Abstract
The integrity of the plasma membrane is maintained through an active repair process, especially for skeletal and cardiac muscle cells, in which contraction-induced mechanical damage frequently occurs in vivo1,2. Muscular dystrophies (MDs) are a group of muscle diseases characterized by skeletal muscle wasting and weakness3,4. An important cause of MD is defective repair of sarcolemmal injuries, and sarcolemma repair requires Ca2+ sensor proteins5–8 and Ca2+-dependent delivery of intracellular vesicles to injury sites5,8,9. TRPML1 (ML1) is an endosomal and lysosomal Ca2+ channel and its human mutations cause Mucolipidosis IV, a neurodegenerative disease with motor disabilities10,11. Here, we report that ML1-null mice develop a primary, early-onset muscular dystrophy independent of neural degeneration. Although the dystrophin-glycoprotein complex and the known membrane repair proteins are normally expressed, membrane resealing was defective in ML1-null muscle fibers or upon acute and pharmacological inhibition of ML1 channel activity or vesicular Ca2+ release. Injury facilitated the trafficking and exocytosis of vesicles by upmodulating ML1 channel activity. In the dystrophic mdx mouse model, overexpression of ML1 decreased muscle pathology. Collectively, we have identified an intracellular Ca2+ channel that regulates membrane repair in skeletal muscle via Ca2+-dependent vesicle exocytosis.
Keywords: Membrane repair, Ca2+, TRP channel, lysosome
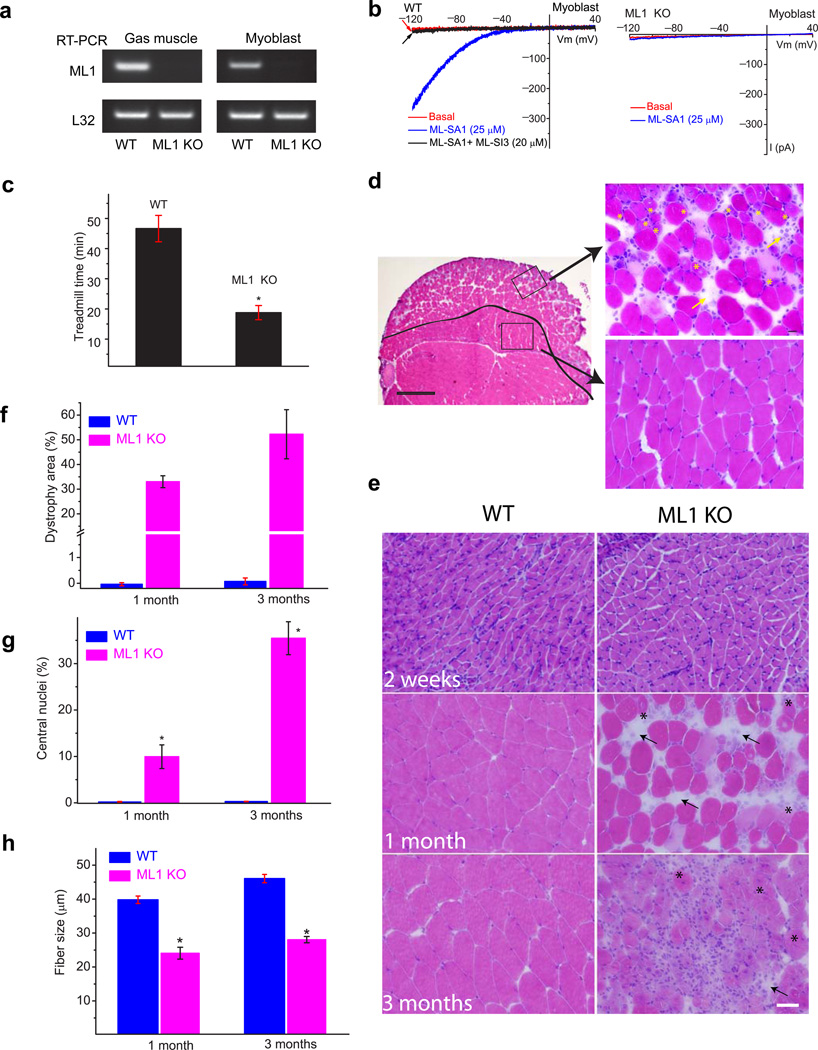
A mouse model with targeted deletion of the ML1 gene (ML1 knockout or KO; ML1−/−)11 was confirmed by PCR genotyping (Supplementary Fig. 1a). In skeletal muscle and cultured myoblasts isolated from ML1 KO mice, reverse transcription-PCR analysis could not detect full-length ML1 transcript (Fig. 1a). Consistently, patch-clamping of the endolysosomal membranes12 showed that whole-endolysosome ML1-like currents (IML1) were activated by ML-SA1, a membrane-permeable TRPML-specific synthetic agonist13 in WT but not ML1 KO primary cultured myoblasts (Fig. 1b). IML1 was potently inhibited by ML-SI compounds (Fig. 1b), which are membrane-permeable TRPML-specific synthetic antagonists14.
Figure 1. ML1-null mice develop early-onset progressive MD.
(a) Reverse transcription-PCR analysis of WT and TRPML1 (ML1) KO Gastroc muscle and primary cultured myoblasts with a primer pair targeting the deleted region (exons 3, 4, and 5) of the TRPML1 gene11. The housekeeping gene L32 served as a loading control. (b) ML-SA1 (25 µM) activated endogenous whole-endolysosome ML1-like currents (IML1) in WT but not ML1 KO myoblasts. ML-SA1-activated currents were inhibited by ML-SI3 (20 µM). Currents were stimulated by using a ramp voltage protocol from −120 to +40 mV. (c) One-month-old ML1-null mice exhibited a shorter running time in a treadmill test compared with WT controls. Animals were trained to run on a 15° downhill treadmill at a speed of 20 m/min. n = 5 animals each. (d) H&E staining of a Gastroc muscle section from a 1-month-old ML1 KO mouse. Scale bar = 400 µm. The black line indicates the boundary between the “dystrophic” region at the periphery and the “normal” region in the center. The representative images on the right are from the boxed areas (left) and show the centrally nucleated fibers (indicated with *) and fibrosis (arrows). Scale bar = 20 µm. (e) H&E staining of Gastroc muscle sections from ML1 KO mice at 2 weeks, and 1 and 3 months of age. Scale bar = 20 µm. (f) The percentage of the dystrophic area in ML1 KO (pink) and WT (blue) muscle. (g) The percentage of centrally nucleated fibers increased with age in ML1 KO. (h) The diameters of muscle fibers were substantially smaller in ML1 KO. For panels f, g, h, n = 3 animals for each condition. Data are presented as the mean ± s.e.m.
At 1 month of age, ML1 KO mice are grossly healthy and do not exhibit any obvious neurodegeneration11. However, when they are challenged with a 15° downhill treadmill test at the speed of 20 m/min, ML1 KO mice show a pronounced defect in their motor abilities and a greatly reduced treadmill staying time (Fig. 1c). Histological analysis of various tissues involved in the movement impairment revealed, unexpectedly, that the skeletal muscles of ML1 KO mice exhibited clear signs of dystrophy even at 1 month (Fig. 1d,e). By 1 month, individual necrotic and centrally nucleated fibers were detectable in ML1−/− skeletal muscle (Fig. 1d,e). In contrast, no obvious dystrophy was seen in WT skeletal muscle (Fig. 1e,f). By three months, central nucleation, fibrosis (fibrous scar tissue and fat replacement), and immune cell infiltration were commonly seen (Fig. 1d,e & Supplementary Fig. 1). As observed in most animal models of MD15, the distribution of the dystrophic area in skeletal muscle was heterogeneous. For example, for the Gastroctrocnemius (Gastroc) muscle, the dystrophic area was mainly concentrated on the periphery of the muscle and the central region remained largely intact (Fig. 1d). A characteristic of MD is muscle regeneration triggered by degeneration, forming a cycle of degeneration and regeneration4,9. Hence, centrally nucleated muscle fibers and smaller-sized fibers are frequently observed, which reflects muscles undergoing active regeneration4,9. Consistent with this finding, ML1−/− fibers were relatively small in size and with a high degree of central nucleation (Fig. 1d, g, h).
ML1 KO mice exhibited progressive MD, with severity increasing with age (Fig. 1e–g). Muscle-specific heterogeneity is commonly seen in MD, potentially due to the variability in their use-dependent physical activity3,4. At 1 month old, only about half of the skeletal muscles, including the triceps, quadriceps, hamstring, and Gastroc muscles, manifested dystrophy (Supplementary Fig. 1c). In contrast, the diaphragm, iliopsoas, gluteus, soleus, and tibialis anterior muscles appeared normal (Supplementary Fig. 1c). However, at 3 months, more skeletal muscles developed dystrophy (Supplementary Fig. 1d). Both type 1 slow-twitch and type 2 fast-twitch muscle fibers were dystrophic (Supplementary Fig. 2a). However, cardiac and smooth muscles did not exhibit obvious pathology (Supplementary Fig. 2b). These results suggest that ML1-null mice exhibited early-onset, progressive, and extensive MD.
Evans blue (EB) dye is a reliable in vivo marker of myofiber damage16. A small but significant percentage of ML1-null Gastroc myofibers were EB-positive at rest (Fig. 2a,b). After a 15° downhill treadmill exercise, the percentage of EB-positive cells in the ML1-null Gastroc muscle increased from 2% to 12% (Fig. 2a,b). In comparison, the percentage of EB-positive cells in WT littermates never exceeded 1%, even after treadmill exercise (Fig. 2a,b). Another measure of myofiber damage is the leakage of muscle proteins to the serum3,4. Consistent with the EB analysis, the serum creatine kinase (CK) levels of ML1-null mice were 2–3-fold higher than those of WT littermates (Fig. 2c). Treadmill exercise further increased serum CK levels (Fig. 2c). Taken together, these results suggest that an increase in muscle membrane damage underlies MD in ML1 KO.
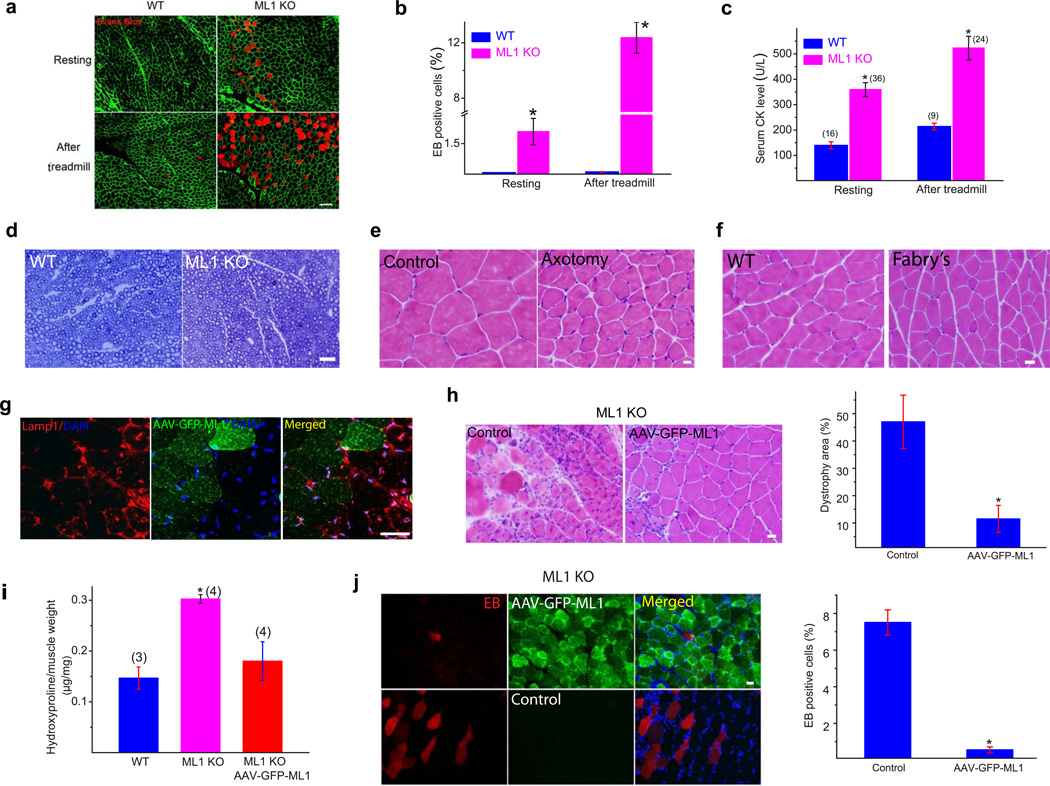
Figure 2. MD and muscle membrane damage of ML1 KO mice are not caused by neural degeneration and can be rescued by muscle expression of ML1.
(a) Evans blue (EB) dye staining in WT and ML1 KO Gastroc muscles either at rest or after a 30-min treadmill test at the speed of 12 meter/min. The immuostaining of α-DG was used to label the sarcolemma. Scale bar = 40 µm. (b) The percentage of EB-positive muscle fibers in WT and ML1 KO mice. n = 3 animals for each condition. (c) Serum CK levels in WT and ML1 KO mice, either before or after treadmill exercise. The number of animals for each condition is indicated in the parentheses. (d) Lack of obvious myelination defects in the sciatic nerve of 1-month-old ML1 KO mice. (e) Sciatic axotomy induced atrophy of WT Gastroc muscle. (f) Atrophic Gastroc muscle of 4-month-old Fabry's mice. Scale bar = 20 µm. (g) AAV-GFP-ML1-infection reduced Lamp1 elevation in ML1-null muscle cells compared with neighboring noninfected controls. Scale bar = 40 µm. (h) The effect of AAV-GFP-ML1 infection on the dystrophic phenotype in ML1 KO muscles; H&E staining revealed deceased levels of necrosis and fibrosis. The right panel shows a decrease in the percentage of the dystrophic area in ML1-null muscle. n = 4 animals for each condition. (i) AAV-GFPML1 infection on collagen content in ML1 KO muscle. The content of hydroxyproline, a marker for collagen content, was measured in Gastroc skeletal muscle from 3-month-old WT and ML1 KO mice. (g) The effect of AAV-GFP-ML1 infection on EB uptake in ML1-null muscle. n = 3 animals for each condition. Data are presented as the mean ± s.e.m. Scale bars = 10 µm for panels d, e, h, and i.
Although muscle pathology and elevated CK levels were initially reported in some ML4 patients17,18, ML4 has generally been considered a disease of neural degeneration10,11, which could explain the motor defects of ML4 patients and ML1 KO mice. Consistent with previous reports11,19, ML1 KO mice did exhibit neuronal cell death, but only at ages >5 months (Supplementary Fig. 2c). At younger ages (1–3 months) motor neurons in the spinal cord did not show any obvious sign of neural degeneration (Supplementary Fig. 1d). Likewise, sciatic nerve myelination was normal in ML1 KOs at one month (Fig. 2d). Furthermore, in conditions mimicking neural degeneration, such as sciatic nerve axotomy, no dystrophic phenotype was observed (Fig. 2e). Instead, axotomy resulted in muscle atrophy, which manifested as a homogeneous decrease in fiber size (Fig. 2e). In addition, Gastroc muscle from the mouse model of Fabry's disease20 exhibited denervation-like effects on the fiber size, without exhibiting MD-like necrosis and central nucleation (Fig. 2f). In 1-month-old ML1-null mice, lysosome storage was barely observed in the dystrophic muscles (Supplementary Fig. 2e). Collectively, muscle dystrophy in the ML1-null mice is unlikely to be a secondary effect of neural degeneration or a lysosome storage defect.
To directly investigate the dystrophic mechanisms caused by ML1 deficiency, we performed a rescue experiment involving intramuscular injection of an adeno-associated virus (AAV) carrying the GFP-ML1 transgene (AAV-GFP-ML1), which typically infected the majority (>85%) of the muscle fibers. As was observed in many LSDs, ML1-null muscle exhibited a compensatory increase in the key lysosomal protein Lamp113, as shown by Lamp1 immunofluorescence staining and western blot analysis (Supplementary Fig. 3a,b). However, when compared with the adjacent noninfected and contralateral uninfected muscle fibers, ML1-null Gastroc muscle infected with AAV-GFP-ML1 (localized in Lamp1-positive compartments; see Supplementary Fig. 3c,d) experienced a dramatic AAV-infection mediated decrease of elevated Lamp1 expression (Fig. 2g & Supplementary Fig. 3e). AAV-ML1-GFP infection reduced the dystrophic area (Fig. 2h), collagen content (Fig. 2i), and the percentage of EB-positive muscle fibers (Fig. 2j). Hence, expression of ML1 in muscle was sufficient to rescue the MD of ML1 KO, suggesting a cell-autonomous mechanism as the underlying cause of MD.
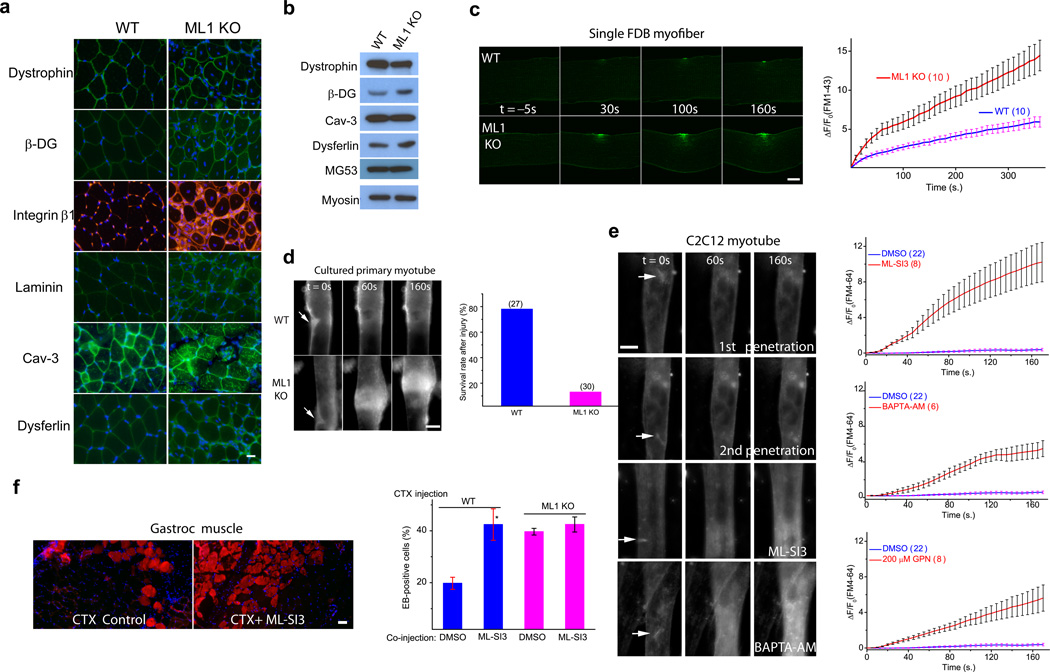
Mechanical stress can cause myofiber necrosis by two separate mechanisms. First, the sarcolemma of a muscle fiber could be more susceptible to damage, as seen in the dystrophin (a core component of DGC) mutant (mdx) mice4,21. Second, a muscle fiber could have a defect in sarcolemma repair, as seen in dysferlin or MG53 KO mice5,9,22. The majority of human MD mutations are linked to defects in the components of the DGC4. However, no obvious decrease in expression was observed for any of core and accessory components of the DGC being examined, which included dystrophin, β-DG, integrin β1, and laminin (Fig. 3a,b). Furthermore, the expression of dysferlin, cav-3, and MG53, three proteins known to be involved in sarcolemma repair and human MD4,5,23, also exhibited no decrease in ML1-null muscle (Fig. 3a,b).
Figure 3. Defective membrane repair capacity in ML1 KO muscle.
(a) Immunofluorescence of dystrophin, β-dystroglycan (β-DG), integrin β1, laminin, caveolin-3 (Cav-3), and dysferlin in ML1-null Gastroc muscle. Scale bar = 10 µm. (b) Western blotting analysis of the DGC components, Cav-3, dysferlin, and MG53 in ML1 KO mice. Myosin served as a loading control. (c) A membrane repair assay performed on single FDB muscle fibers isolated from WT and ML1 KO mice. Membrane damage was induced with a two-photon laser at t = 0 s. Scale bar = 10 µm. The right panel shows the time-dependent changes (ΔF) in FM1–43 fluorescence intensity normalized to the basal fluorescence (F0) for WT (blue) and ML1 KO (red) fibers. (d) Representative images of in vitro differentiated myotubes in response to mechanical damage elicited by a microelectrode (arrows). The lower panel shows the percentage of “surviving” myotubes in response to microelectrode penetration. The experiments were performed in the presence of extracellular Ca2+, and defective membrane resealing led to excessive Ca2+ influx that triggered prolonged muscle contraction. The “surviving” cells are those without prolonged contraction. Scale bar = 50 µm. (e) Responses of C2C12-derived myotubes to microelectrode penetration in Tyrode's solution (2 mM Ca2+) in the presence of DMSO (0.1%; 1st and 2nd penetration), ML-SI3 (20 µM), BAPTA-AM (20 µM), and GPN (200 µM). The right panels show the time course of FM4–64 accumulation (ΔF/F0) at injury sites following microelectrode penetration. Scale bar = 50 µm. Note that the same set of DMSO control data was compared with all groups of drug treatment. (f) The effect of ML-SI3, a TRPML-specific synthetic inhibitor, on EB dye uptake in WT Gastroc muscles injected with the cardiotoxin VII4 (CTX), a cytolytic toxin that disrupts cell membrane in living animals24. The lower panel shows that intramuscular coinjection of ML-SI3 with CTX on EB dye uptake in WT and ML1 KO muscle. Scale bar = 10 µm. Data are presented as the mean ± s.e.m, n = 3 animals for each condition.
To create plasma membrane disruptions and to evaluate the resealing efficiency, single myofibers isolated from the flexor digitorum brevis (FDB) muscle were irradiated with a two-photon laser5,9. FM1–43, a membrane-impermeable fluorescent dye that preferentially adheres to lipids, is commonly used to detect membrane disruptions5. Rapid FM dye entry and accumulation were observed within seconds after laser irradiation (Fig. 3c & Supplementary Fig. 4a). However, in WT muscle fibers, dye entry ceased shortly (1–2 min) after irradiation, suggesting successful membrane resealing (Fig. 3c). In contrast, upon identical laser irritation, ML1-null fibers continued FM dye uptake at the injury sites for several minutes (Fig. 3c), suggesting failed membrane resealing. Removal of extracellular Ca2+ caused rapid accumulation of FM dyes at the injury sites indistinguishably for WT or ML1-null myofibers (Supplementary Fig. 4b). Defective membrane resealing was also observed in ML1-null myotubes that were exposed to mechanical damage elicited by microelectrode penetration into the sarcolemmal membrane (Fig. 3d). Unlikely most WT myotubes, the majority (>80%) of ML1-null myotubes were not able to “survive” the prolonged contractions caused by continuous Ca2+ entry (see below).
Next, pharmacological experiments were performed on C2C12-derived myotubes with the microelectrode penetration assay24. No significant FM4–64 dye was seen at injury sites, even with repeated penetrations (Fig. 3e). In contrast, removal of extracellular Ca2+ significantly increased the entry of FM dyes (Supplementary Fig. 4c), suggesting that the influx of extracellular Ca2+ is essential for repair8. In the presence of BAPTA-AM to chelate intracellular Ca2+ or glycyl-L-phenylalanine 2-naphthylamide (GPN), a lysosome-targeted cathepsin C substrate, to specifically deplete the lysosomal Ca2+ store13, significant dye entry was observed even under normal extracellular Ca2+ concentrations (2 mM; Fig. 3e). These results suggest that lysosomal Ca2+ also has a role in membrane resealing. To monitor the Ca2+ levels at injury sites, myoblasts and C2C12 cells were transfected with Lamp1-GCaMP3, a lysosome-targeted genetically encoded Ca2+ indicator13. Transient Ca2+ increases were observed at injury sites in the presence or absence of external Ca2+ (Supplementary Fig. 4d–g). However, intracellular Ca2+ release was less in ML1 KO myoblasts or in the presence of ML-SI compounds25 (Supplementary Fig. 4f, g), suggesting a contribution to Ca2+ release from lysosomes during membrane damage or early stage of membrane repair. In the presence of external Ca2+, prolonged Ca2+ influx was observed in ML1 KO myoblasts or ML-SI3-treated C2C12 myoblasts (Supplementary Fig. 4d, e). Hence, ML1 plays a dual role in promoting the initial phase of Ca2+ increase (within seconds) but inhibiting the prolonged phase of Ca2+ increase (lasting minutes).
To test whether ML1 plays a direct role in membrane repair, we acutely inhibited ML1 channel function using ML-SIs. Notably, in experiments performed by researchers who were blind to experimental conditions, significant FM4–64 dye uptake was seen in the presence of three structurally independent ML-SI compounds (Fig. 3e & Supplementary Fig. 4c). We also employed ML1 inhibitors to test the role of ML1 in sarcolemma repair in vivo. Cardiotoxin VII4 (CTX) is a toxin that can induce membrane damage in vivo to cause EB dye accumulation in muscle cells24. EB-positive muscle cells were more numerous in CTX-treated ML1-null muscle than in CTX-treated WT controls (Supplementary Fig. 4h). Notably, co-injection of ML-SI3 with CTX markedly increased the percentage of EB-positive muscles in CTX-treated WT mice, to the same level as that of CTX-treated ML1-null mice (Fig. 3f & Supplementary Fig. 4h).
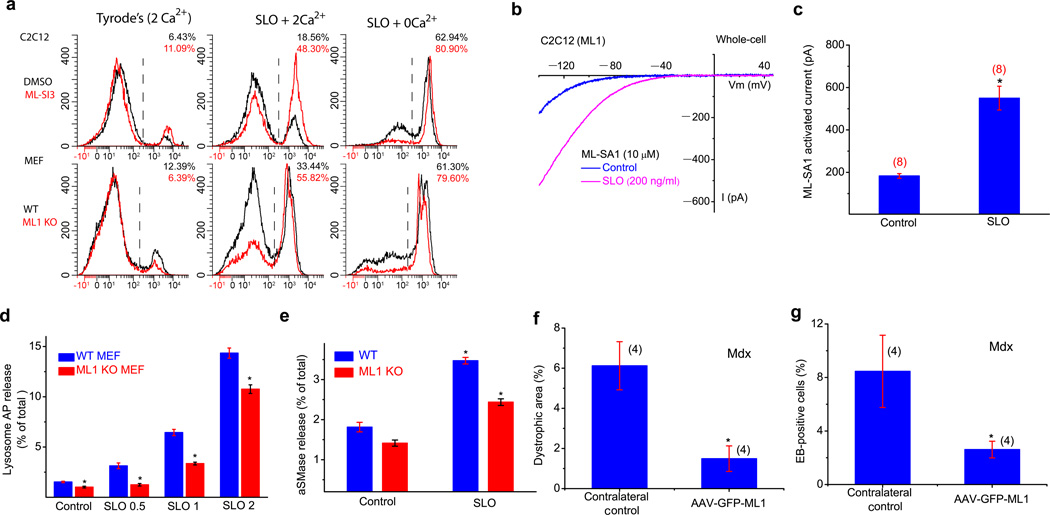
Chemical injuries from streptolysin O (SLO) toxin is commonly employed to induce membrane repair responses, and propidium iodide (PI) staining is a common readout for membrane damage and cell viability26. In non-muscle cells, including mouse embryonic fibroblasts (MEFs) and bone marrow-derived macrophages (BMMs), SLO treatment caused more PI-positive cells for ML1 KO (Fig. 4a & Supplementary Fig. 5). In addition, ML-SI3 increased PI staining in WT, but not KO cells (Fig. 4a & Supplementary Fig. 5). Conversely, ML1 overexpression decreased PI staining (Supplementary Fig. 5). These results suggest that ML1 may be a core component of the membrane repair machinery in both muscle and non-muscle cells.
Figure 4. An essential role of ML1 in lysosomal exocytosis, membrane repair, and protection of muscle damage in vivo.
(a) C2C12 and MEF cells were treated with pore-forming toxin streptolysin O (SLO; 2–5 µg/ml), and stained with PI, a marker for membrane damage. FACS quantification of PI staining was performed in cells with or without SLO or external Ca2+ (2 mM). (b, c) SLO (0.2 µg/ml) treatment increased whole-cell IML1 in C2C12 cells transfected with GFP-ML1. d, SLO (0.5, 1 and 2 µg/ml for 15 min) treatment on the release of lysosomal acid phosphatase (AP; determined using an AP activity colorimetric assay kit) into the culture medium in WT and ML1 KO MEFs. The data are presented as the percentage of the activity of the released vs. total cell-associated enzymes. e, SLO (0.5 µg/mL for 15 min) treatment on the release of lysosomal acid SMase (aSMase; determined using an aSMase activity assay kit) in WT and ML1 KO MEFs. (f, g) The effect of AAV-GFP-ML1 infection on EB uptake and dystrophic area in mdx Gastroc muscle. Data are presented as the mean ± s.e.m.
Membrane resealing requires the fusion and exocytosis of intracellular vesicles at sarcolemma injury sites8. Upon SLO treatment, pHluorin TIRF imaging27 showed that ML1 and Vamp7 doubly-positive vesicles underwent exocytosis (Supplementary Fig. 6a & Video 1). Exocytosis of ML1-resident vesicles may lead to the appearance of ML1 proteins at the plasma membrane. Hence, measurement of whole-cell ML1 currents may provide a readout for exocytosis14. Consistently, SLO treatment also increased whole-cell IML1 by 2–3-fold in C2C12 cells (Fig. 4b, c). ML-SI3-mediated inhibition of ML1 and ML1 KO, on the other hand, significantly reduced SLO-induced lysosomal exocytosis as measured by Lamp1 surface staining28 (Supplementary Fig. 6b,c) and lysosomal enzyme (AP or acid SMase29) release (Fig. 4d, e) in muscle cells and MEFs. Collectively, these results suggest ML1-mediated lysosomal exocytosis may play an important role in membrane resealing.
Consistent with the possibility that overexpression of ML1 promotes membrane repair in mdx muscle4,21, AAV-GFP-ML1-infected muscle exhibited a significant reduction in both EB dye uptake and dystrophic area compared with the contralateral noninjected control muscle (Fig. 4f, g).
ML1-null mice exhibit a primary early-onset MD that is caused by defective membrane resealing. Similar but less dramatic dystrophic phenotype was observed in mice lack MG53 or dysferlin, two proteins known to be involved in membrane repair5,9. By interacting with Ca2+ sensors in the vesicles, for example, dysferlin and Syt-VII5,6, ML1 may mediate membrane repair by promoting vesicle exocytosis in both muscle and non-muscle cells (Supplementary Fig. 7). The initial rapid Ca2+ increase, within 10–20 seconds, is essential for triggering the resealing process. Depending on the nature of the damage and size of the wound, three different mechanisms may be employed for membrane repair: membrane patching, endocytosis, and shedding8,29,30. Previous studies have unequivocally established the roles of extracellular Ca2+ in all in vitro assays and models of membrane repair5,28,30. We show in the current study that lysosomal Ca2+ is also essential for this process, even in the presence of extracellular Ca2+, suggesting an involvement of more than one Ca2+ source. Consistently, multiple Ca2+ sensors are implicated in sarcolemma repair, including dysferlin, myoferlin, annexin, Syt-VII, Alix/ALG-2, and calcineurin8,30. Although lysosomes have long been implicated in membrane repair in non-muscle cells28, their role in sarcolemma repair has remained unclear. Notably, the expression level of the housekeeping lysosomal protein Lamp1 that is compensatorily upregulated in many LSDs is also elevated in dysferlin-null mice31. In addition, mice lacking Syt-VII, a lysosome-specific Ca2+ sensor, exhibit a MD-like phenotype6. Hence, the sarcolemma repair system may have a close relationship with the late endocytic pathway.
ONLINE METHODS
Reverse transcription-PCR
Total RNA was extracted from muscle tissues or cultured myoblasts and dissolved in TRIzol (Invitrogen). First-strand cDNA, synthesized with Superscript III RT (Invitrogen), was used for reverse transcription-PCR analysis of ML1 expression based on the following intron-spanning primer pair (and L32 control):
Forward: AAACACCCCA GTGTCTCCAG; Reverse: GAATGACACC GACCCAGACT
L32: Forward: TGGTGAAGCC CAAGATCGTC; Reverse: CTTCTCCGCA CCCTGTTGTC
Plasmid construction
Generation of the plasmids used in this study (GFP-ML1, mCherry-ML1, CFP-ML1, GCaMP3-ML1, Lamp1-GCaMP3, and Vamp7-pHluorin) was reported previously13,27.
Western blot and immunoprecipitation
Standard western blotting protocol was used. Gastroctrocnemius muscle was lysed with ice-cold RIPA buffer in the presence of 1X protease inhibitor cocktail (Sigma). For each sample, 10 µg to 100 µg of total protein was loaded onto 4–12% SDS-polyacrylamide gradient gels (Invitrogen), and transferred to PVDF membranes. The membranes were blocked for 1 h with 1% BSA in PBST and incubated with various antibodies against dystrophin (Abcam), β-dystroglycan (β-DG) (Santa Cruz), caveolin-3 (BD Biosciences), dysferlin (Abcam), myosin (Iowa Hybridoma Bank), MG53 (Novus Biologicals), and Lamp1 (ID4B; Iowa Hybridoma Bank) in PBST. The blots were detected by using Peroxidase-conjugated anti-rabbit/mouse/rat secondary antibody with an enhanced chemiluminescence reagent (Amersham Pharmacia Biotech).
Mouse lines
The generation and characterization of ML1 KO mice (in a B6:129 mixed genetic background) were described previously11,14. Mdx mice were ordered from Jackson Laboratories. Fabry’s mice32 were a gift from Dr. James Shayman. Mice were used under approved animal protocols and Institutional Animal Care Guidelines at the University of Michigan. Littermates were used as controls in the mouse experiments. Mice were randomly assigned to both control and testing groups, each typically contain 3–5 animals.
Treadmill exercise
Mice were trained to run on an Exer-6M treadmill (Columbus Instruments) with a 15° downhill angle at the speed of 12–20 m/min.
Muscle cell culture
Murine myoblasts were prepared and cultured as described previously33. Briefly, limb muscles were isolated and dissociated with 1% collagenase and 0.25% trypsin treatment at 37 °C. After pre-plating on a standard Petri dish (non-tissue culture-coated) for 60–90 min to remove fibroblasts, muscle cells were maintained at 37 °C and 5% CO2 in F10 medium with 20% FBS (Gibco). To induce differentiation, myoblasts were grown to confluence before switching to DMEM containing 5% horse serum. For transient transfections, myoblasts were plated at 70% confluence and transfected by using Lipofectamine 2000 reagent (Invitrogen). Live cell imaging experiments were performed 24–48 h after transfection or at the times indicated for individual experiments.
The myogenic C2C12 murine myoblast cell line was purchased from the American Type Culture Collection (Manassas, VA). Cells were grown in a humidified environment at 37 °C and 5% CO2 in DMEM medium supplemented with 20% FBS and penicillin (100 units/ml) and streptomycin (100 µg/ml). C2C12 cell differentiation was induced by switching to DMEM medium containing 5% horse serum.
Fluorescence and time-lapse confocal imaging
Live imaging of C2C12 or primary myoblasts was performed on a heated stage on a spinning disk confocal imaging system, which consisted of an Olympus IX81 inverted microscope, a 60X or 100X objective (Olympus), a CSU-X1 scanner (Yokogawa), an iXon EM-CCD camera (Andor), and MetaMorph Advanced Imaging acquisition software v.7.7.8.0 (Molecular Devices). For immunofluorescence detection, cells or muscle sections were fixed with 4% PFA, permeabilized with 0.03% Triton X-100, and stained with various primary antibodies: EEA1 (Abcam), Lamp1 (ID4B), dystrophin, β-DG, integrin β1 (Iowa Hybridoma Bank), and laminin (Chemicon).
Whole-endolysosome electrophysiology
Endolysosomal electrophysiology was performed on isolated endolysosomes with a patch clamp method as described previously12,34. Briefly, cells were treated with 1 µM vacuolin-1 for 2–5 h to increase the size of endosomes and lysosomes. Whole-endolysosome recordings were performed on isolated enlarged endolysosomes. The bath (internal/cytoplasmic) solution contained 140 mM K-gluconate, 4 mM NaCl, 1 mM EGTA, 2 mM Na2-ATP, 2 mM MgCl2, 0.39 mM CaCl2, 0.2 mM GTP, and 10 mM HEPES [pH adjusted with KOH to 7.2; the free [Ca2+]i was estimated to be ~100 nM on Maxchelator software (http://maxchelator.stanford.edu/)]. The pipette (luminal) solution consisted of a ‘low pH Tyrode’s solution’ with 145 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, 10 mM MES, and 10 mM glucose (pH 4.6). All bath solutions were applied via a perfusion system to achieve a complete solution exchange within a few seconds. Data were collected with an Axopatch 2A patch clamp amplifier, Digidata 1440, and pClamp 10.0 software (Axon Instruments). Currents were digitized at 10 kHz and filtered at 2 kHz. All experiments were conducted at room temperature (21–23 °C), and all recordings were analyzed with pClamp 10.0 and Origin 8.0 (OriginLab, Northampton, MA, USA).
Whole-cell electrophysiology
Whole-cell recordings were performed as described previously13,35. The pipette solution contained 147 mM Cs, 120 mM methane sulfonate, 4 mM NaCl, 10 mM EGTA, 2 mM Na2-ATP, 2 mM MgCl2, and 20 mM HEPES (pH 7.2; free [Ca2+]i < 10 nM). The standard extracellular bath solution (modified Tyrode’s solution) contained 153 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 20 mM HEPES, and 10 mM glucose (pH 7.4).
GCaMP3 Ca2+ imaging
GCaMP3 Ca2+ imaging was performed in primary myoblasts or C2C12 cells that were transfected with Lamp1-GCaMP3 and GCaMP3-ML1, which are lysosome-targeted genetically encoded Ca2+ sensors13. The fluorescence intensity at 488 nm (F488) was recorded with the spinning disk confocal imaging system.
H&E and histochemical staining
H&E staining was performed in 12-µm-thick sections that were prepared from freshly frozen tissues and fixed with 4% PFA. Myosin ATPase staining was performed at pH 4.3; at this pH, type 1 slow-twitch fibers are dark-colored and type 2 fast-twitch fibers are light-colored36.
Collagen content measurement
The level of hydroxylproline, a major component of collagen6, was measured using Hydroxyproline Assay Kit (Sigma).
Sciatic axotomy
Right sciatic nerves of 3-month-old WT mice were exposed and a 5-mm segment of the nerve was removed surgically. The wound was closed with silk sutures. The Gastroctrocnemius muscle was collected for H&E staining 2 weeks after the axotomy.
Myofiber damage assay
After mice were sacrificed by cervical dislocation, FDB muscles were surgically removed to be digested in a Tyrode's solution containing type I collagenase (2 mg/ml; Sigma) at 37 °C for 60 min. After re-suspension in the Tyrode's solution, single FDB fibers were mounted on a glass bottom chamber in Tyrode's or zero Ca2+ solution in the presence of 2.5 µM green-colored FM1–43 dye (Molecular Probes). To induce damage to the muscle fibers, a selected region (5 µm × 5 µm) of the plasma membrane was irradiated for 5 s with a two-photon laser (laser power 3700 at wavelength 820 nm) in a Leica Fluoview 300 confocal microscope system. Images were captured at 10-s intervals. For every image taken, the fluorescence intensity at the site of the damage was measured with ImageJ software. Fibers that had defective membrane resealing showed dye accumulation at the injury sites throughout the time course of the experiment, whereas for resealed fibers, dye influx stopped typically within 1 min.
Microelectrode penetration damage
C2C12 myoblasts or primary myoblasts were allowed to differentiate into myotubes for 3–5 days before use. Microelectrodes (borosilicate glass capillaries; World Precision Instruments, Sarasota, FL, USA) were connected to a 3-axis micromanipulator (MHH-103 Narishige International, Tokyo, Japan) to precisely control the penetration of the cell membrane in the presence of 2.5 µM red-colored FM4–64 dye (Molecular Probes). After a microelectrode was gently attached to a myotube, a further displacement of ~2 µm was considered penetration. Images were taken on an Olympus microscope equipped with a QIClick digital CCD Camera (QImaging, Surrey, BC, Canada). Live imaging of myoblasts was performed on a heated stage with the spinning disk confocal imaging system described above. Microelectrodes attached to the 3-axis micromanipulator MP-285 (Sutter Instruments, Novato, CA, USA) were used to induce penetration.
Evans Blue dye uptake
Evans blue dye (1%; 10 ml per kg body weight; Sigma) was injected into the intraperitoneal space of 1-month-old mice 8–16 h before tissue collection. Dissected Gastroctrocnemius muscles were frozen for cryosectioning.
Flow cytometry PI staining
His-tagged SLO (carrying a cysteine deletion that eliminates the need for thiol activation26) was provided by R. Tweeten (University of Oklahoma, Norman, OK) and purified using Ni-nitrilo-triacetic acid (NTA) agarose resin (Qiagen) in BL21 Ecoli expression system. The protein concentration was measured by using the Bradford assay, and stored at −80 °C until use. For each experiment, about 2 × 106 cells were trypsinized, washed with Tyrode’s solution, and incubated with SLO (2–5 µg/ml) for 10 min at 37°C. Titration of SLO was performed to determine the minimum concentration required for cell permeabilization in 0 Ca2+ and the maximum concentration not causing significant cell loss. SLO-treated cells were then stained with 200 µg/ml propidium iodide (PI; Sigma-Aldrich) for 5 min and analyzed by FACS Flow Cytometry (iCyt Synergy; Sony). More than 10, 000 cells were used for each condition. Data were analyzed using WinList 3D software (Verity Software House).
Lysosomal enzyme release/activity
Upon SLO treatment for 15 min, the conditioned culture medium was collected for acid phosphatase (AP) and acid sphingomyelinase (aSMase) activity measurement using an AP colorimetric assay kit (Sigma) and an acid sphingomyelinase activity assay Kit (Echelon Biosciences), respectively.
Lamp1 surface staining
C2C12 were incubated with 500–800 ng/mL SLO (a concentration that causes no significant PI staining) at 37°C for 30 min. Cells were stained with PI, re-suspended in 1% BSA with anti-mouse Lamp1 (1D4B) antibody on ice for 45 min, and fixed in 2% PFA for 30 min, and were finally incubated with Alexa-488 conjugated anti-rat secondary antibody (Invitrogen) at room temperature for 1h. After re-suspension in 0.5 ml PBS, cells were analyzed on a FACS Flow Cytometer (MoFlo Astrios, Beckman Coulter). At least 10,000 cells per experiment were analyzed for the forward angle scatter, the right angle scatter, and the fluorescence intensity.
PHluorin TIRF imaging
TIRF imaging was performed at 37°C using a VisiTech Infinfity 3 array-scanning confocal microscope (VisiTech International Ltd.; Sunderland, United Kingdom) equipped with a 100× APO TIRF objective oil-immersion (Nikon, NA 1.49). MetaMorph v7.71 image acquisition software was used to process the data.
Electron microcopy analysis
For electron microcopy analysis, animals were perfused with 4% PFA and 2.5% glutaraldehyde in PBS. Muscles were surgically removed with intact tendons. Samples were prepared with standard embedding and sectioning procedures.
AAV generation and infection
CAG promoter-driven AAV1/2-GFP-ML1 virus was generated by the Gene Transfer Vector Core of the University of Iowa. Virus injection was performed on one side of the Gastroctrocnemius muscle of 1-month-old ML1 KO or 10-day-old mdx mice. The other side of the Gastroctrocnemius muscle was used as the contralateral control. Injected and noninjected contralateral leg muscles were examined after 4 weeks.
Data analysis
Data are presented as the mean ± s.e.m. Statistical comparisons were performed with analysis of variance (ANOVA). A P value < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgements
This work was supported by NIH grants (NS062792, MH096595, and AR060837 to H.X; HL116546 and AR064241 to R.H.). We are grateful to Dr. Susan Slaugenhaupt for the ML1 KO mice, Dr. Loren Looger for the GCaMP3 construct, Robert Edwards for the Vamp7-pHluorin construct, the Center for Live-imaging at the University of Michigan for the help on TIRF Imaging, and Richard Hume and Mohammad Akaaboune for comments on the manuscript. We appreciate the encouragement and helpful comments of other members of the Xu laboratory.
Footnotes
The authors have no competing interests as defined by Nature Publishing Group, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Author contribution:
X.C. initiated the project; H.X., X.C., J.D. and R.H. designed the research; X.C., X.Z., Q.G., M.A.S., M.A., W.L.T. and L.D. performed the research; N.S., X.L., A.G.G., X.W., M.F. and L.X. contributed the new regents; X.C., X.Z, Q.G., M.A.S., M.A., W.L.T., J.D., R.H. and H.X. analyzed the data; and H.X. and X.C. wrote the paper with inputs from all the authors.
References
- 1.Clarke MS, Khakee R, McNeil PL. Loss of cytoplasmic basic fibroblast growth factor from physiologically wounded myofibers of normal and dystrophic muscle. J Cell Sci. 1993;106(Pt 1):121–133. doi: 10.1242/jcs.106.1.121. [DOI] [PubMed] [Google Scholar]
- 2.McNeil PL, Khakee R. Disruptions of muscle fiber plasma membranes. Role in exercise-induced damage. Am J Pathol. 1992;140:1097–1109. [PMC free article] [PubMed] [Google Scholar]
- 3.Davies KE, Nowak KJ. Molecular mechanisms of muscular dystrophies: old and new players. Nat Rev Mol Cell Biol. 2006;7:762–773. doi: 10.1038/nrm2024. [DOI] [PubMed] [Google Scholar]
- 4.Rahimov F, Kunkel LM. The cell biology of disease: cellular and molecular mechanisms underlying muscular dystrophy. J Cell Biol. 2013;201:499–510. doi: 10.1083/jcb.201212142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bansal D, et al. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarti S, et al. Impaired membrane resealing and autoimmune myositis in synaptotagmin VII-deficient mice. J Cell Biol. 2003;162:543–549. doi: 10.1083/jcb.200305131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barresi R, et al. LARGE can functionally bypass alpha-dystroglycan glycosylation defects in distinct congenital muscular dystrophies. Nat Med. 2004;10:696–703. doi: 10.1038/nm1059. [DOI] [PubMed] [Google Scholar]
- 8.McNeil P. Membrane repair redux: redox of MG53. Nat Cell Biol. 2009;11:7–9. doi: 10.1038/ncb0109-7. [DOI] [PubMed] [Google Scholar]
- 9.Cai C, et al. MG53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol. 2009;11:56–64. doi: 10.1038/ncb1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun M, et al. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum Mol Genet. 2000;9:2471–2478. doi: 10.1093/hmg/9.17.2471. [DOI] [PubMed] [Google Scholar]
- 11.Venugopal B, et al. Neurologic, gastric, and opthalmologic pathologies in a murine model of mucolipidosis type IV. Am J Hum Genet. 2007;81:1070–1083. doi: 10.1086/521954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong XP, et al. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature. 2008;455:992–996. doi: 10.1038/nature07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen D, et al. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat Commun. 2012;3:731. doi: 10.1038/ncomms1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samie M, et al. A TRP Channel in the Lysosome Regulates Large Particle Phagocytosis via Focal Exocytosis. Dev Cell. 2013;26:511–524. doi: 10.1016/j.devcel.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Figueiredo P, Brown WJ. A role for calmodulin in organelle membrane tubulation. Mol Biol Cell. 1995;6:871–887. doi: 10.1091/mbc.6.7.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Straub V, Rafael JA, Chamberlain JS, Campbell KP. Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J Cell Biol. 1997;139:375–385. doi: 10.1083/jcb.139.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weitz R, et al. Muscle involvement in mucolipidosis IV. Brain Dev. 1990;12:524–528. doi: 10.1016/s0387-7604(12)80220-8. [DOI] [PubMed] [Google Scholar]
- 18.Zlotogora J, Ben Ezra D, Livni N, Ashkenazi A, Cohen T. A muscle disorder as presenting symptom in a child with mucolipidosis IV. Neuropediatrics. 1983;14:104–105. doi: 10.1055/s-2008-1059563. [DOI] [PubMed] [Google Scholar]
- 19.Venugopal B, et al. Chaperone-mediated autophagy is defective in mucolipidosis type IV. J Cell Physiol. 2009;219:344–353. doi: 10.1002/jcp.21676. [DOI] [PubMed] [Google Scholar]
- 20.Abe A, et al. Reduction of globotriaosylceramide in Fabry disease mice by substrate deprivation. J Clin Invest. 2000;105:1563–1571. doi: 10.1172/JCI9711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell KP. Three muscular dystrophies: loss of cytoskeleton-extracellular matrix linkage. Cell. 1995;80:675–679. doi: 10.1016/0092-8674(95)90344-5. [DOI] [PubMed] [Google Scholar]
- 22.Cai C, et al. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. J Biol Chem. 2009;284:15894–15902. doi: 10.1074/jbc.M109.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bansal D, Campbell KP. Dysferlin and the plasma membrane repair in muscular dystrophy. Trends Cell Biol. 2004;14:206–213. doi: 10.1016/j.tcb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Weisleder N, et al. Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Sci Transl Med. 2012;4:139ra185. doi: 10.1126/scitranslmed.3003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samie M, et al. A TRP Channel in the Lysosome Regulates Large Particle Phagocytosis via Focal Exocytosis. Dev Cell. 2013 doi: 10.1016/j.devcel.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Idone V, et al. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J Cell Biol. 2008;180:905–914. doi: 10.1083/jcb.200708010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hua Z, et al. v-SNARE composition distinguishes synaptic vesicle pools. Neuron. 2011;71:474–487. doi: 10.1016/j.neuron.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell. 2001;106:157–169. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 29.Corrotte M, et al. Caveolae internalization repairs wounded cells and muscle fibers. Elife. 2013;2:e00926. doi: 10.7554/eLife.00926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jimenez AJ, et al. ESCRT machinery is required for plasma membrane repair. Science. 2014;343:1247136. doi: 10.1126/science.1247136. [DOI] [PubMed] [Google Scholar]
- 31.Demonbreun AR, et al. Impaired muscle growth and response to insulin-like growth factor 1 in dysferlin-mediated muscular dystrophy. Hum Mol Genet. 2011;20:779–789. doi: 10.1093/hmg/ddq522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Method References
- 32.Bolsover FE, Murphy E, Cipolotti L, Werring DJ, Lachmann RH. Cognitive dysfunction and depression in Fabry disease: a systematic review. J Inherit Metab Dis. 2013 doi: 10.1007/s10545-013-9643-x. [DOI] [PubMed] [Google Scholar]
- 33.Springer ML, Rando TA, Blau HM. Gene delivery to muscle. Curr Protoc Hum Genet. 2002;Chapter 13(Unit13):14. doi: 10.1002/0471142905.hg1304s31. [DOI] [PubMed] [Google Scholar]
- 34.Dong XP, et al. PI(3,5)P(2) Controls Membrane Traffic by Direct Activation of Mucolipin Ca Release Channels in the Endolysosome. Nat Commun. 2010;1 doi: 10.1038/ncomms1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, et al. TPC proteins are phosphoinositide-activated sodium-selective ion channels in endosomes and lysosomes. Cell. 2012;151:372–383. doi: 10.1016/j.cell.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brooke MH, Kaiser KK. Muscle fiber types: how many and what kind? Arch Neurol. 1970;23:369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.