Abstract
Background
Multipeptide vaccines for melanoma may cause inflammatory adverse events (IAE). We hypothesize that IAE's are associated with a higher rate of immune response to vaccination (IR) and improved clinical outcomes.
Methods
Adult patients with resected, high-risk (stage IIB-IV) melanoma were vaccinated with a combination of 12 Class I MHC-restricted melanoma epitopes (12MP) and IAE's were recorded. A separate category for hypopigmentation (vitiligo) was also assessed. CD8+ T cell immune response was assessed by direct IFN-γ ELIspot. Overall survival and disease-free survival were analyzed by Cox proportional hazards modeling.
Results
Out of 332 patients, 57 developed IAE's, the majority of which were dermatologic (minimum CTCAE grade 3). Most non-dermatologic IAE's were CTCAE grade 1 and 2. Vitiligo developed in 23 patients (7%). 174 patients (53%) developed a CD8+ response. Presence of IAE was significantly associated with development of IR (70% vs 49%, p = 0.005) and with disease-free survival (HR 0.54, p = 0.043). There were no significant associations relating vitiligo or immune response alone with clinical outcomes.
Conclusions
Inflammatory adverse events are associated with a higher rate of CD8+ T-cell response following vaccination therapy for high-risk melanoma. Our findings suggest either that antitumor activity induced by Class I-restricted peptide vaccines may depend on immunologic effects beyond simple expansion of CD8+ T-cells or that the intrinsic inflammatory response of patients contributes to clinical outcome in melanoma.
Keywords: immunotherapy, T lymphocytes, vitiligo, cytokines
INTRODUCTION
Immune therapies can induce durable clinical benefit in patients with advanced melanoma, but autoimmune toxicities can limit their use and have been lethal in some patients 1. Cancer vaccines may induce protective antitumor immunity in high-risk patients and augment this clinical benefit by combination with other effective immune therapies. Melanoma vaccines can elicit cellular immune responses from CD8+ cytotoxic T cells and CD4+ helper T cells, and may induce clinical responses alone or in combination 2-4. Toxicities with vaccine therapies are rare, but can include local or systemic inflammatory responses, including ulcerations, dyspnea, and autoimmune toxicities 5,6. While evidence exists linking delayed-type hypersensitivity following vaccination with the induction of a cellular immune response 7,8, there is a paucity of research assessing the relationship between vaccine toxicity and clinical effect in melanoma immunotherapy.
We have previously reported immune response patterns and clinical outcomes of vaccination with a combination of 12 Class I MHC-restricted peptides administered with Montanide ISA-51 adjuvant among patients with resected stage IIB-IV melanoma 9-13. While each formulation was safe overall, significant inflammatory adverse events did occur for some patients 11,13. Although it is tempting to attribute these events to a cellular immune response, there exist no conclusive data to support this. There is, however, evidence to suggest that hypopigmentation (vitiligo) following melanoma immunotherapy may be a predictor of clinical response 14,15. The purpose of the present study is to test the hypothesis that inflammatory adverse events and vitiligo are associated with CD8+ T cell response and clinical outcomes following vaccine immunotherapy for resected, high-risk melanoma.
METHODS
This study included patients with stage IIB-IV high-risk, resected melanoma receiving a vaccine comprised of 12 Class I MHC-restricted melanoma peptides (12MP) in one of three prospective, phase II clinical trials. All patients had complete ELIspot results. Clinical trial design and assay protocols are reported in detail through prior publications 9-13. In brief, all three trials assess the CD8+ T lymphocyte response to 12MP, administered in Montanide ISA-51 with or without GM-CSF or cyclophosphamide (Table 1). For all trials, T cell response was assessed through peripheral blood mononuclear cell sampling (PBMC) using ELIspot analysis with the following definitions of results:
Table 1.
Class I MHC-restricted vaccine (12MP) trial protocols for patients with high-risk melanoma.
| Trial | Stage | Peptides | Adjuvant | N | %IAE |
|---|---|---|---|---|---|
| Mel43a | IIB-IV Resected | 12MP; tetanus helper | Montanide ISA-51 + GM-CSF | 60 | 23% |
| Montanide ISA-51 | 61 | 36% | |||
| Mel44a | IIB-IV Resected | 12MP; tetanus helper | Montanide ISA-51 +/− cyclophosphamide | 82 | 13% |
| 12MP; 6MHPc | Montanide ISA-51 +/− cyclophosphamide | 85 | 2% | ||
| Mel48a | IIB-IV Resectedb | 12MP; tetanus helper | Montanide ISA-51 | 45 | 18% |
| Total | 332d | 17% | |||
10 vaccinations were administered for Mel43 and Mel44; 6 for Mel48
patients with non-measurable disease or equivocal findings may be enrolled in Mel48.
6MHP, 6 melanoma helper peptide vaccine
one participant belonged to both trials 43 and 48
Nvax = number T-cells responding to vaccine peptide; Nneg = number T-cells responding to maximum negative control; Rvax = Nvax/Nneg.
An immune response to the 12MP vaccine was present if all of the following conditions were met: (1) Nvax – Nneg ≥ 20 cells/100,000 CD8+ T cells, (2) Rvax ≥ 2, (3) (Nvax – 1 SD) ≥ (Nneg + 1 SD), and (4) Rvax post-vaccination ≥ 2 x Rvax pre-vaccination.
Following vaccination, patients underwent scheduled follow-up over a maximum of 10 years based on previously-described protocols. Follow-up sessions assessed melanoma progression and adverse events which were reported as treatment-related toxicities. Inflammatory adverse event (IAE) categories are defined in Table 2, and severity criteria were based on Common Terminology Criteria for Adverse Events (CTCAE v3.0). For each event, date of symptom onset was recorded; when this was not available, date of follow-up with first toxicity documentation was used as the proxy for date of onset.
Table 2.
Definitions of inflammatory adverse events based on Common Terminology Criteria for Adverse Events (CTCAE)
| Category | Inflammatory Adverse Events | CTCAE grade |
|---|---|---|
| Dermatologica | Injection site reaction Pruritus/itching Rash/desquamation Ulceration/urticaria |
III |
| Pulmonary | Adult respiratory distress syndrome Bronchospasm Cough Dyspnea Pneumonitis/pulmonary infiltrates |
I, II, III |
| Constitutional | Flu-like syndrome Rigors/chills Fever Fatigue, sweating |
II, III |
Hypopigmentation analyzed separately as a non-inflammatory dermatologic adverse event
Statistical analysis
We compared the rate of immune response (IR) between patients who developed IAE's and patients who did not using a chi-squared test of association. Summary statistics were used to describe the chronologic relationship between IAE's and IR's, comparing the date of IAE onset to the date of peripheral blood sampling that yielded the first evidence of CD8+ T lymphocyte response.
Kaplan-Meier survival estimates, the log rank test and Cox proportional hazards models (PROC PHREG in SAS 9.2) were used to perform landmark time-to-event analyses of overall survival (OS) and disease-free survival (DFS). Landmark analysis focused on IAE's and IR's that occurred within 8 weeks of initiating the vaccination protocol. Overall survival was calculated as the time elapsed from the eight week post-registration date to either death or loss to follow up, while DFS was calculated as the time elapsed from the eight week post-registration date to death, recurrence/progression or loss to follow up. The following baseline characteristics were included in the models: stage (2 vs. 3 vs. 4), IAE (Y/N), IR (Y/N), age, and gender. Stage was based on patient status at study entry. Patients experiencing the outcome of interest (OS or DFS) prior to the eight week window were excluded from landmark analysis. For this reason, one and fourteen individuals were excluded from the analysis of OS and DFS, respectively. Assumptions of the Cox proportional hazards models were assessed using graphical methods. A two-sided Type I error rate of 0.05 was used.
The present study was conducted under the sanction of the Institutional Review Board of the University of Virginia (HIC#10524, 11491, and 13498).
RESULTS
A total of 332 patients met inclusion criteria for the present study. Median age was 56 years, and 31.3% (104/332) were female. Forty-four patients were enrolled with stage IIB/IIC disease, 45 with stage IIIA, 182 with stage IIIB/IIIC, and 61 with stage IV. Median follow-up time was 5.4 years. Across all trials, 52.7% (174/330) of patients produced a CD8+ T cell response by ELIspot criteria, 17.2% (57/332) developed an IAE, and 6.9% (23/332) developed vitiligo. Of patients who developed IAE's, 42 presented with dermatologic, 19 with constitutional, and 9 with pulmonary symptoms (supplemental table). Patients with nodular melanoma growth patterns were more likely to develop an IAE than patients with the superficial spreading pattern (31.1% vs 10.7%, p = 0.012). IAE rate was lower in the in the 6MHP arms of Mel44 compared to the other trial protocol arms (p=0.008, Table 1). Within Mel43, the rate of IAE's among patients who received GM-CSF was 23% (14/60), compared to 36% (22/61) among those who did not (p = 0.16). Within Mel44, patients who received cyclophosphamide had an IAE rate of 8% (7/84), compared to 7% (6/83) among those who did not (p = 1.0). One patient belonged to two vaccine trials; this patient did not develop an IAE within either trial, and only data from their initial study was used in analysis.
Overall, patients who were IAE+ had a higher rate of immune response (OR 2.36, p = 0.005, Table 3). Under subgroup analysis, this significant association held true for Mel43 (OR 2.75, p = 0.017) and Mel44 (OR 3.90, p = 0.033). Under subgroup analysis by IAE type, only dermatologic IAE's were associated with a significantly increased rate of CD8+ T cell response (OR 2.75, p = 0.005). These dermatologic IAEs were principally local vaccine site reactions. Conversely, among patients who developed an immune response, rate of IAE occurrence was twice that of patients who did not have an immune response (22% vs 12%, p = 0.005, not shown). Temporally, IAE's tended to occur after the onset of immune responses, with a median delay of 1.0 (IQR 0.4 – 2.3), 0.2 (IQR −0.1 – 0.4), and 0.8 (IQR 0.4 – 1.1) months for dermatologic, constitutional, and pulmonary subtypes, respectively (supplemental figure). Because vitiligo was considered to represent an autoimmune response, the timing relationship between peripheral immune response and vitiligo onset was not calculated; however, median onset of vitiligo was at 9 months, considerably delayed following IR positivity.
Table 3.
Association between CD8+ T cell response by direct IFN-γ ELIspot and inflammatory adverse events following vaccination for high-risk melanoma.
| IAE | IR | Total | OR | CI | p-value | |
|---|---|---|---|---|---|---|
| Mel 43 | + | 25 | 35 | 2.75 | 1.18 – 6.43 | .017 |
| - | 40 | 84 | ||||
| Mel 44 | + | 10 | 13 | 3.90 | 1.03 – 14.7 | .033 |
| - | 71 | 154 | ||||
| Mel 48 | + | 4 | 8 | 0.48 | 0.10 – 2.26 | .35 |
| - | 25 | 37 | ||||
| Dermatologic | + | 30 | 41 | 2.75 | 1.33 – 5.69 | .005 |
| - | 144 | 289 | ||||
| Pulmonary | + | 4 | 9 | 0.71 | 0.19 – 2.69 | 0.61 |
| - | 170 | 321 | ||||
| Constitutional | + | 12 | 19 | 1.57 | 0.60 – 4.11 | .35 |
| - | 162 | 311 | ||||
| All Trials | + | 39 | 56 | 2.36 | 1.27 – 4.38 | .005 |
| - | 135 | 274 | ||||
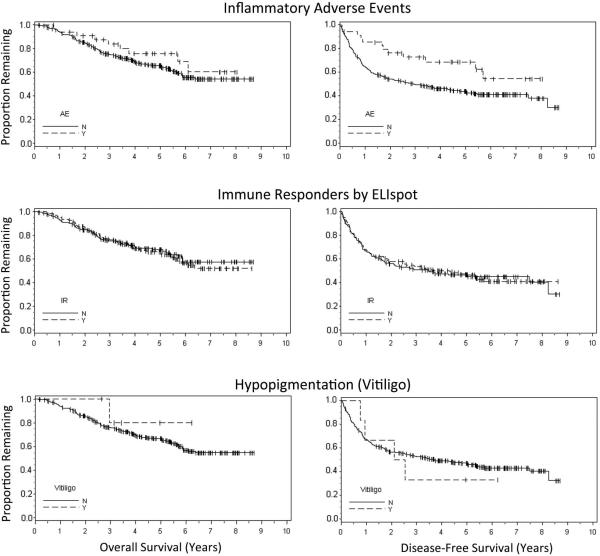
Associations of IAE, IR, and vitiligo with overall survival and disease-free survival, as assessed through Kaplan-Meier estimation, are presented in Figure 1. There was no significant association of OS with IR, IAE, or vitiligo. While IR and vitiligo also were not associated with DFS, IAE was significantly associated with improved DFS (log rank p = 0.024). However, with only 7% of patients experiencing vitiligo, our ability to detect significant associations with this variable was low. Assessing univariate relationships by symptom subgroups, patients experiencing dermatologic IAE's trended toward improved DFS, however, this relationship did not reach statistical significance (p = 0.096). Constitutional and pulmonary symptom subgroups were too small for independent analysis. Results from the proportional hazard models indicated only younger age and male gender were associated with improved OS, while only the presence of IAE was associated with improved DFS (HR 0.54, Wald p = 0.043, Table 4).
Figure 1.
Kaplan-Meier curves for landmark analysis of overall survival (left) and disease-free survival (right). Inflammatory adverse events were associated with a significant increase in disease-free survival (p = 0.024). CD8+ T cell response by direct ELIspot was not associated with overall or disease-free survival.
Table 4.
Multivariable predictors of overall survival and disease-free survival among patients receiving 12MP vaccine therapy for high-risk melanoma.
| Hazard Ratio | Confidence Interval | p-value | |
|---|---|---|---|
| Overall Survival (OS) | |||
| Stage III | 1.54 | 0.80 – 2.98 | 0.20 |
| Stage IV | 1.09 | 0.50 – 2.34 | 0.84 |
| Immune Response | 1.11 | 0.76 – 1.63 | 0.58 |
| Inflamm Adverse Event | 0.77 | 0.38 – 1.53 | 0.45 |
| Age | 1.02 | 1.00 – 1.03 | 0.020 |
| Male Sex | 0.67 | 0.45 – 0.99 | 0.046 |
| Disease Free Survival (DFS) | |||
| Stage III | 1.16 | 0.72 – 1.88 | 0.53 |
| Stage IV | 1.14 | 0.65 – 2.01 | 0.65 |
| Immune Response | 1.04 | 0.76 – 1.42 | 0.81 |
| Inflamm Adverse Event | 0.54 | 0.30 – 0.98 | 0.043 |
| Age | 1.01 | 1.00 – 1.02 | 0.061 |
| Male Sex | 0.76 | 0.54 – 1.06 | 0.10 |
DISCUSSION
As treatment protocols for high-risk melanoma continue to evolve, the balance between clinical benefit and dose-dependent toxicity is continuously scrutinized. Therapies that provide the highest overall response rates are at times limited to treatment for refractory cases or adjusted to less efficacious doses due to risks of serious organ injury 1,16-18.
Our results highlight an alternative viewpoint of adverse events, suggesting that an association is present between IAE's and cellular immune response following administration of a combination Class I MHC-restricted peptide vaccine. This relationship is most evident for dermatologic IAE's. Moreover, there exists an association between these adverse events and disease-free survival that is not explained entirely by the development of peripheral CD8+ T lymphocyte responses, and IAE's confer a stronger prediction of clinical outcome after vaccination than does the presence of a CD8+ T lymphocyte response. The benefit toward DFS among IAE+ patients following immunotherapy appears to be conferred during the initial two years, which argues for consideration of more intensive monitoring among patients who do not develop an early IAE. By documenting progression early, interventions for refractory disease, such as additional vaccines or targeted therapies, might be pursued to impact overall survival.
The connection between post-vaccination adverse events and CD8+ T lymphocyte proliferation has not been an area of intensive research. Our findings point to the potential presence of a group of intrinsic “hyper-responsive” patients for whom antitumor vaccine therapy may be particularly efficacious. The concept of intrinsic hyper-responders is evident in the association between local dermatologic reactions and TH2 response among children vaccinated with DTaP 8. While we did not assess for CD4 T lymphocyte proliferation in response to melanoma antigens across this study, our results are in line with findings that effector T cell proliferation follows onset of rash among patients experiencing herpes zoster reactivation 19.
A potential relationship between delayed-type hypersensitivity (DTH) and peripheral T-cell response following vaccination has been explored in cancer immunotherapy. Our finding of an association between dermatologic IAE and peripheral IR suggests that peripheral CD8+ T cells may be trafficking to vaccine sites and contributing to DTH and dermal inflammation 20,21. This mechanism is supported by other studies associating DTH and T cell proliferation following administration of vaccines derived from HER-2/neu and colon cancer cells 22,23.
Vaccine-induced effector-memory CD8+ T lymphocyte proliferation has been shown to predict immunotherapy efficacy against tumors 24-26. By demonstrating improved disease-free survival associated with IAE's but not IR's, we allude to mechanisms of antitumor activity through 12MP vaccination beyond induction of a CD8+ cytotoxic lymphocyte response. Local and systemic adverse events following vaccination are most commonly attributed to proinflammatory cytokine release by macrophages, T lymphocytes, and other cells. Within our series, IAE's were most commonly either constitutional or dermatologic. Mediators of flu-like syndromes are typically attributed to acute phase reactants such as TNF-α, which has cellular-level effects of macrophage activation, neutrophil migration, and cell-death signaling. TNF-α surge has been noted following smallpox vaccination, correlating with constitutional symptoms 27,28. Within the dermis, TNF-α is stored in mast cells and is released in response to immune stimulus 29, resulting in erythema, induration, and pain typical of dermatologic IAE's within our series. Our finding that IAE's following vaccination are associated with DFS suggests a potential connection with antitumor effects of TNFα in melanoma 30,31.
Other cytokines up-regulated following vaccination include IFN-γ, IL-2 and IL-4 32, each offering additional mechanisms for antitumor activity independent of CD8+ T lymphocyte proliferation. IFN-γ promotes cellular immunity via TH1 lymphocyte differentiation and increased MHC Class I expression on antigen-presenting cells. Although IL-4 has down-regulatory effects on TH1 differentiation and IFN-γ expression, its induction of an IgE response via plasma cell differentiation may contribute to durable clinical responses given IgE's applications as a passive immunotherapeutic in solid tumors 33,34. Finally, treatment of advanced-stage melanoma with IL-2 alone or in combination with other immunotherapeutic agents has been on-going with durable clinical benefit in a small subset of patients 15,35-39.
Although we did not find a significant relationship between hypopigmentation and clinical outcomes, there was an early divergence between survival curves for patients with vitiligo and patients without (Figure 1). With only 23 cases of vitiligo captured, our study volume may be underpowered to detect this relationship. Bystryn and colleagues first reported significantly better five-year survival among melanoma patients who developed vitiligo 14. Furthermore, patients with melanoma who develop vitiligo also show an improved rate of objective response to immunotherapy with IL-2 15. Our findings are in concordance with these data, and prompt further exploration with a larger patient sample.
The present study has several limitations. Because each vaccine contains several components, we are not able to identify which component is responsible for each IAE. GM-CSF and cyclophosphamide may promote IAE's through dendritic cell activation and regulatory T-cell inhibition, respectively. Interestingly, within Mel43, patients who were treated with GM-CSF actually trended toward fewer IAE's (23%) than those who were not (36%). This is consistent with our prior report that, among vaccinated patients, T cell responses were negatively associated with GM-CSF. Similarly, there was no significant effect of cyclophosphamide on IAEs, which is consistent with the lack of effect on measured T cell responses 11,13. Vaccine regimens also differed in whether they included helper peptides, and, for reasons that are unclear, there was a lower incidence of IAE's with administration of 6MHP in Mel44. Host factors—such as melanoma growth pattern—may also contribute to adverse events for reasons that are not clear. Overall, however, we believe that patient groups were large enough to dilute these variations, and there were no significant differences in DFS or OS when comparing the three trials under univariate analysis. Moreover, analyzing the clinical outcomes of intrinsic hyper-responders is valid regardless of the inciting agent that exposed this characteristic. The relationship between IAE's and nodular growth pattern is somewhat surprising, as nodular melanomas tend to have worse prognosis and higher local recurrence rates 40. Statistical modeling was performed using time-to-event analysis with a landmark adjustment. Repeating analyses without the landmark adjustment did not change the association between IAE and DFS. While time-dependent analysis is more common, it would have accounted for events occurring years after vaccination, which are less likely to be truly vaccine-related. Lastly, despite being the largest study of its kind, our patient volume was insufficient to generate conclusions regarding the proliferative response implications of pulmonary IAEs.
CONCLUSIONS
Inflammatory adverse events following vaccination with the combination 12MP Class I MHC-restricted melanoma vaccine are associated with a higher rate of CD8+ T cell response and better disease-free survival among patients with high-risk melanoma. Their prognostic utility may be harnessed to influence monitoring protocols during the first two years following treatment. Interestingly, the positive association between disease-free survival and inflammatory adverse events does not appear to be directly related to CD8+ T cell response, and may involve unanticipated effects of vaccine components, possibly including effects of one or more vaccine-induced cytokines.
Supplementary Material
Supplemental Figure: Timing of inflammatory adverse events, shown as months following the first onset of CD8 T cell immune response. Overall, inflammatory adverse events most frequently developed between 0.5 to 1.0 months after the onset of immune responses.
Synopsis.
Inflammatory adverse events following Class-I MHC-restricted vaccine treatment for high-risk melanoma immunotherapy is associated with improved disease-free survival. This relationship may be independent of vaccine-induced CD8 T lymphocyte proliferation.
ACKNOWLEDGEMENTS
Financial Support: This study was funded by NIH/NCI grants NIH R01 CA057653 and CA118386, and NIH R21 CA103528 (to C.L.S), and NIH T32 CA163177 (to YH). Support was also provided by the University of Virginia Cancer Center Support Grant (NIH/NCI P30 CA44579, Clinical Trials Office, Tissue Procurement Facility, and Biomolecular Core Facility); and the UVA General Clinical Research Center (NIH M01 RR00847). Peptides used in the vaccines were prepared with philanthropic support from the Commonwealth Foundation for Cancer Research and Alice and Bill Goodwin. Additional philanthropic support was provided from Frank and Jane Batten, the James and Rebecca Craig Foundation, George S. Suddock, Richard and Sherry Sharp, and the Patients and Friends Research Fund of the University of Virginia Cancer Center.
ABREVIATION LIST
- IR
immune response
- IAE
inflammatory adverse event
- 12MP
vaccine comprised of 12 Class I MHC-restricted melanoma peptides
- 6MHP
vaccine comprised of 6 Class II MHC-restricted melanoma helper peptides
- PBMC
peripheral blood mononuclear cells
- DFS
disease-free survival
- OS
overall survival
- OR
odds ratio
- DTH
delayed-type hypersensitivity
Footnotes
Disclosures: Dr. Slingluff is an inventor for peptides included in these trials; the patents are held by the University of Virginia Licensing and Ventures Group.
REFERENCES
- 1.Callahan MK, Wolchok JD. At the bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J Leukoc Biol. 2013;94(1):41–53. doi: 10.1189/jlb.1212631. doi: 10.1189/jlb.1212631; 10.1189/jlb.1212631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slingluff CL, Jr, Petroni GR, Olson W, et al. Helper T-cell responses and clinical activity of a melanoma vaccine with multiple peptides from MAGE and melanocytic differentiation antigens. J Clin Oncol. 2008;26(30):4973–4980. doi: 10.1200/JCO.2008.17.3161. doi: 10.1200/JCO.2008.17.3161; 10.1200/JCO.2008.17.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slingluff CL, Jr, Lee S, Zhao F, et al. A randomized phase II trial of multiepitope vaccination with melanoma peptides for cytotoxic T cells and helper T cells for patients with metastatic melanoma (E1602). Clin Cancer Res. 2013;19(15):4228–4238. doi: 10.1158/1078-0432.CCR-13-0002. doi: 10.1158/1078-0432.CCR-13-0002; 10.1158/1078-0432.CCR-13-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartzentruber DJ, Lawson DH, Richards JM, et al. Gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364(22):2119–2127. doi: 10.1056/NEJMoa1012863. doi: 10.1056/NEJMoa1012863; 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woodson EM, Chianese-Bullock KA, Wiernasz CJ, et al. Assessment of the toxicities of systemic low-dose interleukin-2 administered in conjunction with a melanoma peptide vaccine. J Immunother. 2004;27(5):380–388. doi: 10.1097/00002371-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Chianese-Bullock KA, Woodson EM, Tao H, et al. Autoimmune toxicities associated with the administration of antitumor vaccines and low-dose interleukin-2. J Immunother. 2005;28(4):412–419. doi: 10.1097/01.cji.0000171314.00924.2b. [DOI] [PubMed] [Google Scholar]
- 7.Barth A, Hoon DS, Foshag LJ, et al. Polyvalent melanoma cell vaccine induces delayed-type hypersensitivity and in vitro cellular immune response. Cancer Res. 1994;54(13):3342–3345. [PubMed] [Google Scholar]
- 8.Rowe J, Yerkovich ST, Richmond P, et al. Th2-associated local reactions to the acellular diphtheria-tetanus-pertussis vaccine in 4- to 6-year-old children. Infect Immun. 2005;73(12):8130–8135. doi: 10.1128/IAI.73.12.8130-8135.2005. doi: 10.1128/IAI.73.12.8130-8135.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slingluff CL, Petroni GR, Smolkin ME, et al. Immunogenicity for CD8+ and CD4+ T cells of 2 formulations of an incomplete freund's adjuvant for multipeptide melanoma vaccines. J Immunother. 2010;33(6):630–638. doi: 10.1097/CJI.0b013e3181e311ac. doi: 10.1097/CJI.0b013e3181e311ac; 10.1097/CJI.0b013e3181e311ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salerno EP, Shea SM, Olson WC, et al. Activation, dysfunction and retention of T cells in vaccine sites after injection of incomplete freund's adjuvant, with or without peptide. Cancer Immunol Immunother. 2013;62(7):1149–1159. doi: 10.1007/s00262-013-1435-5. doi: 10.1007/s00262-013-1435-5; 10.1007/s00262-013-1435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slingluff CL, Jr, Petroni GR, Olson WC, et al. Effect of granulocyte/macrophage colony-stimulating factor on circulating CD8+ and CD4+ T-cell responses to a multipeptide melanoma vaccine: Outcome of a multicenter randomized trial. Clin Cancer Res. 2009;15(22):7036–7044. doi: 10.1158/1078-0432.CCR-09-1544. doi: 10.1158/1078-0432.CCR-09-1544; 10.1158/1078-0432.CCR-09-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaefer JT, Patterson JW, Deacon DH, et al. Dynamic changes in cellular infiltrates with repeated cutaneous vaccination: A histologic and immunophenotypic analysis. J Transl Med. 2010;8:79–5876-8-79. doi: 10.1186/1479-5876-8-79. doi: 10.1186/1479-5876-8-79; 10.1186/1479-5876-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slingluff CL, Jr, Petroni GR, Chianese-Bullock KA, et al. Randomized multicenter trial of the effects of melanoma-associated helper peptides and cyclophosphamide on the immunogenicity of a multipeptide melanoma vaccine. J Clin Oncol. 2011;29(21):2924–2932. doi: 10.1200/JCO.2010.33.8053. doi: 10.1200/JCO.2010.33.8053; 10.1200/JCO.2010.33.8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bystryn JC, Rigel D, Friedman RJ, Kopf A. Prognostic significance of hypopigmentation in malignant melanoma. Arch Dermatol. 1987;123(8):1053–1055. [PubMed] [Google Scholar]
- 15.Rosenberg SA, White DE. Vitiligo in patients with melanoma: Normal tissue antigens can be targets for cancer immunotherapy. J Immunother Emphasis Tumor Immunol. 1996;19(1):81–84. [PubMed] [Google Scholar]
- 16.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. doi: 10.1056/NEJMoa1003466; 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: Recent successes and next steps. Nat Rev Cancer. 2011;11(11):805–812. doi: 10.1038/nrc3153. doi: 10.1038/nrc3153; 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: A randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11(2):155–164. doi: 10.1016/S1470-2045(09)70334-1. doi: 10.1016/S1470-2045(09)70334-1; 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 19.Weinberg A, Levin MJ. VZV T cell-mediated immunity. Curr Top Microbiol Immunol. 2010;342:341–357. doi: 10.1007/82_2010_31. doi: 10.1007/82_2010_31; 10.1007/82_2010_31. [DOI] [PubMed] [Google Scholar]
- 20.Salerno EP, Shea SM, Olson WC, et al. Activation, dysfunction and retention of T cells in vaccine sites after injection of incomplete freund's adjuvant, with or without peptide. Cancer Immunol Immunother. 2013;62(7):1149–1159. doi: 10.1007/s00262-013-1435-5. doi: 10.1007/s00262-013-1435-5; 10.1007/s00262-013-1435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hailemichael Y, Dai Z, Jaffarzad N, et al. Persistent antigen at vaccination sites induces tumor-specific CD8(+) T cell sequestration, dysfunction and deletion. Nat Med. 2013;19(4):465–472. doi: 10.1038/nm.3105. doi: 10.1038/nm.3105; 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bloemena E, Gall H, Ransom JH, et al. Delayed-type hypersensitivity reactions to tumor-associated antigens in colon carcinoma patients immunized with an autologous tumor cell/bacillus calmette-guerin vaccine. Cancer Res. 1993;53(3):456–459. [PubMed] [Google Scholar]
- 23.Disis ML, Schiffman K, Gooley TA, McNeel DG, Rinn K, Knutson KL. Delayed-type hypersensitivity response is a predictor of peripheral blood T-cell immunity after HER-2/neu peptide immunization. Clin Cancer Res. 2000;6(4):1347–1350. [PubMed] [Google Scholar]
- 24.van Duikeren S, Fransen MF, Redeker A, et al. Vaccine-induced effector-memory CD8+ T cell responses predict therapeutic efficacy against tumors. J Immunol. 2012;189(7):3397–3403. doi: 10.4049/jimmunol.1201540. doi: 10.4049/jimmunol.1201540. [DOI] [PubMed] [Google Scholar]
- 25.Slingluff CL, Jr, Lee S, Zhao F, et al. A randomized phase II trial of multiepitope vaccination with melanoma peptides for cytotoxic T cells and helper T cells for patients with metastatic melanoma (E1602). Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-13-0002. doi: 10.1158/1078-0432.CCR-13-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Duikeren S, Arens R. Predicting the efficacy of cancer vaccines by evaluating T-cell responses. Oncoimmunology. 2013;2(1):e22616. doi: 10.4161/onci.22616. doi: 10.4161/onci.22616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen JI, Hohman P, Fulton R, et al. Kinetics of serum cytokines after primary or repeat vaccination with the smallpox vaccine. J Infect Dis. 2010;201(8):1183–1191. doi: 10.1086/651453. doi: 10.1086/651453; 10.1086/651453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rock MT, Yoder SM, Talbot TR, Edwards KM, Crowe JE., Jr. Adverse events after smallpox immunizations are associated with alterations in systemic cytokine levels. J Infect Dis. 2004;189(8):1401–1410. doi: 10.1086/382510. doi: 10.1086/382510. [DOI] [PubMed] [Google Scholar]
- 29.Walsh LJ, Trinchieri G, Waldorf HA, Whitaker D, Murphy GF. Human dermal mast cells contain and release tumor necrosis factor alpha, which induces endothelial leukocyte adhesion molecule 1. Proc Natl Acad Sci U S A. 1991;88(10):4220–4224. doi: 10.1073/pnas.88.10.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lienard D, Ewalenko P, Delmotte JJ, Renard N, Lejeune FJ. High-dose recombinant tumor necrosis factor alpha in combination with interferon gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin Oncol. 1992;10(1):52–60. doi: 10.1200/JCO.1992.10.1.52. [DOI] [PubMed] [Google Scholar]
- 31.Lienard D, Eggermont AM, Schraffordt Koops H, et al. Isolated perfusion of the limb with high-dose tumour necrosis factor-alpha (TNF-alpha), interferon-gamma (IFN-gamma) and melphalan for melanoma stage III. results of a multi-centre pilot study. Melanoma Res. 1994;4(Suppl 1):21–26. [PubMed] [Google Scholar]
- 32.Garcia-Pineres A, Hildesheim A, Dodd L, et al. Cytokine and chemokine profiles following vaccination with human papillomavirus type 16 L1 virus-like particles. Clin Vaccine Immunol. 2007;14(8):984–989. doi: 10.1128/CVI.00090-07. doi: 10.1128/CVI.00090-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karagiannis SN, Josephs DH, Karagiannis P, et al. Recombinant IgE antibodies for passive immunotherapy of solid tumours: From concept towards clinical application. Cancer Immunol Immunother. 2012;61(9):1547–1564. doi: 10.1007/s00262-011-1162-8. doi: 10.1007/s00262-011-1162-8; 10.1007/s00262-011-1162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vercelli D, Jabara HH, Arai K, Geha RS. Induction of human IgE synthesis requires interleukin 4 and T/B cell interactions involving the T cell receptor/CD3 complex and MHC class II antigens. J Exp Med. 1989;169(4):1295–1307. doi: 10.1084/jem.169.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atzpodien J, Neuber K, Kamanabrou D, et al. Combination chemotherapy with or without s.c. IL-2 and IFN-alpha: Results of a prospectively randomized trial of the cooperative advanced malignant melanoma chemoimmunotherapy group (ACIMM). Br J Cancer. 2002;86(2):179–184. doi: 10.1038/sj.bjc.6600043. doi: 10.1038/sj.bjc.6600043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slingluff CL, Jr, Petroni GR, Yamshchikov GV, et al. Immunologic and clinical outcomes of vaccination with a multiepitope melanoma peptide vaccine plus low-dose interleukin-2 administered either concurrently or on a delayed schedule. J Clin Oncol. 2004;22(22):4474–4485. doi: 10.1200/JCO.2004.10.212. doi: 10.1200/JCO.2004.10.212. [DOI] [PubMed] [Google Scholar]
- 37.Weiss GR, Grosh WW, Chianese-Bullock KA, et al. Molecular insights on the peripheral and intratumoral effects of systemic high-dose rIL-2 (aldesleukin) administration for the treatment of metastatic melanoma. Clin Cancer Res. 2011;17(23):7440–7450. doi: 10.1158/1078-0432.CCR-11-1650. doi: 10.1158/1078-0432.CCR-11-1650; 10.1158/1078-0432.CCR-11-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elias EG, Zapas JL, McCarron EC, Beam SL, Hasskamp JH, Culpepper WJ. Sequential administration of GM-CSF (sargramostim) and IL-2 +/− autologous vaccine as adjuvant therapy in cutaneous melanoma: An interim report of a phase II clinical trial. Cancer Biother Radiopharm. 2008;23(3):285–291. doi: 10.1089/cbr.2007.0438. doi: 10.1089/cbr.2007.0438; 10.1089/cbr.2007.0438. [DOI] [PubMed] [Google Scholar]
- 39.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 40.Zogakis TG, Essner R, Wang HJ, et al. Melanoma recurrence patterns after negative sentinel lymphadenectomy. Arch Surg. 2005;140(9):865–71. doi: 10.1001/archsurg.140.9.865. discussion 871-2. doi: 140/9/865 [pii] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure: Timing of inflammatory adverse events, shown as months following the first onset of CD8 T cell immune response. Overall, inflammatory adverse events most frequently developed between 0.5 to 1.0 months after the onset of immune responses.