Abstract
The renin-angiotensin system (RAS) is a major determinant of blood pressure regulation. It consists of a cascade of enzymatic reactions involving three components: angiotensinogen (Agt), renin, and angiotensin-converting enzyme (ACE), which generate angiotensin II (Ang II) as a biologically active product. Agt is largely produced in the liver, acting as a major determinant of the circulating RAS, which exerts acute hemodynamic effects on blood pressure regulation. How the expression of Agt is regulated is not completely understood. Here we hypothesize that Agt is regulated by forkhead transcription factor forkhead box class O1 (Foxo1), an insulin-suppressed transcription factor, and thereby controls blood pressure in mice. We generated liver-specific Foxo1 knockout mice (L-F1KO mice), which exhibited a reduction in plasma Agt and Ang II levels and a significant decrease in blood pressure. Using hepatocyte cultures, we demonstrated that overexpression of Foxo1 increased Agt expression, while hepatocytes lacking Foxo1 demonstrated a reduction of Agt gene expression and partially impaired insulin inhibition on Agt gene expression. Furthermore, mouse Agt prompter analysis demonstrated that the Agt promoter region contains a functional Foxo1 binding site, which is responsible for both Foxo1 stimulation and insulin suppression on the promoter activity. Together, these data demonstrate that Foxo1 regulates hepatic Agt gene expression and controls plasma Agt and Ang II levels, modulating blood pressure control in mice.
Keywords: Foxo1, insulin/insulin-like growth factor (IGF-1), liver angiotensinogen, hypertension
Angiotensinogen (Agt) is a precursor of angiotensin II (Ang II), an octapeptide pressor hormone that has a key role in mediating vascular constriction and regulating salt and fluid homeostasis 1. Elevation of Ang II contributes to hypertension, atherosclerosis, cardiac hypertrophy, and heart failure 2, 3. Agt is expressed and synthesized largely in the liver and secreted into the blood circulation where it is processed by the kidney-derived aspartyl protease renin to produce angiotensin I (Ang I), which is in turn hydrolyzed by angiotensin-converting enzyme (ACE) to generate Ang II.
Overexpression of the Agt gene in mice increases blood pressure 4, while mice lacking the Agt gene systemically, exhibited hypotension 5, 6. Mice lacking Agt in liver have a significant reduction in plasma Agt and Ang II levels, and have reduced blood pressure. Thus, liver is a major source for plasma Agt and Ang II in control of blood pressure 7. Determining the mechanisms by which Agt gene expression is regulated, is important for understanding the molecular basis for blood pressure control.
Insulin is known to decrease Agt gene expression in hepatocytes 8, though other hormones and nutrients, such as high glucose, increase Agt gene expression 9, 10. Over the past decade, we have identified an insulin-regulated forkhead transcription factor, Foxo1, which stimulates expression of target genes encoding gluconeogenic enzymes and hence promotes hepatic glucose production 11–13. The forkhead transcription factor family has more than 80 members and each contains a conserved DNA binding domain that forms a forkhead winged/helix structure interacting with DNA. They are grouped into 19 classes from FoxA to FoxS based on DNA sequence homology 14. The O class of forkhead transcription factor family (FoxO) consists of Foxo1, Foxo3, Foxo4, and Foxo6 and each has three consensus Akt phosphorylation motifs (RxRxxT/S, R-arginine, x-any amino acid, and T/S- threonine/serine- target of Akt) 11, 12. Foxo1 is involved in a wide range of events, including embryonic development, cell metabolism, survival, and diseases 13, 15.
Insulin suppresses the gene transcription via a conserved insulin response element (IRE) in the promoter region of target genes 11. The IRE sequence that we have identified is 5′CAAAACAA3′, which Foxo1 binds to in the promoter region and activates target gene transcription 11, 16. In response to insulin stimulation, Akt is activated and then inhibits Foxo1 by phosphorylating threonine/serine residues at T24, S256, and S319. These phosphorylation events control Foxo1 protein turnover and promote Foxo1 cytoplasmic localization and ubiquitination, impairing Foxo1 interactions with the IRE on the target gene’s promoter region and suppressing gene transcription 11, 12.
At first, we performed an online analysis of mouse Agt promoter region with a transcription element search system (TESS- http://www.cbil.upenn.edu), and found that there are two potential IREs or Foxo1 binding sites, prompting us to test the hypothesis that Foxo1 may regulate Agt gene expression, in the liver; thereby controlling plasma Agt and Ang II and further modulating blood pressure in animals. This study provides in vitro and genetic evidence that demonstrates the role of liver Foxo1 in regulating Agt gene expression and blood pressure in mice.
Materials and Methods
Mice
All animal experiments were performed according to procedures approved by the the Texas A&M Health Science Center Institutional Animal Care and Use Committee. The floxed Foxo1 mice (Foxo1L/L) and albumin-Cre mice, in which cre-recombinase is specifically expressed in the liver, were previously described 13. All of the mice were on a C57BL/6 and 129 Sv mixed background and were maintained on regular chow (Prolab Isopro 5P76).
DNA Cloning, Mutagenesis, and Reporter Gene Assay
Mouse Agt promoter regions were amplified by PCR, using mouse tail DNA and cloned into a luciferase reporter gene. The Agt promoter region-1 spanning 1.5kb upstream from the transcriptional initiation site (−1.5kb) was amplified with PCR primers: 5′-ttttggtaccgcggagtctatacagccag-3′ and 5′-ttttaagcttgtggagatggatctattcctg-3′; an 0.8kb region upstream from the 1.5 kb promoter, designated as the Agt promoter region-2, and amplified by PCR, with the primers: 5′-agtttggtaccgctgcatgtgcacactagg-3′ and 5′-agagtaagctttacagcacaggctgctggtc-3′; and an 0.66kb region upstream from the 0.8kb promoter region was designated as the Agt promoter region-3, and amplified by PCR, with primers: 5′-actttggtacccatgacagactgcacgcagtc-3 and 5′-tgtttaagcttcctagtgtgcacatgcagc-3′. The three PCR fragments were cloned into the pGL3-luciferase reporter gene (Promega), generating Agtp-1.5kb, Agtp-800bp, and Agtp-660bp luciferase reporter constructs. The mutation of the Foxo1 binding site on the Agtp-800bp promoter was achieved by in vitro mutagenesis, with PCR primers 5′-ctctttcttggctgcagcaagcttcgtcaaagaccctctgttc-3′ and 5′-gaacagagggtctttgacgaagcttgctgcagccaagaaagag-3′, using a site-specific mutagenesis kit (Stratagen). In the Agtp-800bp promoter region, three reporter constructs containing 5′ deletion of 200bp, 400bp, and 600bp were generated and designated as Agtp-600, Agtp-400, and Agtp-200bp, respectively. All cloned DNA fragments and mutations were confirmed by DNA sequencing. HepG2cells were cultured in DMEM/10%FBS and transfected by pAlter-Max plasmid DNA, with or without expression of Foxo1, using TransIT-293 transfection reagent (Mirus, Madison, WI), as previously described 13.
Chemicals and Antibodies
Foxo1, pFoxo1-S253, Akt, pAkt-T308, ERK1/2, pERK1/2-T202Y204, GAPDH, and α-actin antibodies were from Cell Signaling Technology (Billerica, MA), and Agt antibody was purchased from Immuno-Biological laboratories, Inc (Japan). Insulin and collagenase were purchased from Sigma, and Percoll from Amersham.
Measurement of Plasma Ang II Concentration
Ang II from plasma was extracted in 1 M acetic acid, passed over a DSC-18 column (Supelco, Bellefonte, PA) and eluted with methanol, as previously described 17. A standard curve was generated from binding of a constant amount of biotinylated angiotensin peptide with increasing concentrations of non-biotinylated peptide.
Measurement of Blood Pressure
Mice were anesthetized with isoflurane and placed on a heating pad, and rectal temperature maintained between 36.0 and 37.5°C. The left carotid artery was cannulated with a catheter (FTH-1212B-4518, 1.2Fr P-V catheter with 4.5mm electrode spacing, Scisense Inc, Canada) and connected to a transducer and data acquisition systems (iWorx IX/228S Data Acquisition System with the Scisense Advantage pV control unit version 5.0). Alternatively, measurement of systolic blood pressure (SBP) was also performed on conscious, restrained mice via the Visitech BP-2000 tail cuff system. Briefly, SBP was quantified for 3 days and each cycle consisted of 10 preliminary tail cuff inflation/deflations for daily acclimation, followed by 10 additional cycles that were recorded. Criteria for inclusion of measurements from individual mice were at least four out of ten successful measurements, with a SD less than 50 required for inclusion, as previously described 18.
Protein Analysis and Western Blot
Immunoblotting analysis using specific antibodies was previously described 19. Signal intensity was measured and analyzed by NIH Image J software. For Agt measurements, plasma samples were analyzed by Western-blot using Agt antibody and total plasma protein by Ponceau Red (Bio-Red).
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assay was performed as described previously 20. Immunoprecipitated DNA was analyzed by PCR with following primers: 5′-gactgaagttgcagcctgctc-3′ and 5′ctcagaagtattcacatatggc-3′.
Primary Hepatocyte Isolation and Cell Culture
Mouse hepatocytes were isolated from 8–12 week-old mice with a protocol previously described 13. After cell attachment, hepatocytes were cultured in serum-free medium overnight, and then treated with or without 100 nmol/L insulin for 18 h prior to further analysis.
Adenovirus Infection of Hepatocytes
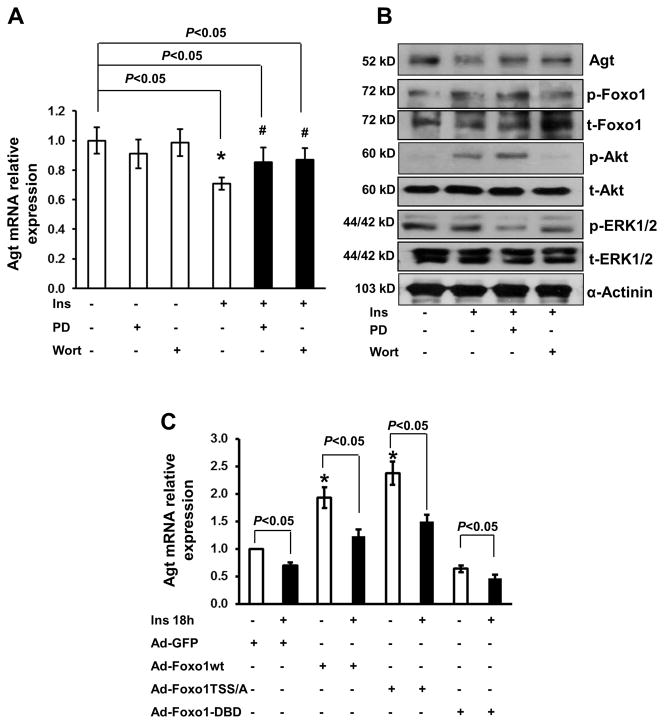
Mouse primary hepatocytes were cultured in DMEM/10% FBS medium for 8 h, then transfected by adding adenovirus expressing green fluorescent protein (GFP), Foxo1-wild type (wt), constitutively active Foxo1-T/S/S-A in which three Akt phosphorylation sites on T24, S256, and S319 are mutated to alanine (A), and Foxo1-DBD that expresses DNA binding domain (DBD), which was previously described 21. Cells were infected with adenovirus with 50 multiplicity of infection (MOI) for 8 h and then changed to fresh DMEM medium with serum for another 8 h of culturing. Cells were serum starved for 8 h prior to insulin intervention. For protein kinase inhibitor treatment, the inhibitor PD98059 or wortmannin was added to cells for 0.5 h prior to addition of insulin, before cellular protein lysates were prepared for immunoblotting.
Quantitative Real-Time PCR Analysis
Mouse liver RNA isolation was extracted with Trizol reagent (Invitrogen) and PCR performed as previously described 13. PCR primers used for Agt were 5′-gcggaggcaaatctgaacaacat -3′ and 5′-gaaggggctgctcagggtcacatc -3′.
Statistical Analysis
All results are presented as the mean ± SEM and analyzed by ANOVA to determine P values. P<0.05 was considered statistically significant, as previously described 22, 23.
Results
Generation of liver-specific Foxo1 gene knockout (L-F1KO) mice
We intercrossed Foxo1L/L with Albumin-Cre transgenic mice (CreAlb), to produce mice without liver Foxo1 (L-F1KO mice) and control littermates (CNTR) that include Foxo1L/L mice or Albumin-Cre mice (Figure 1A). All of the mice were born in Mendelian ratios and F1KO mice were indistinguishable in appearance from control littermates. At the age of 10 weeks, F1KO mice exhibited a 15–20% reduction in blood glucose concentrations and no change in serum insulin compared to control littermates. The metabolic phenotype of F1KO mice was extensively characterized in our previous study 13.
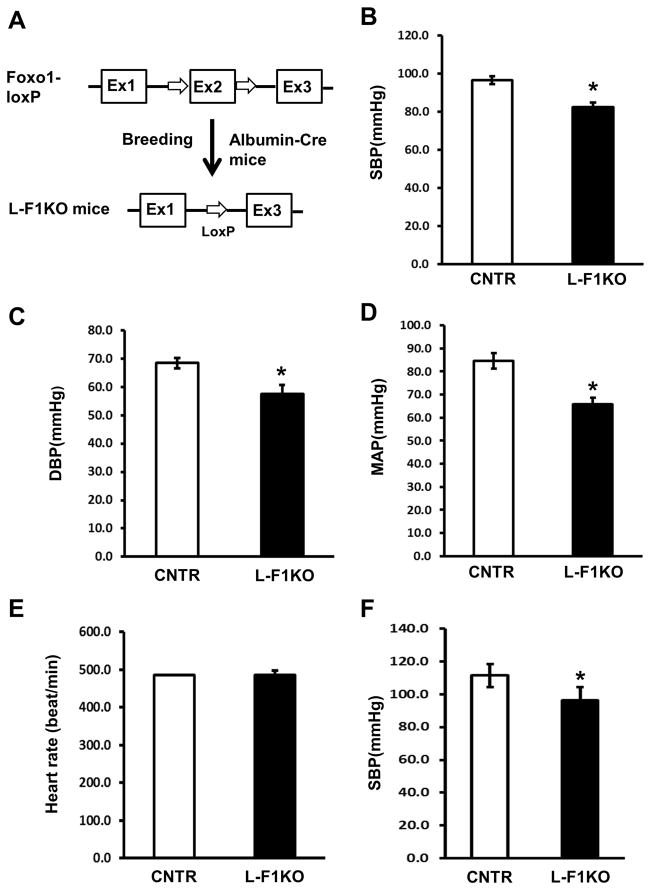
Figure 1.
Generation of liver–specific Foxo1 knockout (L-F1KO) mice and measurement of blood pressure. A, Location of loxP sites on the Foxo1 gene in Foxo1-loxP mice and deletion of exon 2 of Foxo1 containing DNA binding domain in L-F1KO mice. B–D, The blood pressure of 12 week-old male F1KO and control littermates under anesthesia were measured by PV-Loop experiments and tail cuff experiments when mice are under conscious conditions (E–F). Data are expressed as the mean ± SEM; * P<0.05 vs. control (n=8–12 mice /group).
L-F1KO mice exhibit lower blood pressure
We examined the blood pressure of mice under anesthesia with the pressure-loop system. At 12 weeks of age, L-F1KO mice had reduced blood pressure: a 14 mmHg reduction of systolic blood pressure (SBP) compared to control mice (P<0.05, n=6); a 11 mmHg reduction in diastolic blood pressure (DBP) (P<0.05, n=6); and a 19 mmHg reduction in the mean arterial pressure (MAP) (P<0.05, n=6), though the L-F1KO mice exhibited no difference in heart rate (Figure 1B–E). To further confirm the reduction in blood pressure in L-F1KO mice, we measured blood pressure of mice under conscious conditions with a tail-cuff. The L-F1KO mice exhibited a reduction of SBP by 15 mmHg (P<0.05, n=6) (Figure 1F). Clearly, the liver-specific Foxo1 deletion resulted in lower blood pressure in mice.
Hepatic deficiency of Foxo1 decreases plasma Agt and Ang II concentration
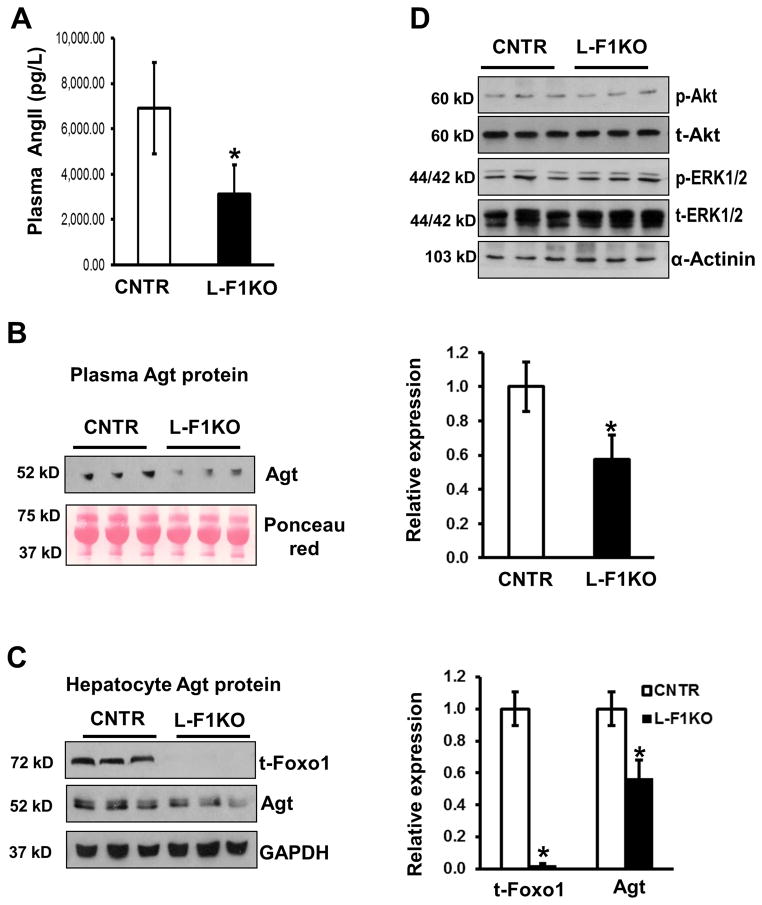
We next determined the plasma Ang II concentration in these mice. The plasma Ang II concentration was reduced by 55% in L-F1KO mice, compared to control (P<0.05, n=6) (Figure 2A). Agt in the plasma was reduced by nearly 50% in L-F1KO mice, as determined by Western-blot (Figure 2B). The Agt, which is largely produced by the liver, demonstrated a 52% reduction in protein expression in L-F1KO liver, compared to control (Figure 2C). In addition, loss of Foxo1 in F1KO liver had no changes in intracellular signaling, including phosphorylation of Akt and ERK1/2, as determined by Western-blot (Figure 2D). Together, these results indicate that Foxo1 regulates Agt gene expression in the liver and significantly controls plasma Agt and Ang II levels.
Figure 2.
Hepatic deficiency of Foxo1 decreases plasma Agt and Ang II concentration. A, Ang II from plasma of 12- week-old male F1KO and control mice was measured by a competitive ELISA kit with a quantification of Ang II. B, 50 μg of plasma protein were loaded on SDS-PAGE to determine Agt protein levels by Western-blot. Levels of plasma proteins were also indicated by Ponceau red staining. C, One hundred μg protein of liver extracts of F1KO and control mice was subjected to Western-blot for determination of Agt, Foxo1, and GAPDH proteins. *P<0.05 vs. control, n=3–6 mice/group. D, Akt and ERK1/2 phosphorylation and total proteins were determined by Western-blot in the sample from (C),
Overexpression of Foxo1 stimulates Agt expression in hepatocytes
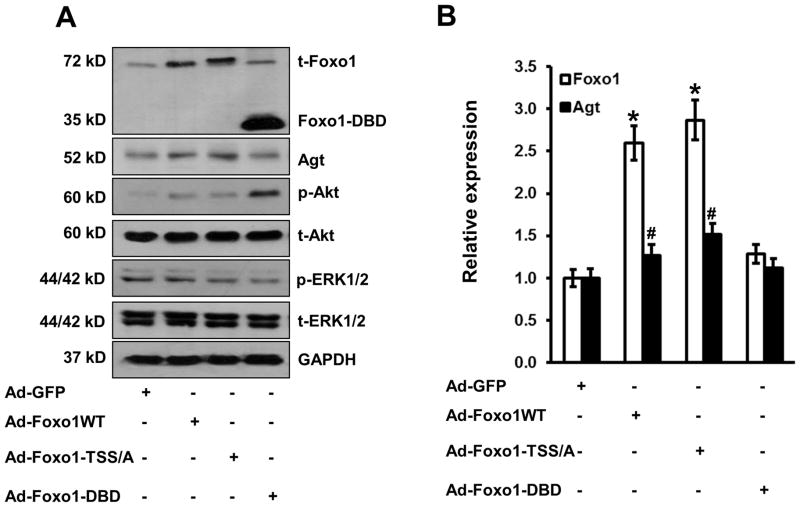
We next examined whether Foxo1 stimulates Agt gene expression in hepatocytes. Forced expression of Foxo1-wt in hepatocytes, by 2.5-fold at the protein level, was achieved by adenovirus infection, resulting in a 1.3-fold increase in Agt protein expression (Figure 3A–B). A similar effect was observed with forced expression of the Foxo1-T/S/S-A mutant that prevents Akt-mediated phosphorylation. However, expression of Foxo1-DBD that contains the DNA binding domain without transcriptional activation domain, did not increase Agt protein expression (Figure 3A–B). Consistent with the previous study 24, overexpression of Foxo1-wt, Foxo1-T/S/S-A, or Foxo1-DBD enhanced Akt phosphorylation with little effect on ERK1/2 phosphorylation, likely owing to Foxo1 interaction with TRB3, an endogenous Akt inhibitor. These results indicate that overexpression of Foxo1 enhances Agt protein expression in hepatocytes.
Figure 3.
Overexpression of Foxo1 stimulates Agt expression in hepatocytes. A, Mouse primary hepatocytes were infected with adenovirus that expresses GFP, Foxo1-wt, Foxo1-T/S/S-A, and Foxo1-DBD (50 moi) and cellular protein collected and expression of Foxo1, Agt, and GAPDH determined by Western-blot. B, The Agt mRNA levels were determined by real-time PCR from (A), * P<0.05 vs. AdGFP for Foxo1; # P<0.05 vs. control for Agt, n=3 experiments.
Foxo1 deficiency impairs insulin inhibition on Agt mRNA expression in hepatocytes
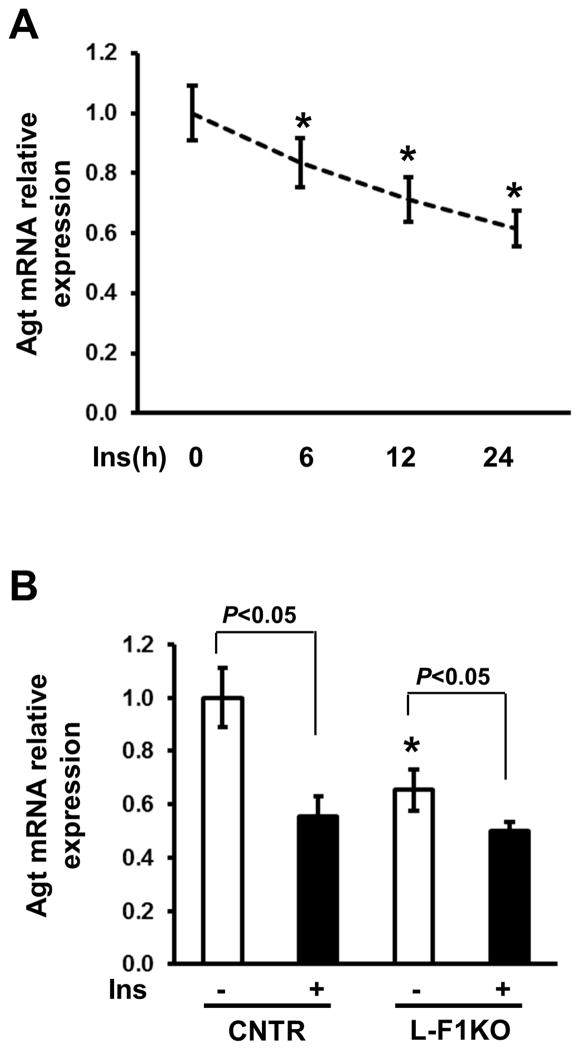
Since insulin decreases Agt mRNA expression in liver 8, we assessed the role of Foxo1 in insulin inhibition on Agt gene expression. We treated control and L-F1KO hepatocytes with 100 nmol/L insulin for 24 h and Agt mRNA expression was analyzed. Agt mRNA expression levels were reduced by 40% in insulin-treated control hepatocytes (Figure 4A). Although Agt mRNA was reduced by 30% in F1KO hepatocytes compared to control, insulin further reduced Agt mRNA levels by 15% in F1KO hepatocytes (P <0.05, Figure 4B). Although the ability of insulin to suppress Agt mRNA expression was not completely disrupted in hepatocytes lacking Foxo1, Foxo1 deletion impaired insulin inhibition on the Agt mRNA expression. These results suggest that insulin inhibits Agt gene expression in both a Foxo1-dependent and independent manner.
Figure 4.
Foxo1 deficiency impairs insulin inhibition on Agt mRNA expression in hepatocytes. A, Mouse primary hepatocytes were treated with 100 nmol/L insulin in different periods of time and RNA collected for real-time analysis. *P<0.05 vs. non-insulin treatment. B, Effect of different doses of insulin treatment for 24 h on Agt mRNA expression. C, Agt mRNA analysis in L-F1KO and control hepatocytes treated with 100 nmol/L insulin for 24 h. *P<0.05 vs. control, n=3 experiments.
Insulin suppresses Agt mRNA expression through PI3K and MAPK pathways
Insulin activates both PI3K and MAP kinases, regulating expression of many genes in control of metabolism and growth. We next determined whether activation of PI3K and MAPK is required for insulin to inhibit Agt mRNA expression. 100 nmol/L insulin treatment for 18 h in control hepatocytes reduced Agt mRNA and protein levels by nearly 40%, while treatment with a kinase inhibitor for PI3K (wortmannin) or ERK1/2 MAPK (PD98059), partially blunted insulin inhibition on the Agt mRNA level (Figure 5A–B). After 18 h of insulin treatment in cells, Akt and Foxo1 phosphorylation, rather than ERK1/2 phosphorylation was evident (Figure 5B), though treatment of insulin for 0.5 h robustly stimulated Akt, Foxo1, and ERK1/2 phosphorylation (data not shown), which was shown in cardiomyocytes 23. To further assess whether insulin regulates Foxo1-stimulated Agt mRNA expression, we measured Agt mRNA expression in hepatocytes infected by adenovirus expressing control GFP, Foxo1-wt, Foxo1-T/S/S-A and Foxo1-DBD. Overexpression of Foxo1-wt and Foxo1-T/S/S-A significantly increased endogenous Agt mRNA by 2.0-fold and 2.5-fold, respectively, compared to expression of GFP control (Figure 5C). However, expression of Foxo1-DBD slightly decreased the basal level of Agt mRNA (P<0.09). Thus, Foxo1 stimulates endogenous Agt gene expression at the gene transcriptional level.
Figure 5.
Insulin suppresses Agt mRNA expression through PI3K and MAPK pathways. A, Mouse primary hepatocytes were treated with 20 μmol/L PD98059 (MAPK inhibitor) and 100 nmol/L wortmannin (PI3K inhibitor) for 30 min prior to 100 nmol/L insulin treatment for 18 h. The Agt mRNA level was analyzed by real-time PCR. # P<0.05 vs. insulin treatment, n=3 experiments. B, One hundred μg protein samples from hepatocytes (A) were analyzed for Foxo1, Akt, and ERK1/2 phosphorylation and total proteins by Western-blot. Representative images are presented. C, Mouse primary hepatocytes were infected by adenovirus, followed by 100 nmol/L insulin treatment for 18 h and the Agt mRNA level analyzed by real-time PCR. * P<0.05 vs. Ad-GFP treatment, n=3 experiments.
Insulin treatment inhibited Foxo1wt-stimulated Agt expression, by 40% (P <0.05), while insulin inhibition on the Agt mRNA level was slightly affected in cells with expression of constitutively active Foxo1-T/S/S-A or Foxo1-DBD (Figure 5C). The results further support the concept that Foxo1 stimulates Agt gene transcription, while blocking Akt-mediated phosphorylation of Foxo1 or inhibition of PI3K partially impaired insulin inhibition on Agt gene transcription.
Identification of a Foxo1 binding site on the Agt promoter region
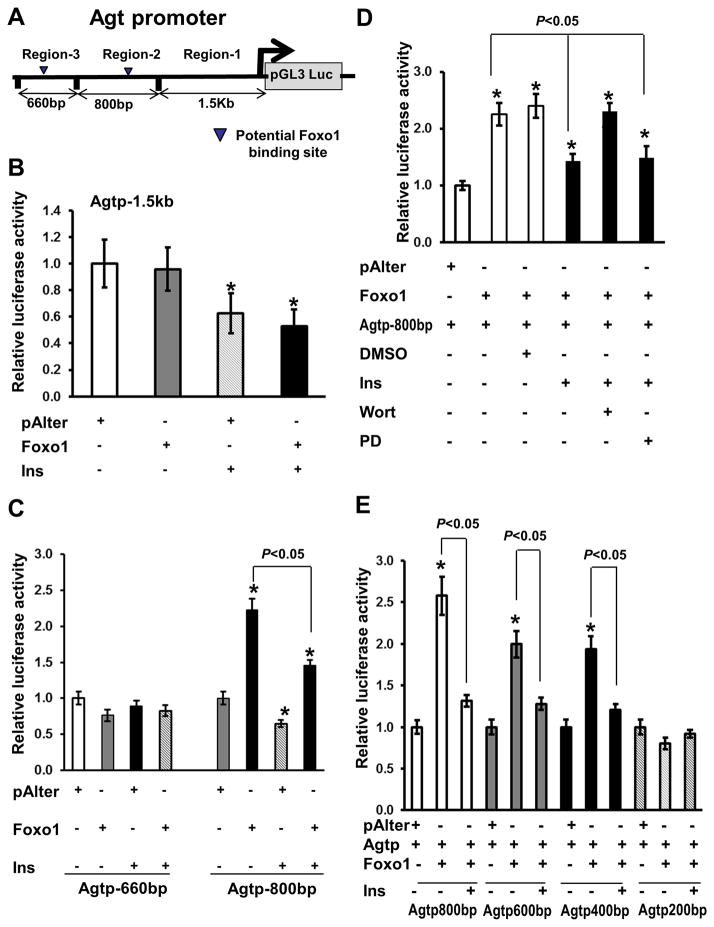
We next tested whether Foxo1 directly regulates Agt gene expression through interactions with the promoter region. Promoter analysis of the mouse Agt gene suggests that two loci are potential Foxo1 binding sites or insulin response elements-CAAAACAA (Figure 6A). To determine whether these promoter regions are responsive to Foxo1 stimulation or insulin suppression, we cloned 3 promoter regions into the pGL3-luciferase reporter gene construct. Promoter region-1 has 1.5 kb upstream of the transcriptional initiation site (−1.5kb), containing an insulin response element distinct from the Foxo1 binding site, mediating insulin suppression through ERK1/2 MAP kinase activation 25. Transfection of the reporter gene in HepG2 cells indicated that −1.5 kb of the promoter region 1 (Agtp-1.5kb) was responsive to insulin inhibition; but, failed to respond to Foxo1 stimulation (Figure 6B). Promoter region-3 (Agtp-660 bp) that contains a potential Foxo1 binding site was neither responsive to Foxo1 stimulation nor to insulin inhibition (Figure 6C). However, promoter region-2 (Agtp-800 bp) which contains a potential Foxo1 binding site was responsive to both Foxo1 stimulation and insulin inhibition in a PI3K-depednent manner (Figure 6C–D). To further map the functional Foxo1 binding site, which can be responsive to both Foxo1 stimulation and insulin inhibition, we made two constructs, each with a potential Foxo1 binding site (Agtp-600bp and Agtp-400bp), and one construct (Agtp-200bp) that does not contain the site (Figure 6A). The Agtp-200bp promoter was unresponsive to Foxo1 stimulation and insulin inhibition, while Agtp600bp and Agtp400bp were responsible for both Foxo1 stimulation and insulin suppression, similar to the Agtp-800bp (Figure 6E).
Figure 6.
Agt promoter analysis by reporter gene assay. A, Mouse Agt promoter regions were cloned into the pGL2-luciferase reporter gene construct (Promega). B–E, HepG2 cells were transfected with different Agt promoter-driven luciferase (Agtp) constructs and control plasmid (pAlter-Max) or plasmid that express Foxo1 (Foxo1), and cells were treated with or without 100 nmol/L insulin for 18 h prior to cellular protein collection and measurement of luciferase activities, n=3 experiments
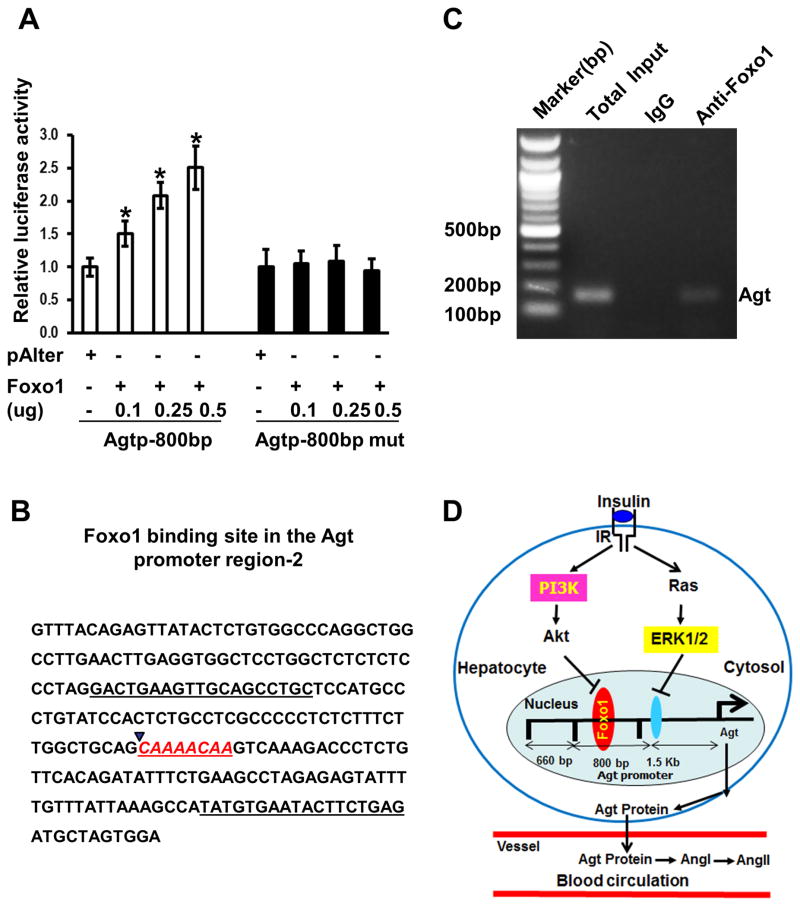
Overexpression of Foxo1 stimulated the Agtp-800bp in a dose-dependent manner (Figure 7A). To further confirm the functional Foxo1 binding site in Agt-p800bp, we mutated the Foxo1 binding site to a non-IRE sequence, as we previously reported 11. These mutations in the IRE in the Agtp-800bp promoter completely abrogated Foxo1 stimulation on promoter activity (Figure 7A). The flanking sequence of the Foxo1 binding site in the promoter region-2 is shown in Figure 7B. We further performed chromatin IP to examine whether Foxo1 binds to the functional Foxo1 binding site in mouse liver. The chromatin IP results indicate that the immunoprecipitate using antibody against Foxo1, rather than IgG, contains the DNA fragment from region-2 (Figure 7C), and that the DNA fragment from promoter region-3 was not observed (data not shown). Together, these results indicate that Foxo1 interacts with a Foxo1 binding site in Agt promoter region-2 and mediates insulin inhibition on Agt gene expression, which is summarized in Figure 7D.
Figure 7.
Determination of Foxo1 binding site of the Agt promoter region. A, HepG2 cells were transfected with the Agtp-800bp and Agtp-800bp mutant promoter-driven luciferase (Agtp) constructs and control plasmid (pAlter-Max) or plasmid expressing Foxo1, and cells were treated with or without 100 nmol/L insulin for 18 h prior cellular protein collection and measurement of luciferase activities, * P< 0.05 vs. pAlter-Max control, n=3 experiments. B, DNA sequence derived from Agtp-800bp containing the potential Foxo1 binding site. C, Binding of Foxo1 on the Agt promoter region-2 by ChIP assay. D, Schematic graph represents the regulation of Agt gene expression by Foxo1 and insulin in the liver in control of blood pressure.
Discussion
In this study, we demonstrate three important and novel findings: 1) L-F1KO mice lacking liver Foxo1 gene exhibit a reduction in plasma Agt and Ang II, and a 19 mmHg reduction in the mean arterial pressure; 2) Foxo1 significantly stimulates Agt gene expression in hepatocytes; and 3) a functional Foxo1 binding site in the mouse Agt promoter region is identified, which is responsible for both Foxo1 stimulation and insulin inhibition. Agt is the most upstream and essential component of the RAS to generate bioactive peptide Ang II, a hormone that exerts potent influence on vascular constriction and cardiovascular remodeling 26. Chemical inhibitors for ACE and AT1 receptor that target Agt downstream actions are effective agents in treating hypertension and cardiac failure 26. Additionally, chemical suppression of hepatic Agt using small interference RNA is sufficient for achieving a sustained lower blood pressure in animal models 27. Our study demonstrated that hepatic Foxo1 regulates Agt gene expression and controls blood pressure, providing a novel regulatory mechanism for the RAS. Of note is that the reduction of blood pressure in F1KO mice may not solely be mediated by decreases in Agt expression and other factors secondary to the loss of liver Foxo1, such as hypoglycemia, may indirectly influence blood pressure.
Transcriptional regulation of Agt gene expression by Foxo1
Multiple hormones and nutrients stimulate expression of Agt at the gene transcriptional level, while insulin is a potent inhibitor for Agt gene transcription 8, 25. Foxo1 is a well-studied transcription factor that is suppressed by insulin/IGF-1 signaling, through activation of PI3K and Akt 11. Foxo1 stimulates Agt gene expression, an effect suppressed by insulin, providing a molecular link via insulin inhibition of Agt gene transcription. Conversely, glucagon, glucocorticoid, isoproterenol, Ang II, estrogen, thyroid hormone, cytokine IL-6, and high glucose, all stimulate Agt gene expression in a variety of cells involving distinct receptors and intracellular protein kinase activation 28. Receptors of the glucocorticoid receptor, estrogen receptor-α, and transcription factors C/EBP, GATA-1, and NFkB interact with the 5′UTR or −1.5kb promoter region of Agt, in which some single nucleotide polymorphisms have been implicated in hypertension 1, 29. In response to hormone stimulation, such as catecholamines and glucocorticoids, elevation of intracellular cAMP and cAMP-dependent protein kinase A (PKA) activate cAMP-responsive element binding protein (CREB), that recruits global transcriptional co-factor p300/CBP enhancing Agt gene expression 28. Foxo1 has been shown to interact with CREB and C/EBP enhancing expression of genes in control of glucose homeostasis 30–32. Thus, we predict that Foxo1 may not only serve as a mediator in insulin/IGF-1→PI3K→Akt signaling, but also mediate effects of other signaling cascades, such as PKA promoting Agt gene expression. Moreover, high glucose promotes glycosylation of Foxo1, thereby enhancing the target gene expression of G6Pase 33, 34. Thus, Foxo1 may also mediate the effect of glucose on inducing Agt, a new target gene expression in cells. Alternatively, high glucose activates p38α MAPK 35–37 and inactivates Akt resulting in Foxo1 activation, by promoting degradation of insulin receptor substrate-1, 2 (IRS1, -2) 23. Taken together, Foxo1 may promote Agt gene transcription through integrating different intracellular signaling cascades.
Role of Foxo1 in suppression of Agt gene expression by insulin
Foxo1 serves as a key component in the insulin/IGF-1 signaling pathway downstream from PI3K 12 and our studies also indicate that insulin inhibits Agt gene expression, through both PI3K and MAPK pathways. Early reports demonstrated that activation of ERK1/2 MAPK, by insulin, mediates the effect of insulin inhibition on Agt gene transcription in kidney cells 38. Moreover, rat Agt promoter region analysis suggested a distinct insulin response element, such as 5′CCTTCCCGCCCTTCA3′, was located within the rat −1.5 kb promoter region, mediating insulin suppression on Agt gene expression, through interacting with heterogeneous nuclear ribonucleoprotein K, downstream from ERK1/2 MAPK 39, 40. In this study, we showed that inhibitors of either ERK1/2 or PI3K partially blocked insulin inhibition on endogenous Agt expression in hepatocytes, and established that the mouse Agt promoter region-2, contains a functional Foxo1 binding site (CAAAACAA) responsible for insulin inhibition and Foxo1 stimulation, providing additional mechanisms for insulin suppression on Agt gene expression.
Agt gene expression is suppressed by insulin, while insulin has a minimal effect on reducing blood pressure. Insulin not only promotes vascular relaxation by activating PI3K and Akt and stimulating eNOS activity in vascular cells, but also stimulates vasoconstrictor action by inducing secretion of endothelin-1 (ET-1), largely via ERK1/2 MAPK activation 41, 42. In a healthy lean individual, physiological concentrations of insulin also increase venous catecholamine levels and sympathetic nervous activity for vasoconstriction 42, 43, which may mask the effect of insulin on reducing blood pressure by decreasing hepatic Foxo1 and associated Agt and plasma Ang II. Thus, insulin has both vasodilator and vasoconstrictor actions, such that the net hemodynamic effect of insulin on blood pressure is minimal in healthy humans 43, 44,
Of note is that IGF-1, an insulin-like growth factor produced by the liver, which shares IRS1 and IRS2, the intracellular signaling molecules of insulin, activating Akt and inhibiting Foxo1 23. An inverse relationship between IGF-1 and blood pressure has recently been reported in humans 45. Thus, a reduction of Agt by suppression of Foxo1 following an increase of IGF-1 and associated Akt activation in liver or other tissues, may also provide a link to reduced blood pressure in humans.
Role of Foxo1 in promoting the RAS in type 2 diabetes and metabolic syndrome
Foxo1 nuclear localization and activation is widely present in tissues of animals with insulin resistance and type 2 diabetes mellitus 46, in which elevation of Agt and other RAS components is concurrent 47. Loss of IRS1 and IRS2, the two major mediators of insulin/IGF-1 signaling, following metabolic stress, chronic hyperinsulinemia, over nutrition, or inflammation, provide a molecular basis of insulin resistance and type 2 diabetes mellitus 23, 34, 48. Loss of IRS1 and IRS2 results in inactivation of PI3K/Akt and Foxo1 activation 22, 23, 34, which may not only disrupt glucose homeostasis via excessive hepatic glucose production, but also contribute to elevation of the circulating RAS components via Agt gene expression. Although tissues of animals with insulin resistance or deficiency often maintain ERK1/2 MAPK activity 34 that reduces Agt gene expression making Agt gene expression complex in diabetes, Foxo1 activation may play a dominant role increasing Agt gene expression in liver and plasma Agt and Ang II, linking to high incidence of hypertension during insulin resistance and/or diabetes mellitus. Hypertension is closely associated with the metabolic syndrome and has become increasingly prevalent over the past several decades together with obesity and type 2 diabetes 34. It is well documented that hypertension occurs in several mouse models with insulin resistance, including high fat-diet and db/db mice 18, 49.
Perspectives
On the basis of the results that hepatic Foxo1 ablation reduces Agt and Ang II in the blood circulation, we believe that the Foxo1→Agt→Ang II axis likely provides a unifying mechanism in many other tissues, such as fat, brain and kidney where Foxo1 is ubiquitously expressed. A variety of tissues have intracellular or local RAS components and Ang II receptors 47, 50. We expect that Foxo1 activation, following insulin resistance, may contribute to an excess of local RAS synthesis by stimulating Agt gene expression. The Foxo1→Agt→Ang II pathway may contribute to the incidence and progression of hypertension, cardiomyopathy, neuropathy, and nephropathy in patients with type 2 diabetes mellitus.
Novelty and Significance.
What Is New?
We have identified Foxo1, a known insulin-suppressed transcription factor through PI3K/Akt activation, involving control of liver angiotensinogen (Agt) gene expression and plasma Agt and angiotensin II (Ang II) levels and modulating blood pressure in mice.
What Is Relevant?
Hypertension is closely associated with insulin resistance, a major mechanism of metabolic syndrome; however, the molecular link between blood pressure control and insulin signaling is lacking.
Summary
This study provides a novel regulatory mechanism for the renin-angiotensin system (RAS) and blood pressure control by the Foxo1→Agt→Ang II pathway.
Acknowledgments
Sources of Funding
This work is supported by American Heart Association grant BGIA-7880040, American Diabetes Association (JF-7-07-27), Faculty Start-up Funds from the Texas A&M University Health Science Center, and in part by NIH RO1DK095118-01 to S. Guo. This material is the result of work supported with resources and use of facilities at the Central Texas Veterans Health Care System, Temple, Texas.
Footnotes
Disclosures
None
References
- 1.Dickson ME, Sigmund CD. Genetic basis of hypertension: Revisiting angiotensinogen. Hypertension. 2006;48:14–20. doi: 10.1161/01.HYP.0000227932.13687.60. [DOI] [PubMed] [Google Scholar]
- 2.Goodfriend TL, Elliott ME, Catt KJ. Angiotensin receptors and their antagonists. N Engl J Med. 1996;334:1649–1654. doi: 10.1056/NEJM199606203342507. [DOI] [PubMed] [Google Scholar]
- 3.Guo S, Lopez-Ilasaca M, Dzau VJ. Identification of calcium-modulating cyclophilin ligand (caml) as transducer of angiotensin ii-mediated nuclear factor of activated t cells (nfat) activation. J Biol Chem. 2005;280:12536–12541. doi: 10.1074/jbc.M500296200. [DOI] [PubMed] [Google Scholar]
- 4.Kimura S, Mullins JJ, Bunnemann B, Metzger R, Hilgenfeldt U, Zimmermann F, Jacob H, Fuxe K, Ganten D, Kaling M. High blood pressure in transgenic mice carrying the rat angiotensinogen gene. EMBO J. 1992;11:821–827. doi: 10.1002/j.1460-2075.1992.tb05119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanimoto K, Sugiyama F, Goto Y, Ishida J, Takimoto E, Yagami K, Fukamizu A, Murakami K. Angiotensinogen-deficient mice with hypotension. J Biol Chem. 1994;269:31334–31337. [PubMed] [Google Scholar]
- 6.Sun Z, Cade R, Zhang Z, Alouidor J, Van H. Angiotensinogen gene knockout delays and attenuates cold-induced hypertension. Hypertension. 2003;41:322–327. doi: 10.1161/01.hyp.0000050964.96018.fa. [DOI] [PubMed] [Google Scholar]
- 7.Matsusaka T, Niimura F, Shimizu A, Pastan I, Saito A, Kobori H, Nishiyama A, Ichikawa I. Liver angiotensinogen is the primary source of renal angiotensin ii. J Am Soc Nephrol. 2012;23:1181–1189. doi: 10.1681/ASN.2011121159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang E, Perlman AJ. Angiotensinogen mrna. Regulation by cell cycle and growth factors. J Biol Chem. 1988;263:5480–5484. [PubMed] [Google Scholar]
- 9.Singh VP, Le B, Bhat VB, Baker KM, Kumar R. High-glucose-induced regulation of intracellular ang ii synthesis and nuclear redistribution in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2007;293:H939–948. doi: 10.1152/ajpheart.00391.2007. [DOI] [PubMed] [Google Scholar]
- 10.Gabriely I, Yang XM, Cases JA, Ma XH, Rossetti L, Barzilai N. Hyperglycemia modulates angiotensinogen gene expression. Am J Physiol Regul Integr Comp Physiol. 2001;281:R795–802. doi: 10.1152/ajpregu.2001.281.3.R795. [DOI] [PubMed] [Google Scholar]
- 11.Guo S, Rena G, Cichy S, He X, Cohen P, Unterman T. Phosphorylation of serine 256 by protein kinase b disrupts transactivation by fkhr and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. J Biol Chem. 1999;274:17184–17192. doi: 10.1074/jbc.274.24.17184. [DOI] [PubMed] [Google Scholar]
- 12.Rena G, Guo S, Cichy SC, Unterman TG, Cohen P. Phosphorylation of the transcription factor forkhead family member fkhr by protein kinase b. J Biol Chem. 1999;274:17179–17183. doi: 10.1074/jbc.274.24.17179. [DOI] [PubMed] [Google Scholar]
- 13.Zhang K, Li L, Qi Y, Zhu X, Gan B, DePinho RA, Averitt T, Guo S. Hepatic suppression of foxo1 and foxo3 causes hypoglycemia and hyperlipidemia in mice. Endocrinology. 2012;153:631–646. doi: 10.1210/en.2011-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papanicolaou KN, Izumiya Y, Walsh K. Forkhead transcription factors and cardiovascular biology. Circ Res. 2008;102:16–31. doi: 10.1161/CIRCRESAHA.107.164186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calnan DR, Brunet A. The foxo code. Oncogene. 2008;27:2276–2288. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- 16.Cichy SB, Uddin S, Danilkovich A, Guo S, Klippel A, Unterman TG. Protein kinase b/akt mediates effects of insulin on hepatic insulin-like growth factor-binding protein-1 gene expression through a conserved insulin response sequence. J Biol Chem. 1998;273:6482–6487. doi: 10.1074/jbc.273.11.6482. [DOI] [PubMed] [Google Scholar]
- 17.Baker KM, Chernin MI, Schreiber T, Sanghi S, Haiderzaidi S, Booz GW, Dostal DE, Kumar R. Evidence of a novel intracrine mechanism in angiotensin ii-induced cardiac hypertrophy. Regulatory peptides. 2004;120:5–13. doi: 10.1016/j.regpep.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Yiannikouris F, Gupte M, Putnam K, Thatcher S, Charnigo R, Rateri DL, Daugherty A, Cassis LA. Adipocyte deficiency of angiotensinogen prevents obesity-induced hypertension in male mice. Hypertension. 2012;60:1524–1530. doi: 10.1161/HYPERTENSIONAHA.112.192690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo S, Dunn SL, White MF. The reciprocal stability of foxo1 and irs2 creates a regulatory circuit that controls insulin signaling. Mol Endocrinol. 2006;20:3389–3399. doi: 10.1210/me.2006-0092. [DOI] [PubMed] [Google Scholar]
- 20.Cheng Z, Guo S, Copps K, Dong X, Kollipara R, Rodgers JT, Depinho RA, Puigserver P, White MF. Foxo1 integrates insulin signaling with mitochondrial function in the liver. Nat Med. 2009;15:1307–1311. doi: 10.1038/nm.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Gan L, Pan H, Guo S, He X, Olson ST, Mesecar A, Adam S, Unterman TG. Phosphorylation of serine 256 suppresses transactivation by fkhr (foxo1) by multiple mechanisms. Direct and indirect effects on nuclear/cytoplasmic shuttling and DNA binding. J Biol Chem. 2002;277:45276–45284. doi: 10.1074/jbc.M208063200. [DOI] [PubMed] [Google Scholar]
- 22.Guo S, Copps KD, Dong X, Park S, Cheng Z, Pocai A, Rossetti L, Sajan M, Farese RV, White MF. The irs1 branch of the insulin signaling cascade plays a dominant role in hepatic nutrient homeostasis. Mol Cell Biol. 2009;29:5070–5083. doi: 10.1128/MCB.00138-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qi Y, Xu Z, Zhu Q, Thomas C, Kumar R, Feng H, Dostal DE, White MF, Baker KM, Guo S. Myocardial loss of irs1 and irs2 causes heart failure and is controlled by p38alpha mapk during insulin resistance. Diabetes. 2013;62:3887–3900. doi: 10.2337/db13-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han S, Liang CP, Westerterp M, Senokuchi T, Welch CL, Wang Q, Matsumoto M, Accili D, Tall AR. Hepatic insulin signaling regulates vldl secretion and atherogenesis in mice. J Clin Invest. 2009;119:1029–1041. doi: 10.1172/JCI36523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang SL, Chen X, Filep JG, Tang SS, Ingelfinger JR, Chan JS. Insulin inhibits angiotensinogen gene expression via the mitogen-activated protein kinase pathway in rat kidney proximal tubular cells. Endocrinology. 1999;140:5285–5292. doi: 10.1210/endo.140.11.7125. [DOI] [PubMed] [Google Scholar]
- 26.Kumar R, Thomas CM, Yong QC, Chen W, Baker KM. The intracrine renin-angiotensin system. Clin Sci (Lond) 2012;123:273–284. doi: 10.1042/CS20120089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olearczyk J, Gao S, Eybye M, Yendluri S, Andrews L, Bartz S, Cully D, Tadin-Strapps M. Targeting of hepatic angiotensinogen using chemically modified sirnas results in significant and sustained blood pressure lowering in a rat model of hypertension. Hypertension research: official journal of the Japanese Society of Hypertension. 2014;37:405–412. doi: 10.1038/hr.2013.155. [DOI] [PubMed] [Google Scholar]
- 28.Ming M, Wu J, Lachance S, Delalandre A, Carriere S, Chan JS. Beta-adrenergic receptors and angiotensinogen gene expression in mouse hepatoma cells in vitro. Hypertension. 1995;25:105–109. doi: 10.1161/01.hyp.25.1.105. [DOI] [PubMed] [Google Scholar]
- 29.Jain S, Tang X, Narayanan CS, Agarwal Y, Peterson SM, Brown CD, Ott J, Kumar A. Angiotensinogen gene polymorphism at −217 affects basal promoter activity and is associated with hypertension in african-americans. J Biol Chem. 2002;277:36889–36896. doi: 10.1074/jbc.M204732200. [DOI] [PubMed] [Google Scholar]
- 30.Nasrin N, Ogg S, Cahill CM, Biggs W, Nui S, Dore J, Calvo D, Shi Y, Ruvkun G, Alexander-Bridges MC. Daf-16 recruits the creb-binding protein coactivator complex to the insulin-like growth factor binding protein 1 promoter in hepg2 cells. Proc Natl Acad Sci U S A. 2000;97:10412–10417. doi: 10.1073/pnas.190326997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sekine K, Chen YR, Kojima N, Ogata K, Fukamizu A, Miyajima A. Foxo1 links insulin signaling to c/ebpalpha and regulates gluconeogenesis during liver development. EMBO J. 2007;26:3607–3615. doi: 10.1038/sj.emboj.7601784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo S, Cichy SB, He X, Yang Q, Ragland M, Ghosh AK, Johnson PF, Unterman TG. Insulin suppresses transactivation by caat/enhancer-binding proteins beta (c/ebpbeta). Signaling to p300/creb-binding protein by protein kinase b disrupts interaction with the major activation domain of c/ebpbeta. J Biol Chem. 2001;276:8516–8523. doi: 10.1074/jbc.M008542200. [DOI] [PubMed] [Google Scholar]
- 33.Housley MP, Rodgers JT, Udeshi ND, Kelly TJ, Shabanowitz J, Hunt DF, Puigserver P, Hart GW. O-glcnac regulates foxo activation in response to glucose. J Biol Chem. 2008;283:16283–16292. doi: 10.1074/jbc.M802240200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo S. Insulin signaling, resistance, and the metabolic syndrome: Insights from mouse models into disease mechanisms. J Endocrinol. 2014;220:T1–T23. doi: 10.1530/JOE-13-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsieh TJ, Zhang SL, Filep JG, Tang SS, Ingelfinger JR, Chan JS. High glucose stimulates angiotensinogen gene expression via reactive oxygen species generation in rat kidney proximal tubular cells. Endocrinology. 2002;143:2975–2985. doi: 10.1210/endo.143.8.8931. [DOI] [PubMed] [Google Scholar]
- 36.Zhang SL, Tang SS, Chen X, Filep JG, Ingelfinger JR, Chan JS. High levels of glucose stimulate angiotensinogen gene expression via the p38 mitogen-activated protein kinase pathway in rat kidney proximal tubular cells. Endocrinology. 2000;141:4637–4646. doi: 10.1210/endo.141.12.7844. [DOI] [PubMed] [Google Scholar]
- 37.Lal H, Verma SK, Golden HB, Foster DM, Smith M, Dostal DE. Stretch-induced regulation of angiotensinogen gene expression in cardiac myocytes and fibroblasts: Opposing roles of jnk1/2 and p38alpha map kinases. J Mol Cell Cardiol. 2008;45:770–778. doi: 10.1016/j.yjmcc.2008.09.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang SL, Chen X, Wei CC, Filep JG, Tang SS, Ingelfinger JR, Chan JS. Insulin inhibits dexamethasone effect on angiotensinogen gene expression and induction of hypertrophy in rat kidney proximal tubular cells in high glucose. Endocrinology. 2002;143:4627–4635. doi: 10.1210/en.2002-220408. [DOI] [PubMed] [Google Scholar]
- 39.Wei CC, Zhang SL, Chen YW, Guo DF, Ingelfinger JR, Bomsztyk K, Chan JS. Heterogeneous nuclear ribonucleoprotein k modulates angiotensinogen gene expression in kidney cells. J Biol Chem. 2006;281:25344–25355. doi: 10.1074/jbc.M601945200. [DOI] [PubMed] [Google Scholar]
- 40.Chen X, Zhang SL, Pang L, Filep JG, Tang SS, Ingelfinger JR, Chan JS. Characterization of a putative insulin-responsive element and its binding protein(s) in rat angiotensinogen gene promoter: Regulation by glucose and insulin. Endocrinology. 2001;142:2577–2585. doi: 10.1210/endo.142.6.8214. [DOI] [PubMed] [Google Scholar]
- 41.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev. 2007;28:463–491. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 42.Anderson EA, Mark AL. The vasodilator action of insulin. Implications for the insulin hypothesis of hypertension. Hypertension. 1993;21:136–141. doi: 10.1161/01.hyp.21.2.136. [DOI] [PubMed] [Google Scholar]
- 43.Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991;87:2246–2252. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Westerbacka J, Wilkinson I, Cockcroft J, Utriainen T, Vehkavaara S, Yki-Jarvinen H. Diminished wave reflection in the aorta. A novel physiological action of insulin on large blood vessels. Hypertension. 1999;33:1118–1122. doi: 10.1161/01.hyp.33.5.1118. [DOI] [PubMed] [Google Scholar]
- 45.Schutte AE, Volpe M, Tocci G, Conti E. Revisiting the relationship between blood pressure and insulin-like growth factor-1. Hypertension. 2014;63:1070–1077. doi: 10.1161/HYPERTENSIONAHA.113.03057. [DOI] [PubMed] [Google Scholar]
- 46.Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, Shelton JM, Gerard RD, Rothermel BA, Gillette TG, Lavandero S, Hill JA. Metabolic stress-induced activation of foxo1 triggers diabetic cardiomyopathy in mice. J Clin Invest. 2012;122:1109–1118. doi: 10.1172/JCI60329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh VP, Le B, Khode R, Baker KM, Kumar R. Intracellular angiotensin ii production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes. 2008;57:3297–3306. doi: 10.2337/db08-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo S. Decoding insulin resistance and metabolic syndrome for promising therapeutic intervention. J Endocrinol. 2014;220:E1–3. doi: 10.1530/JOE-13-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Su W, Guo Z, Randall DC, Cassis L, Brown DR, Gong MC. Hypertension and disrupted blood pressure circadian rhythm in type 2 diabetic db/db mice. Am J Physiol Heart Circ Physiol. 2008;295:H1634–1641. doi: 10.1152/ajpheart.00257.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu F, Brezniceanu ML, Wei CC, Chenier I, Sachetelli S, Zhang SL, Filep JG, Ingelfinger JR, Chan JS. Overexpression of angiotensinogen increases tubular apoptosis in diabetes. J Am Soc Nephrol. 2008;19:269–280. doi: 10.1681/ASN.2007010074. [DOI] [PMC free article] [PubMed] [Google Scholar]