Abstract
Whether long-term blood pressure variability (BPV) throughout young adulthood is associated with cognitive function in midlife remains uncertain. Using data from the Coronary Artery Risk Development in Young Adults (CARDIA), which recruited healthy young adults ages 18 to 30 years (mean age 25 years) at baseline (Year 0), we assessed BPV by standard deviation (SD) and average real variability (ARV) over 25 years (8 visits). Cognitive function was assessed with the Digit Symbol Substitution Test (DSST, psychomotor speed test), the Rey Auditory Verbal Learning Test (RAVLT, verbal memory test), and the modified Stroop test (executive function test) at follow-up (Year 25). At the Year 25 examination, participants (n=2,326) with a mean age of 50.4 years, 43% were men, and 40% were black. In multivariable-adjusted linear regression models, higher ARVSBP, ARVDBP, and SDDBP were significantly associated with lower scores of DSST (β [standard error: SE]: −.025[.006], −.029[.007], and −.029[.007] respectively; all P<.001) and RAVLT (β [SE]: −.016[.006], −.021[.007], and −.019[.007] respectively; all P<.05) after adjustment for demographic and clinical characteristics and with cumulative exposure to BP through Year 0 to Year 25. Neither SDBP nor ARVBP was associated with the Stroop score. The associations between ARVBP or SDBP and each cognitive function test were similar between blacks and whites except for one significant interaction between race and SDSBP on the DSST (P<.05). Long-term BPV over 25 years beginning in young adulthood was associated with worse psychomotor speed and verbal memory tests in midlife, independent of cumulative exposure to BP during follow-up.
Keywords: Blood pressure variability, Blood pressure, Cognitive function, Young adults, Long-term follow-up
Introduction
The association between higher blood pressure (BP) and lower cognitive function has been well established.1–3 In addition to average BP values, BP variability (BPV) may be associated with cognitive function.4 BPV consists of the short-term BPV (e.g., beat-to-beat and within 24-hours) and long-term BPV (e.g., day-by-day and visit-to-visit BPV).5,6 The implications of long-term BPV are less defined, particularly its effects on cognition.
The association between higher long-term BPV and lower cognitive function has been recently reported.7–9 These studies were conducted on middle-aged/older persons and/or high-risk populations, suggesting that BPV itself could be influenced substantially by comorbidities including (silent) cerebrovascular diseases. The Coronary Artery Risk Development in Young Adults (CARDIA) Study provides a unique opportunity to study these issues, since CARDIA enrolled only young adults (18–30 years) with very few comorbidities.
Using CARDIA data, we assessed whether long-term BPV throughout young adulthood is associated with measures of cognitive function in midlife. We also examined whether diverse domains of cognition, including psychomotor speed, verbal memory, and executive function, are differentially associated with long-term BPV.
Methods
Study population
The CARDIA Study is a multicenter longitudinal study of 5,115 young adults ages 18 to 30 years (mean age 25 yeas) in 1985–1986 (see Supplemental Data).10 All participants provided written informed consent at each examination, and institutional review boards from each field center and the coordinating center approved the study annually.
Among 5,115 participants, we excluded 1,617 participants who did not attend the follow-up examination at Year 25 (Y25), 175 participants with missing data on cognitive function at Y25, and 997 participants with at least one missing BP measurement during follow-up or any missing covariates. As a result, we included 2,326 participants who attended Y0 (baseline), Y2, Y5, Y7, Y10, Y15, Y20, and Y25 examinations and completed cognitive testing at Y25 (median 24.9 years of follow-up). At baseline, the included participants were slightly older (25.3 vs. 24.5 years), and had a lower percentage of blacks (40.2 vs. 61.1 %), higher educational attainment (14.3 vs. 13.4 years), and lower baseline SBP (109.8 vs. 110.9 mmHg; all P<.01) compared to those not included in this study (n=2,789), Supplementary Table S1.
BP and other measurements
At each examination, research staff measured right-arm brachial artery BP 3 times after the participant had been sitting in a quiet room for 5 minutes. Three measurements were taken at 1-minute interval, and the average of the 2nd and 3rd measurements was used for the analysis.11 Members of the CARDIA research staff took measurements by using a Hawksley random-zero sphygmomanometer (Hawksley, Sussex, United Kingdom) until the Y20 examination, when concerns about mercury contained in the apparatus required a switch to an automated oscillometric BP monitor (Omron HEM-907XL; Online Fitness, Santa Monica, CA). A calibration study was performed, and values standardized to the sphygmomanometric measures were used for the Y20 and Y25 BP measurements, so that no machine bias remained (see Supplemental Data).12
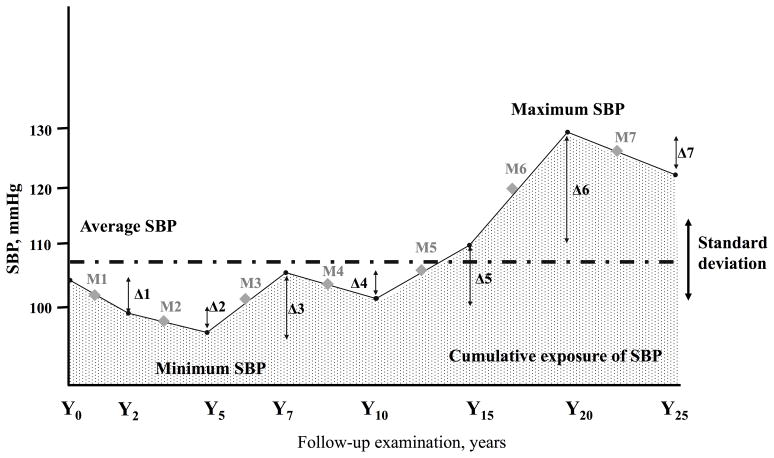
For the BPV index, we calculated standard deviation (SDSBP, SDDBP), coefficient of variation (CVSBP, CVDBP), the maximum and minimum BP difference (MMDSBP, MMDDBP), and average real variability (ARVSBP, ARVDBP) across 8 visits (Y0–25); all of these measures have been used in prior studies of BPV.5–9,13 ARV is the average absolute difference between successive BP measurements, and in contrast with SD and CV, it takes the order of the BP measurements into account.13 Here we only report BPV using SD and ARV, since CV and MMD are strongly correlated with SD (r=0.96–0.98, both P<0.001; Supplementary Table S2). We also calculated baseline BP (Y0), change of BP from Y25 to Y0 (Y25-Y0), average BP from Y0 to Y25 (Y0–25), and cumulative exposure to BP from Y0 to Y25 (Y0–25) (mmHg×year) to use as adjusted variables (Figure 1).
Figure 1. The formula of BP index.
The figure shows one example of individual follow-up data of BP across 8 visits (Y0–25). The absolute differences of BP between successive BP measurements are shown as Δ1−Δ7. For example, Δ1 represents the difference in SBP between the Y0 and Y2 values. Average real variability (ARV) is calculated as (Δ1+Δ2+Δ3+Δ4+Δ5+Δ6+Δ7)/7. The mean BP between successive BP measurements is shown as M1−M7. Cumulative exposure to BP was calculated as (M1×2year+M2×3year+M3×2year+M4×3year+M5×5year+M6×5year+M7×5year), and is shown by the dotted area, representing in mmHg×years. Maximum and minimum BP difference (MMD) was calculated as maximum BP minus minimum BP over the entire follow-up. Average BP and standard deviation (SD) were calculated from all 8 BP values from Y0 to Y25 for each individual, and coefficient of variation was calculated as SD/average BP.
Data on other factors including education, physical activity, and laboratory values were collected using standardized protocols and quality control across study centers and examinations (see Supplementary Data).
Cognitive function assessment
A battery of standardized tests to measure cognitive function was performed at the Y25 examination.14 The Digit Symbol Substitution Test (DSST), a subtest of the Wechsler Adult Intelligence Scale (third edition), assesses an array of cognitive domains, most prominently visual motor speed, sustained attention, and working memory. The range of scores is 0 to 133, with increasing scores indicating better performance. The Rey Auditory Verbal Learning Test (RAVLT) assesses the ability to memorize and to retrieve words (verbal memory) after several presentations of the word list immediately one after another, and then after a delay of 10 minutes. Results from the long-delay (10 minutes) free recall were used in analyses. The range of scores is 0 to 15, with increasing scores indicating better performance. The Stroop test evaluates the ability to view complex visual stimuli and to respond to one stimulus dimension while suppressing the response to another dimension, an executive skill largely attributed to frontal lobe function.15 The interference score provides a measure of how much additional executive processing is needed to respond to an incongruent trial; thus, a higher interference score indicates worse performance on the task. Each trial was scored by summing the number of errors and the time required to complete each trial. An interference score was calculated by subtracting the score on the incongruent trial from the second congruent trial.
Statistical Analysis
Statistical analyses were performed using SAS software version 9.3 (SAS Institute Inc, Cary, NC). The associations between individual variables were calculated by Pearson’s correlation method adjusted by age, sex, and race. To show the distribution of BPV over all participants, the range of each BPV index was calculated by decile. Differences in cognitive function scores among the decile groups were assessed using analysis of covariance with adjustment for age, sex, race, educational attainment, and study site. Unadjusted and multivariable-adjusted linear regression models were used to assess the association of BPV with cognitive function. In the first step, we carried out unadjusted analyses (Model 1). In the second step, we added age (Y0), sex, race, educational attainment (years), and study center as adjustment covariates (Model 2). In the last step, we further adjusted for clinical characteristics at Y25 (i.e., body mass index [BMI], smoking, alcohol, physical activity, glucose and lipid parameters, use of antihypertensive drugs, and incidence of stroke) plus BP (Y0) and change of BP (Y25-Y0) (Model 3) or cumulative exposure to BP through Y0 to Y25 (Model 4). These covariates were selected since they have significant correlations with BPV (Supplementary Table S4) or as relevant factors related with cognitive function, shown in previous literature.1 As a sensitivity analysis, BPV was calculated across 6 (Y0–15) or 7 (Y0–20) visits or to assess the effects of exposure to long-term BPV through Y0 to Y15 or to Y20 on cognitive function at Y25. Statistical significance was defined by a P value of < 0.05 on 2-sided tests.
Results
Table 1 provides the demographic and clinical characteristics at Y0 and Y25 of the included participants. For the 2,326 participants, mean SBP and DBP across 8 visits were 110.8±9.7 mmHg (range=86.1–157.0) and 70.5±7.3 mmHg (range=50.5–99.5), respectively. The mean and range of each BPV index stratified by deciles is shown in Supplementary Table S3. Table S4 shows the associations between each BPV index and clinical characteristics adjusted for age, sex, and race.
Table 1.
Clinical characteristics of study cohort (n=2,326)
| Descriptive variable | Baseline (Y0) | Follow-up time (Y25) |
|---|---|---|
| Age, years | 25.3±3.5 | 50.4±3.6 |
| Men, % | 43.3 | - |
| Blacks, % | 40.2 | - |
| Education, years | 14.3±2.2 | - |
| Body mass index, kg/m2 | 24.4±4.7 | 29.9±7.1 |
| Current smoker, % | 5.7 | 1.8 |
| Current drinker, % | 61.5 | 56.3 |
| Physical activity, exercise units | 424.7±296.9 | 345.5±274.4 |
| Antihypertensive medication, % | 1.9 | 25.4 |
| SBP, mmHg | 109.8±10.8 | 118.8±15.8 |
| DBP, mmHg | 68.5±9.2 | 74.1±11.1 |
| Fasting glucose, mg/dL | 82.1±11.4 | 99.3±28.7 |
| Total cholesterol, mg/dL | 177.7±32.8 | 192.4±36.2 |
| High-density lipoprotein, mg/dL | 53.3±12.6 | 58.1±17.5 |
Data are expressed as the means ± SD or percentage. SBP indicates systolic blood pressure; DBP, diastolic blood pressure.
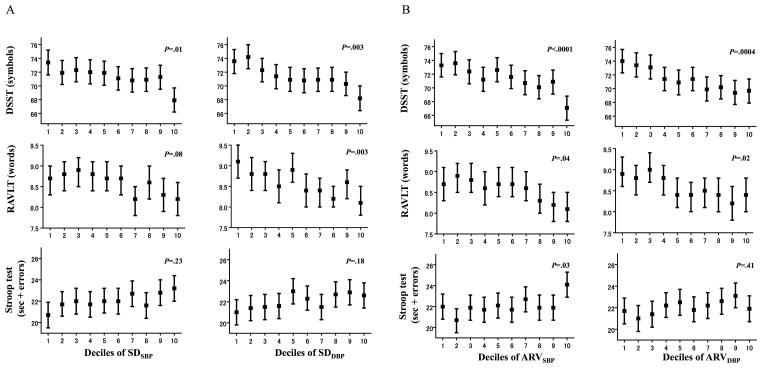
Among participants, mean scores on the DSST, RAVLT, and Stroop test were 71.4±15.7 symbols (range=8–125), 8.6±3.2 words (range=0–15), and 22.1±10.1 sec+errors (range=−2 to 127), respectively. Figure 2 shows mean (95% confidence intervals) scores of cognitive function test with adjustment for age, sex, race, education, and study site in deciles of SDBP and ARVBP. Higher SDBP and ARVBP were associated with lower scores of DSST and RAVLT but not with Stroop test (Figure 2A and 2B).
Figure 2. Scores of cognitive function test in deciles of SDBP (.
Figure 2A) and ARVBP (Figure 2B)
Bars represent means (95% confidence intervals) with adjustment for age, sex, race, education, and study site. P values were calculated by analysis of covariance.
Table 2 shows linear regression models to examine the associations of SDBP and ARVBP with cognitive function. Higher SDBP and ARVBP were associated with lower DSST and RAVLT scores, and with higher Stroop score (Model 1 in Table 2). Adjustment for the demographic variables attenuated the associations (Model 2), but SDDBP, ARVSBP, and ARVDBP remained significantly associated with DSST and RAVLT scores, with adjustment also for clinical characteristics at Y25, and BP (Y0) and change of BP (Y25-Y0) (Model 3) or cumulative exposure to BP (Y0–25) (Model 4). When we used average BP (Y0–25) instead of cumulative exposure to BP (Y0–25) as adjustment factors, results were similar (data not shown). When we used pulse pressure, defined as SBP minus DBP at baseline or Y25, and clinical characteristics at Y0 instead of those at Y25 as adjusted factors, results were unchanged (Supplementary Table S5 and S6). Neither SDBP nor ARVBP was associated with the Stroop score in adjusted models. In Model 4, the standardized β values for age ranged between −0.033 and −0.038 for the DSST (all P<.001) and between −0.007 and −0.009 for the RAVLT (P=NS). When we used CVBP and MMDBP instead of SDBP as primary exposures, results were similar (Supplementary Table S7).
Table 2.
Unadjusted and multivariable-adjusted linear regression models to examine the associations of long-term BPV throughout young adulthood with cognitive function in midlife (n=2,326)
| Model 1 (Unadjusted) | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Variables | β(SE) | R2,% | β(SE) | R2,% | β(SE) | R2,% | β(SE) | R2,% |
| DSST (symbols) | ||||||||
| SDSBP | −.047(.005)§ | 3.9 | −.022(.004)§ | 27.4 | −.022(.006)‡ | 29.1 | −.017(.005)† | 29.1 |
| SDDBP | −.068(.007)§ | 4.1 | −.034(.006)§ | 27.5 | −.030(.008)§ | 29.1 | −.029(.007)§ | 29.2 |
| ARVSBP | −.064(.006)§ | 5.0 | −.033(.005)§ | 27.8 | −.026(.006)§ | 29.2 | −.025(.006)§ | 29.3 |
| ARVDBP | −.067(.007)§ | 3.6 | −.034(.006)§ | 27.5 | −.028(.007)§ | 29.2 | −.029(.007)§ | 29.2 |
| RAVLT (words) | ||||||||
| SDSBP | −.029(.005)§ | 1.5 | −.013(.005)† | 21.4 | −.010(.007) | 21.9 | −.008(.005) | 21.9 |
| SDDBP | −.056(.007)§ | 2.7 | −.024(.007)‡ | 21.6 | −.018(.008)* | 22.1 | −.019(.007)† | 22.1 |
| ARVSBP | −.044(.006)§ | 2.3 | −.021(.005)§ | 21.7 | −.016(.006)* | 22.0 | −.016(.006)* | 22.1 |
| ARVDBP | −.055(.007)§ | 2.4 | −.026(.007)‡ | 21.6 | −.020(.007)† | 22.2 | −.021(.007)† | 22.2 |
| Stroop test (sec + errors) | ||||||||
| SDSBP | .042(.005)§ | 3.2 | .013(.005)† | 17.9 | .002(.007) | 18.7 | .004(.005) | 18.7 |
| SDDBP | .051(.007)§ | 2.3 | .013(.007) | 17.8 | .004(.008) | 18.5 | .004(.007) | 18.5 |
| ARVSBP | .045(.006)§ | 2.4 | .015(.006)† | 17.9 | .004(.006) | 18.7 | .004(.006) | 18.7 |
| ARVDBP | .043(.007)§ | 1.5 | .011(.007) | 17.7 | .004(.007) | 18.5 | .004(.007) | 18.5 |
β means standardized regression coefficient, and R2 means a measure for the model prediction. As adjustment factors, model 2 includes demographic variables (age at baseline, sex, race, education, and study center), model 3 includes demographic variables+clinical characteristics at Y25 (BMI, smoking, alcohol, physical activity, fasting glucose, total cholesterol/HDL, use of antihypertensive drugs, incidence of stroke)+BP (Y0) and change of BP (Y25-Y0), and model 4 included demographic variables+clinical characteristics at Y25+cumulative exposure to BP (Y0–25). DSST indicates digit symbol substitution test; RAVLT, rey auditory verbal learning test; SD, standard deviation; ARV, average real variability. Statistical significance was defined as P <.05.
P<.05;
P<.01;
P<.001;
P<.0001.
Overall patterns of the associations between measures of BPV and cognitive function were similar between blacks and whites (Supplementary Table S8). There was one significant interaction between race and SDSBP in the model for DSST (P<.05); the association was moderately stronger in blacks than whites, but the direction of the association was the same.
When participants who had antihypertensive drugs at Y25 or incident stroke during follow-up (n=595) were excluded, the significant association of SDBP and ARVBP with cognitive function remained similar (Supplementary Table S9). There were no significant interactions between SDBP or ARVBP and antihypertensive medication use in association with cognitive function (all P=NS). When BPV was calculated across 6 (Y0–15) or 7 (Y0–20) visits, the results were mostly similar (Supplementary Table S10 and S11).
Discussion
The main findings here are that higher long-term BPV from young adulthood to midlife (from 25 to 50 years old) was associated with worse psychomotor speed (as measured by the DSST) and verbal memory (as measured by the RAVLT) in midlife, independent of cumulative exposure to BP during follow-up.
At least three studies show longitudinal associations between long-term BPV and cognitive function.7–9 Sabayan et al. reported that, among 5,461 elderly (mean age 75 years and 44% had vascular disease at baseline), higher SDSBP and SDDBP over 3 years of follow-up (13 visits) were associated with poor performance in selective attention, processing speed, immediate verbal memory, and delayed memory.7 Alpérovitch et al. showed that, among 6,506 elderly (mean age 74 years and 80 % were hypertensive patients), higher CVSBP and CVDBP over 3 years of follow-up (3 visits) were associated with higher risk for dementia.8 Matsumoto et al. suggested that, among 485 participants (mean age 63 years; 31% on antihypertensive medications), higher SDSBP in day-to-day BP measured at home over 8 years of follow-up was associated with higher risk for cognitive decline. All studies were conducted among middle-aged/older persons and/or high-risk populations,7–9 raising a possibility that BPV might be affected by comorbidities. For example, even in studies of the stroke-free elderly, silent cerebral infarction has been identified in 20–30% and white matter disease in 80%,16 which could cause both higher BPV and cognitive dysfunction.17,18 Since CARDIA enrolled healthy young adults with very few comorbidities, our findings complement and strengthen the longitudinal association of long-term BPV with cognitive function. When we focused on the effects of exposure to long-term BPV through Y0 to Y20 on cognitive function at Y25, avoiding the inclusion of late assessments of BP at the end of follow-up that already might have been affected by atherosclerotic changes, the conclusions were similar (Supplementary Table S10).
The contributors to short-term BPV include baroreflex and autonomic function as well as responses to environmental and behavioral stimuli, whereas the factors contributing to long-term BPV, particularly in younger adults, remain uncertain.5,6 Chen et al. suggested that lower birth weight was related to higher long-term BPV from childhood to adulthoods; higher sympathetic nervous activity could in part account for the phenomenon.19,20 Behavioral factors and large and small artery stiffening associated with aging may also contribute to long-term BPV.5,6 We found that less educational attainment, higher BMI, and less physical activity were associated with higher long-term BPV. Further clinical implications depend on whether lifestyle interventions in young adults can stabilize long-term BPV throughout young adulthood, and whether such interventions can prevent age-related cognitive decline. Inconsistent BP control by non-adherence and insufficient efficacy of antihypertensive medications are other important contributors to long-term BPV.5 Exclusion of those who used antihypertensive medications at follow-up did not change the association between long-term BPV and cognition (Supplementary Table S9).
We found significant associations of BPV with RAVLT and DSST scores but not with Stroop test scores. The RAVLT and DSST reflect functions of hippocampal neurons.21 Hippocampal neurons are highly vulnerable to disturbances of the cerebral circulation by systemic vascular disease.22 Higher long-term BPV with chronic occurrence of periods of higher and lower BP levels could enhance cerebrovascular damage and/or cerebral hypoperfusion,23 which may result in neuronal injury particularly at the vulnerable area.7 It remains unclear, however, whether such BP fluctuations can overcome the cerebrovascular autoregulation in younger adults. Another explanation is that there may be a common etiology between higher BPV and lower cognitive function. Both BPV and hippocampal neurons are susceptible to hyperactivation of the hypothalamic-pituitary-adrenal axis or hypoxia.5,6,22 Adverse stressors (e.g., psychosocial stress, sleep deprivation, and minor illness) and sleep-disordered breathing are uncontrolled confounders in this study.
Apparent differences in the clinical implications of ARVBP and SDBP were not found, but we noted that ARVBP was more consistently associated than SDBP with RAVLT and DSST scores (Table 2). ARVBP was shown to be a better predictor of cardiovascular outcomes than SDBP and CVBP,5,6,13 while the differences in relation to cognitive function have been less frequently examined.7–9 To identify which BPV indices best identify those with a potential risk of lower cognitive function, further investigations comparing the effects of different BPV indices on cognitive function are warranted.
The associations of BPV with cognitive function were similar between blacks and whites, but one significant interaction of SDSBP with DSST was observed (Supplementary Table S8). No studies have assessed the influence of BPV on cognitive function in racially diverse populations. Blacks show higher BPV compared to whites,24 and therefore it is worthwhile to further examine whether the impact of BPV on cognitive function differs by race.
The major strengths of this study include the study cohort of healthy young adults, application of a comprehensive standardized cognitive test battery, and identical numbers and equal interval of BP measurements by individual. However, there are limitations. First, we could not assess changes in cognitive function from baseline to follow-up, and we cannot conclude whether the low cognitive function scores reflect cognitive decline. Further CARDIA examinations including cognitive function would enable us to explore the time course of cognitive function in participants with higher long-term BPV. Second, a number of people from the original cohort were not included in the present analysis (55%). There is a potential bias in the study participants included for the analysis; less educational attainment and higher SBP at baseline were observed in those not included in this study, suggesting that the included participants might have a lower risk of cognitive dysfunction than those not included. This might have led us to underestimate the true association between BPV and cognitive function. Third, the shift from auscultatory to oscillometric BP readings might affect and bias the assessment of BPV over time. A calibration study was performed, which showed no machine bias, and a sensitivity analysis by BPV across 6 visits (Y0–15) showed similar results (Supplementary Table S11). Fourth, the associations between BPV and cognitive function were significant, but the effect sizes were small. The effect sizes were relatively similar to those associated with age, however. Last, our sample consists of blacks and whites, thus our findings cannot be generalized to other race/ethnic group.
Perspective
The present study provided a clinical implication of BPV throughout young adulthood on cognitive function in midlife. Our results emphasize the importance of focusing not only on BP values alone but also on visit-to-visit BPV to identify younger adults who may be at risk for developing lower cognitive function later. Replication in different studies/cohorts is warranted. Additional follow-up in CARDIA will help to determine the influence of BPV beginning in young adulthood on aging-related cognitive decline and dementia through older age. Such evidence may help to direct potential strategies for preventing lower cognitive function in those middle-aged or over.
Supplementary Material
Novelty and Significance.
What Is New
Higher long-term BP variability throughout young adulthood is associated with lower cognitive function in midlife.
What Is Relevant?
Longitudinal associations between long-term BP variability and cognitive function have been reported in the elderly and/or high-risk populations.
The effects of long-term BPV throughout young adulthood on cognition function in midlife remain uncertain.
Summary
Long-term BPV over 25 years beginning in young adulthood was associated with worse psychomotor speed and verbal memory tests in midlife, independent of cumulative exposure to BP during follow-up.
Acknowledgments
Funding/Support: The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the CARDIA Data Analysis & Publications and Presentations Policies. University of Alabama at Birmingham (HHSN268201300025C & HHSN268201300026C), Northwestern University (HHSN268201300027C), University of Minnesota (HHSN268201300028C), Kaiser Foundation Research Institute (HHSN268201300029C), and Johns Hopkins University School of Medicine (HHSN268200900041C). CARDIA is also partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intra-agency agreement between NIA and NHLBI (AG0005). This manuscript has been reviewed by CARDIA for scientific content.
Footnotes
Conflict of Interest Disclosures: No other author reported disclosures.
References
- 1.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maillard P, Seshadri S, Beiser A, Himali JJ, Au R, Fletcher E, Carmichael O, Wolf PA, DeCarli C. Effects of systolic blood pressure on white-matter integrity in young adults in the Framingham Heart Study: a cross-sectional study. Lancet Neurol. 2012;11:1039–1047. doi: 10.1016/S1474-4422(12)70241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. JAMA. 1995;274:1846–1851. [PubMed] [Google Scholar]
- 4.Tzourio C, Laurent S, Debette S. Is hypertension associated with an accelerated aging of the brain? Hypertension. 2014;63:894–903. doi: 10.1161/HYPERTENSIONAHA.113.00147. [DOI] [PubMed] [Google Scholar]
- 5.Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood-pressure variability. Nat Rev Cardiol. 2013;10:143–155. doi: 10.1038/nrcardio.2013.1. [DOI] [PubMed] [Google Scholar]
- 6.Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375:938–948. doi: 10.1016/S0140-6736(10)60309-1. [DOI] [PubMed] [Google Scholar]
- 7.Sabayan B, Wijsman LW, Foster-Dingley JC, Stott DJ, Ford I, Buckley BM, Sattar N, Jukema JW, van Osch MJ, van der Grond J, van Buchem MA, Westendorp RG, de Craen AJ, Mooijaart SP. Association of visit-to-visit variability in blood pressure with cognitive function in old age: prospective cohort study. BMJ. 2013;347:f4600. doi: 10.1136/bmj.f4600. [DOI] [PubMed] [Google Scholar]
- 8.Alpérovitch A, Blachier M, Soumaré A, Ritchie K, Dartigues JF, Richard-Harston S, Tzourio C. Blood pressure variability and risk of dementia in an elderly cohort, the Three-City Study. Alzheimers Dement. 2013 Aug 14; doi: 10.1016/j.jalz.2013.05.1777. pii: S1552-5260(13)02474-6. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto A, Satoh M, Kikuya M, et al. Day-to-Day Variability in Home Blood Pressure Is Associated With Cognitive Decline: The Ohasama Study. Hypertension. 2014;63:1333–1338. doi: 10.1161/HYPERTENSIONAHA.113.01819. [DOI] [PubMed] [Google Scholar]
- 10.Hughes GH, Cutter G, Donahue R, et al. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (Cardia) Study. Control Clin Trials. 1987;8(4 Suppl):68S–73S. doi: 10.1016/0197-2456(87)90008-0. [DOI] [PubMed] [Google Scholar]
- 11.National Heart Lung and Blood Institute. [Accessed July 1, 2014.];Coronary Artery Risk Develop ment in Young Adults (CARDIA) study manuals of operation. 1985 http://www.cardia.dopm.uab.edu/exam-materials2/manual-of-operations/year-0.
- 12.Jacobs DR, Jr, Yatsuya H, Hearst MO, Thyagarajan B, Kalhan R, Rosenberg S, Smith LJ, Barr RG, Duprez DA. Rate of decline of forced vital capacity predicts future arterial hypertension: the Coronary Artery Risk Development in Young Adults Study. Hypertension. 2012;59:219–225. doi: 10.1161/HYPERTENSIONAHA.111.184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hastie CE, Jeemon P, Coleman H, McCallum L, Patel R, Dawson J, Sloan W, Meredith P, Jones GC, Muir S, Walters M, Dominiczak AF, Morrison D, McInnes GT, Padmanabhan S. Long-term and ultra long-term blood pressure variability during follow-up and mortality in 14 522 patients with hypertension. Hypertension. 2013;62:698–705. doi: 10.1161/HYPERTENSIONAHA.113.01343. [DOI] [PubMed] [Google Scholar]
- 14.Reis JP, Loria CM, Launer LJ, Sidney S, Liu K, Jacobs DR, Jr, Zhu N, Lloyd-Jones DM, He K, Yaffe K. Cardiovascular health through young adulthood and cognitive functioning in midlife. Ann Neurol. 2013;73:170–179. doi: 10.1002/ana.23836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- 16.Price TR, Manolio TA, Kronmal RA, Kittner SJ, Yue MC, Robbins J, Anton-Culver H, O’Leary DH. Silent brain infarction on magnetic resonance imaging and neurological abnormalities in community-dwelling older adults: the Cardiovascular Health Study. Stroke. 1997;28:1158–1164. doi: 10.1161/01.str.28.6.1158. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein IB, Bartzokis G, Hance DB, Shapiro D. Relationship between blood pressure and subcortical lesions in healthy elderly people. Stroke. 1998;29:765–772. doi: 10.1161/01.str.29.4.765. [DOI] [PubMed] [Google Scholar]
- 18.Brown JP, Sollers JJ, 3rd, Thayer JF, Zonderman AB, Waldstein SR. Blood pressure reactivity and cognitive function in the Baltimore Longitudinal Study of Aging. Health Psychol. 2009;28:641–646. doi: 10.1037/a0015215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen W, Srinivasan SR, Yao L, Li S, Dasmahapatra P, Fernandez C, Xu J, Berenson GS. Low birth weight is associated with higher blood pressure variability from childhood to young adulthood: the Bogalusa Heart Study. Am J Epidemiol. 2012;176 (Suppl 7):S99–S105. doi: 10.1093/aje/kws298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen W, Srinivasan SR, Hallman DM, Berenson GS. The relationship between birthweight and longitudinal changes of blood pressure is modulated by beta-adrenergic receptor genes: the Bogalusa Heart Study. J Biomed Biotechnol. 2010;2010:543514. doi: 10.1155/2010/543514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raji CA, Lopez OL, Kuller LH, Carmichael OT, Longstreth WT, Jr, Gach HM, Boardman J, Bernick CB, Thompson PM, Becker JT. White matter lesions and brain gray matter volume in cognitively normal elders. Neurobiol Aging. 2012;33:834.e7–e16. doi: 10.1016/j.neurobiolaging.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalaria RN. Cerebrovascular disease and mechanisms of cognitive impairment: evidence from clinicopathological studies in humans. Stroke. 2012;43:2526–2534. doi: 10.1161/STROKEAHA.112.655803. [DOI] [PubMed] [Google Scholar]
- 23.Havlik RJ, Foley DJ, Sayer B, Masaki K, White L, Launer LJ. Variability in midlife systolic blood pressure is related to late-life brain white matter lesions: the Honolulu-Asia Aging study. Stroke. 2002;33:26–30. doi: 10.1161/hs0102.101890. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Snieder H, Su S, Harshfield GA, Treiber FA, Wang X. A longitudinal study of blood pressure variability in African-American and European American youth. J Hypertens. 2010;28:715–722. doi: 10.1097/HJH.0b013e328336ed5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.