Fonds de la Recherche en Santé du Québec AIDS and Infectious Disease Network (SIDA-MI). Supplementary and unconditional funding support was provided by Merck-Frosst Canada Ltd and Merck & Co. Ltd. A. N. B. is supported by a Canadian Institutes of Health Research (CIHR) New Investigator Award. The funders played no role in the writing of the manuscript or the decision to submit it for publication.
Potential conflicts of interest. E. L. F. is a board member for Merck and has received consultancies from Roche and Scimetrika. P. P. T. has received payment for lectures by Merck-Frosst Canada and Bayers. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813–9. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 2.Giuliano AR, Lazcano-Ponce E, Villa LL, et al. The human papillomavirus infection in men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev. 2008;17:2036–43. doi: 10.1158/1055-9965.EPI-08-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nielson CM, Flores R, Harris RB, et al. Human papillomavirus prevalence and type distribution in male anogenital sites and semen. Cancer Epidemiol Biomarkers Prev. 2007;16:1107–14. doi: 10.1158/1055-9965.EPI-06-0997. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez BY, Wilkens LR, Zhu X, et al. Circumcision and human papillomavirus infection in men: a site-specific comparison. J Infect Dis. 2008;197:787–94. doi: 10.1086/528379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Agency for Research on Cancer (IARC) Lyon, France: IARC; 2007. Monographs on the evaluation of carcinogenic risks to humans. Report No.: Volume 90. [PMC free article] [PubMed] [Google Scholar]
- 6.Giuliano AR, Tortolero-Luna G, Ferrer E, et al. Epidemiology of human papillomavirus infection in men, cancers other than cervical and benign conditions. Vaccine. 2008;26(Suppl 10):K17–28. doi: 10.1016/j.vaccine.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosch FX, Burchell AN, Schiffman M, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26(Suppl 10):K1–16. doi: 10.1016/j.vaccine.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 8.Morris M, Holmes K. Sexual partnership effects on STIs/HIV transmission. In: Holmes K, Sparling P, Stamm W, et al., editors. Sexually transmitted diseases. 4th ed. New York: McGraw-Hill Medical; 2008. pp. 127–36. [Google Scholar]
- 9.Herrero R, Castle PE, Schiffman M, et al. Epidemiologic profile of type-specific human papillomavirus infection and cervical neoplasia in Guanacaste, Costa Rica. J Infect Dis. 2005;191:1796–807. doi: 10.1086/428850. [DOI] [PubMed] [Google Scholar]
- 10.Muñoz N, Méndez F, Posso H, et al. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis. 2004;190:2077–87. doi: 10.1086/425907. [DOI] [PubMed] [Google Scholar]
- 11.Moscicki AB, Hills N, Shiboski S, et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA. 2001;285:2995–3002. doi: 10.1001/jama.285.23.2995. [DOI] [PubMed] [Google Scholar]
- 12.Lu B, Wu Y, Nielson CM, et al. Factors associated with acquisition and clearance of human papillomavirus infection in a cohort of US men: a prospective study. J Infect Dis. 2009;199:362–71. doi: 10.1086/596050. [DOI] [PubMed] [Google Scholar]
- 13.Nielson CM, Harris RB, Dunne EF, et al. Risk factors for anogenital human papillomavirus infection in men. J Infect Dis. 2007;196:1137–45. doi: 10.1086/521632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho GYF, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–8. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 15.UNAIDS Reference Group on Estimates, Modelling, and Projections: Working Group on Measuring Concurrent Sexual Partnerships. HIV: consensus indicators are needed for concurrency. The Lancet. 2010;375:621–2. doi: 10.1016/S0140-6736(09)62040-7. [DOI] [PubMed] [Google Scholar]
- 16.Kraut-Becher JR, Aral SO. Gap length: an important factor in sexually transmitted disease transmission. Sex Transm Dis. 2003;30:221–5. doi: 10.1097/00007435-200303000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Foxman B, Newman M, Percha B, Holmes KK, Aral SO. Measures of sexual partnerships: lengths, gaps, overlaps, and sexually transmitted infection. Sex Transm Dis. 2006;33:209–14. doi: 10.1097/01.olq.0000191318.95873.8a. [DOI] [PubMed] [Google Scholar]
- 18.Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine. 2006;24(Suppl 1):S1–15. doi: 10.1016/j.vaccine.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 19.Burchell AN, Tellier P-P, Hanley J, Coutlée F, Franco EL. Human papillomavirus infections among couples in new sexual relationships. Epidemiol. 2010;21:31–7. doi: 10.1097/EDE.0b013e3181c1e70b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright TC, Jr, Denny L, Kuhn L, Pollack A, Lorincz A. HPV DNA testing of self-collected vaginal samples compared with cytologic screening to detect cervical cancer. JAMA. 2000;283:81–6. doi: 10.1001/jama.283.1.81. [DOI] [PubMed] [Google Scholar]
- 21.Sellors JW, Lorincz AT, Mahony JB, et al. Comparison of self-collected vaginal, vulvar and urine samples with physician-collected cervical samples for human papillomavirus testing to detect high-grade squamous intraepithelial lesions. CMAJ. 2000;163:513–8. [PMC free article] [PubMed] [Google Scholar]
- 22.Burchell AN, Tellier P-P, Hanley J, Coutlée F, Franco EL. Influence of partner's infection status on prevalent human papillomavirus among persons with a new sex partner. Sex Transm Dis. 2010;37:34–40. doi: 10.1097/OLQ.0b013e3181b35693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weaver BA, Feng Q, Holmes KK, et al. Evaluation of genital sites and sampling techniques for detection of human papillomavirus DNA in men. J Infect Dis. 2004;189:677–85. doi: 10.1086/381395. [DOI] [PubMed] [Google Scholar]
- 24.Coutlée F, Rouleau D, Petignat P, et al. Enhanced detection and typing of human papillomavirus (HPV) DNA in anogenital samples with PGMY primers and the Linear array HPV genotyping test. J Clin Microbiol. 2006;44:1998–2006. doi: 10.1128/JCM.00104-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens—part B: biological agents. Lancet Oncol. 2009;10:321–2. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 26.Barros AJD, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winer RL, Lee S-K, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218–26. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 28.Giuliano AR, Harris R, Sedjo RL, et al. Incidence, prevalence, and clearance of type-specific human papillomavirus infections: the Young Women's Health Study. J Infect Dis. 2002;186:462–9. doi: 10.1086/341782. [DOI] [PubMed] [Google Scholar]
- 29.Partridge JM, Hughes JP, Feng Q, et al. Genital human papillomavirus infection in men: incidence and risk factors in a cohort of university students. J Infect Dis. 2007;196:1128–36. doi: 10.1086/521192. [DOI] [PubMed] [Google Scholar]
- 30.Castellsagué X, Bosch FX, Muñoz N, et al. Male circumcision, penile human papillomavirus infection, and cervical cancer in female partners. N Engl J Med. 2002;346:1105–12. doi: 10.1056/NEJMoa011688. [DOI] [PubMed] [Google Scholar]
- 31.Collins SI, Mazloomzadeh S, Winter H, et al. Proximity of first intercourse to menarche and the risk of human papillomavirus infection: a longitudinal study. Int J Cancer. 2005;114:498–500. doi: 10.1002/ijc.20732. [DOI] [PubMed] [Google Scholar]
- 32.Hernandez BY, Wilkens LR, Zhu X, et al. Transmission of human papillomavirus in heterosexual couples. Emerg Infect Dis. 2008;14:888–94. doi: 10.3201/eid1406.070616.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Widdice L, Ma Y, Jonte J, et al. Concordance and transmission of human papillomavirus within heterosexual couples observed over short intervals. J Infect Dis. 2013;207:1286–94. doi: 10.1093/infdis/jit018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burchell AN, Coutlée F, Tellier P-P, Hanley J, Franco EL. Genital transmission of human papillomavirus in recently formed heterosexual couples. J Infect Dis. 2011;204:1723–9. doi: 10.1093/infdis/jir644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giuliano AR, Lee J-H, Fulp W, et al. Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet. 2011;377:932–40. doi: 10.1016/S0140-6736(10)62342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaccarella S, Lazcano-Ponce E, Castro-Garduño JA, et al. Prevalence and determinants of human papillomavirus infection in men attending vasectomy clinics in Mexico. Int J Cancer. 2006;119:1934–9. doi: 10.1002/ijc.21992. [DOI] [PubMed] [Google Scholar]
- 37.Baldwin SB, Wallace DR, Papenfuss MR, Abrahamsen M, Vaught LC, Giuliano AR. Condom use and other factors affecting penile human papillomavirus detection in men attending a sexually transmitted disease clinic. Sex Transm Dis. 2004;31:601–7. doi: 10.1097/01.olq.0000140012.02703.10. [DOI] [PubMed] [Google Scholar]
- 38.Winer RL, Hughes JP, Feng Q, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med. 2006;354:2645–54. doi: 10.1056/NEJMoa053284. [DOI] [PubMed] [Google Scholar]
- 39.Manhart LE, Koutsky LA. Do condoms prevent genital HPV infection, external genital warts, or cervical neoplasia? A meta-analysis. Sex Transm Dis. 2002;29:725–35. doi: 10.1097/00007435-200211000-00018. [DOI] [PubMed] [Google Scholar]
- 40.Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: a systematic review of the literature. J Infect Dis. 2006;194:1044–57. doi: 10.1086/507432. [DOI] [PubMed] [Google Scholar]
- 41.Burchell A, Tellier P, Coutlée F, Hanley J, Franco E. Evidence for the protective effect of condoms against male-to-female transmission. Poster P-622 presented at the 26th International Papillomavirus Conference; July 3–8; Montreal, Canada: 2010. http://www.hpvmedia.org/index.php?option=com_hpv&view=presentation&id=995 . (Accessed 2011) [Google Scholar]
- 42.Bleeker MCG, Hogewoning CJA, Voorhorst FJ, et al. Condom use promotes regression of human papillomavirus-associated penile lesions in male sexual partners of women with cervical intraepithelial neoplasia. Int J Cancer. 2003;107:804–10. doi: 10.1002/ijc.11473. [DOI] [PubMed] [Google Scholar]
- 43.Schiller JT, Castellsagué X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30(Suppl 5):F123–38. doi: 10.1016/j.vaccine.2012.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sonnex C, Strauss S, Gray JJ. Detection of human papillomavirus DNA on the fingers of patients with genital warts. Sex Transm Infect. 1999;75:317–9. doi: 10.1136/sti.75.5.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D'Souza G, Agrawal Y, Halpern J, Bodison S, Gillison ML. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infect Dis. 2009;199:1263–9. doi: 10.1086/597755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heck JE, Berthiller J, Vaccarella S, et al. Sexual behaviours and the risk of head and neck cancers: a pooled analysis in the International Head and Neck Cancer Epidemiology (INHANCE) consortium. Int J Epidemiol. 2010;39:166–81. doi: 10.1093/ije/dyp350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wasserheit JN, Aral SO. The dynamic topology of sexually transmitted disease epidemics: implications for prevention strategies. J Infect Dis. 1996;174(Suppl 2):S201–13. doi: 10.1093/infdis/174.supplement_2.s201. [DOI] [PubMed] [Google Scholar]