Abstract
Background
Medium chain triglycerides (MCT) enhance thermogenesis and may reduce food intake relative to long chain triglycerides (LCT). The goal of this study was to establish the effects of MCT on appetite and food intake and determine whether differences were due to differences in hormone concentrations.
Methods
Two randomized, crossover studies were conducted in which overweight men consumed 20 g of MCT or corn oil (LCT) at breakfast. Blood samples were obtained over 3 h. In Study 1 (n=10), an ad lib lunch was served after 3 h. In Study 2 (n=7), a pre-load containing 10 g of test oil was given at 3 h and lunch was served 1 h later. Linear mixed model analyses were performed to determine the effects of MCT and LCT oil on change in hormones and metabolites from fasting, adjusting for body weight. Correlations were computed between differences in hormones just before the test meals and differences in intakes after the two oils for Study 1 only.
Results
Food intake at the lunch test meal after the MCT pre-load (Study 2) was (mean ± SEM) 532 ± 389 kcal vs. 804 ± 486 kcal after LCT (P < 0.05). MCT consumption resulted in a lower rise in triglycerides (P = 0.014) and glucose (P = 0.066) and a higher rise in peptide YY (P = 0.017) and leptin (P = 0.036) compared to LCT (combined data). Correlations between differences in hormone levels (GLP-1, PYY) and differences in food intake were in the opposite direction to expectations.
Conclusions
MCT consumption reduced food intake acutely but this does not seem to be mediated by changes in GLP-1, PYY, and insulin.
Keywords: Dietary fat, medium chain triglycerides, appetite, food intake, peptide YY, ghrelin, triglycerides, insulin, glucagon-like peptide 1
INTRODUCTION
Functional foods, “those foods that encompass potentially healthful products including any modified food or ingredient that may provide a health benefit beyond the traditional nutrients it contains”(ref. 1), have been suggested to provide benefits for weight management (ref. 2) via decreased lipid storage and uptake, enhanced rates of fat oxidation, and increased satiety (ref. 3, 4, 5). One functional food that has been proposed to act on both energy expenditure and energy intake is medium chain triglycerides (MCT). MCT bypass chylomicron incorporation for lymphatic transportation, providing the liver with a ready supply of energy and reducing peripheral fat deposition into adipose tissue (ref. 6,7). In humans, MCT consumption enhances reductions in adiposity (ref. 8,9).
Ex vivo studies have shown that the rate of medium chain fatty acid oxidation is 10-fold faster than that of long chain fatty acids (ref. 10). Indeed, MCT consumption produces a greater thermic effect when compared to LCT(ref. 11, 12, 13, 14, 15, 16) and promotes satiety in animal models (ref. 17, 18) and humans (ref. 19, 20). Considering these effects of MCT consumption, it has been hypothesized that substituting MCT oil for LCT oils could potentially be used as an adjunct in weight-loss programs (ref. 21).
While an abundance of research on MCT’s effects on energy expenditure and body composition is available (ref. 16, 21), their role in modulating food intake has not been extensively studied (ref. 8, 20, 22) beyond their effect on cholecystokin (CCK). Studies have shown that long chain fatty acids, but not medium chain fatty acids, stimulate CCK release to reduce food intake (ref. 23). However, a study by Drewe et al. (ref. 24) does not support the role of endogenous CCK as being responsible for the food intake reduction after LCT infusion. One study showed that both MCT and LCT stimulated the release of peptide YY (PYY), when infused intraduodenally, but that LCT did so to a greater extent (ref. 25). The authors suggested that the greater effect of LCT may be due to its stimulation of CCK release, which then stimulates PYY release. Of note is that those studies have used fat duodenal infusions rather than oral intakes. In this study, we chose to provide oils as would be consumed in a typical diet.
The main purpose of this study was to determine whether (1) MCT consumption suppresses food intake relative to LCT; (2) MCT induces a profile of gut hormone responses indicating increased satiety/reduced appetite signaling relative to LCT; and (3) whether the hormonal response to MCT is related to the differences in food intake after the consumption of MCT or LCT. We hypothesized (1) lower food intake after a pre-load high in MCT compared to LCT; (2) lower circulating levels of ghrelin and higher circulating levels of peptide YY (PYY) and glucagon-like peptide 1 (GLP-1) after MCT consumption relative to LCT; and (3) that the effect of MCT on hormones would be related to the difference in food intake observed after the consumption of either MCT or LCT. Gibbons et al. have reported that post-meal levels of ghrelin and GLP-1 were correlated with food intake at an ad libitum meal 3 h later (ref. 26).
PARTICIPANTS AND METHODS
Study Participants
Adult men, age 19–50 y, with a BMI of 25–29.9 kg/m2 were recruited to participate in 2 separate studies (Study 1, n=10; Study 2, n=7) during the summers of 2009 and 2010. Men were recruited from the Columbia University/St. Luke’s-Roosevelt Hospital area (New York, NY) via flyers and online advertisements. Smokers and those with recent weight change (>10 lbs in the previous 3 mo), excessive caffeine use (>6 caffeinated beverages/d), severe chronic health conditions, allergies to any of the food products or ingredients provided in this study or taking medications known to affect energy expenditure or gastrointestinal function were excluded from the study. These studies were approved by the St. Luke’s-Roosevelt Hospital Institutional Review Board and all participants provided informed consent prior to the start of the study.
Protocol details
Both studies employed a 2-arm, randomized, single-blind, cross-over design with each arm consisting of one test day differing in the type of oil incorporated in the breakfast: MCT oil (Neobee 1053, Stepan Company, Northfield, IL) or corn oil (LCT, Mazola, ACH Food Companies, Cordova, TN). A random digit table was used to determine test oil sequence. The random allocation sequence and participant enrollment were determined by a study investigator. Test days were held at least 3 d, but no more than 14 d, apart.
For 2 d prior to test day, participants were asked to refrain from alcohol consumption. In addition, they were asked not to participate in any structured exercise the day before each test day and to record their food intake at dinner and consume the same meal on the night prior to their second test day. They were also instructed to drink approximately 1.9 L of water the day before each test day to ensure proper hydration. These precautions were implemented to ensure a greater degree of consistency between test periods. Participants were asked to fast overnight for 12 h prior to testing.
Each test day was performed at the St. Luke’s/Roosevelt Hospital Outpatient Clinical Research Resource of the Irving Center for Translational Research (Columbia University, New York) in the morning. Upon arrival, anthropometric measurements were taken and a catheter was inserted in an antecubital vein for frequent blood sampling. Participants then consumed the breakfast meal containing 20g of oil over a 10-min period. Immediately before and at fixed time points after breakfast, blood samples were drawn from the catheter for hormone and metabolite measurements. At the end of the 3 h blood sampling protocol, participants from Study 1 were served an ad libitum single item lunch test meal. Participants from Study 2 were given a pre-load containing 10 g of the test oil, followed 1 h later by the ad libitum single item lunch. For the ad libitum lunch test meal, men were instructed to eat as much as they wanted until they were satisfied. Total food intake was measured by weighing the food portion before and after lunch.
Test Breakfast meals and pre-load
Breakfast meals differed in nutrient composition between Study 1 and Study 2 (Table 1) but contained the same amount of test oil and were consumed within 10 min in both studies. Participants in Study 1 received a muffin (20 g of test oil) and 5 oz (148 mL) of orange juice. Muffins were made from 71.5 g of fat-free muffin mix, either raisin bran or spice apple bran (Bob’s Red Mill, Milwaukie, OR). Participants in Study 2 received a liquid meal replacement (14 oz of Boost®, Nestle Healthcare Nutrition, Fremont, MI) to which 20 g of test oil was added. This dose of oil was used since we have previously showed that consumption of 18–24 g/d of MCT oil enhances weight loss relative to LCT (ref. 27) and Dulloo et al. have shown that 15–30 g/d of MCT raise 24-h energy expenditure relative to LCT (ref. 11). The oils did not differ in taste. In Study 1, participants were not given instructions on how to consume the breakfast and the order of intake of the juice and muffin may have differed between participants and between test days. This may have affected transit time and hormone release. The breakfast meal for Study 2 was switched to a liquid meal to ensure greater consistency in gastric emptying time and a more uniform consumption and nutrient absorption pattern.
Table 1.
Characteristics of study meals.
| Meal Characteristics | Study 1 | Study 2 |
|---|---|---|
| Breakfast Meal | Muffin with orange juice | Boost® shake with test oil |
| Total Energy (kJ) | 2 671 | 2 510 |
| Carbohydrate (g) | 105 | 71.6 |
| Protein (g) | 9.6 | 17.5 |
| Fat (g) | 20 | 27 |
| Test oil (g) | 20 | 20 |
| Pre-Load | n/a | Yogurt with 10 g test oil added |
| Total Energy (kJ) | 728 | |
| Carbohydrate (g) | 16 | |
| Protein (g) | 5 | |
| Fat (g) | 10 | |
| Test oil (g) | 10 | |
| Lunch meal | Stouffer’s macaroni and beef | Trader Joe’s Penne Arrabiatta |
| Total Energy (kJ) | 5 146 | 4971 |
| Carbohydrate (g) | 135 | 174 |
| Protein (g) | 66 | 42 |
| Fat (g) | 48 | 36 |
Participants in Study 2 were given a preload of yogurt (Dannon Light & Fit yogurt, Dannon, All entown, PA; Table 1) containing 10 g of the test oil 3 h after breakfast consumption. Provision of a pre-load is commonly done to assess the satiating properties of a food. We chose to provide 711.6 kJ as this is consistent with a snack, which typically provides 795.3–1 172.1 kJ (ref. 28).
Blood sampling protocol
For both Study 1 and 2, blood samples were obtained in the fasted state immediately before (−15 min) and after test breakfast consumption (0 min) and at 30, 45, 60, 120, and 180 min. Study 2 participants provided additional samples at 15, 75, 90, and 150 min. This 3-h sampling period has been used by others to assess the impact of meal nutrient composition on appetite-related hormones (ref. 29, 30,31). Samples were collected in EDTA-coated chilled tubes for the measurement of gut hormones. Tubes were pre-treated with a protinin (0.6 TIU per mL of blood) and dipeptidyl peptidase 4 inhibitor (10 μL per mL of blood, for GLP-1 and total PYY assay only) to prevent degradation of gut hormones. Upon collection, blood was immediately placed on ice. Plasma was separated within 60 to 180 min of collection via centrifugation for 20 min at 4 °C. Samples to be analyzed for active ghrelin were acidified with 50 μL of 1N HCl and then frozen at −80 °C until assayed.
All hormone analyses were performed in duplicate in the Hormone and Metabolite Core Laboratory of the New York Obesity Nutrition Research Center. Glucose measurements were performed with a glucose analyzer (Analox Instruments USA Inc, Lunenburg, MA). Insulin and total and active ghrelin were assayed using RIA (EMD Millipore, Billerica, MA). Serum leptin was measured in duplicate aliquots using a double-antibody RIA (Linco Research Products Inc., St. Charles, MO). Total PYY was determined according to Millipore procedure using an antibody that recognizes both 1–36 and 3–36 forms of human PYY. Total GLP-1 was measured by RIA (Phoenix Pharmaceutical, Belmont, CA) after plasma extraction with 95% ethanol. This assay is 100% specific for GLP-17-36, GLP-19-36, and GLP-17-37 and does not cross react with glucagon (0.2%), GLP-2 (<0.001%), or exend in (< 0.0.1%). Triglyceride (TG) levels were assessed using an Ektachem DT II System (Johnson and Johnson Clinical Diagnostics, Rochester, NY) with appropriate standards and reagents for Study 2 participants only. All other hormones and metabolites were assessed in both studies.
Food intake measurement
At the end of the 3 h testing period, participants in Study 1 were served a one item ad libitum lunch test meal (Stouffer’s macaroni and beef, Nestle USA, Wilkes-Barre, PA). In Study 2, a pre-load was served at 3 hours and the single item ad libitum lunch (Penne Arrabiata, Trader Joe’s, Monrovia, CA) was provided 1 h later. One hour was noted as the time where the difference in hunger ratings was seemingly greatest between MCT and LCT and has been used by others previously (ref. 32). In both studies, participants were served in excess of their predicted energy intakes and instructed by the investigator to eat “as much as you would like of this meal until you are satisfied”. Total food intake at the ad libitum lunch test meal was recorded by weighing the food pre- and post-meal. Water was available to drink with the meal but its intake was not recorded.
Anthropometric measurements
Body weight was measured on a standard balance beam scale to the nearest 0.5 kg with the participant wearing light clothing and without shoes on each test day, in the fasted state. Height was measured using a wall-mounted stadiometer to the nearest 0.5 cm with the participants shoeless. Waist circumference was measured halfway between the lowest rib and the iliac crest using a non-stretchable measuring tape (ref. 33). The average of two measurements was used in the analyses. These measurements were taken in the fasted state when the participant arrived at the laboratory.
Statistical analyses
Appetite and hormone data were analyzed using a linear mixed model analysis using R software (http://cran.r-project.org) and SAS (version 9.2, SAS Institute, Cary, NC). Each response measure was tested using a likelihood ratio test to determine if log-transformation would significantly improve the normal approximation of the measure. It was established that the normal approximation would not improve due to log-transformation for any of the measures. Normality of our test-statistics (t-test and F-test used in linear mixed model analyses) was ensured as 14 measures each taken from 17 (7 in some cases) participants gave us at least 98, and at most 238, data-points. Although we have performed multiple tests in this study, an adjustment for multiple comparisons was not required because it is only necessary when multiple tests are used for testing a single hypothesis. In this study, each hypothesis was examined using a single test.
Test oil (LCT vs. MCT) was used as a fixed effect and time as a linear variable in hormone data. However, when fasting values were used as response measures, time was not used as an independent variable. Body weight was used as a covariate and subject was treated as a random effect. A test oil x time interaction was included in the models initially but was removed if it was not significant. Data are reported with test oil and time as fixed effects, subject as a random effect, and body weight as a covariate.
Statistical analyses were performed for both studies combined (n = 17) and also for Study 2 (n = 7) separately because the composition of the breakfast meals was too different between studies: liquid and solid in Study 1 vs. liquid only in Study 2. Hormone data from Study 2 are presented in the figures. Food intake data are reported separately for each study as the difference in protocol for this measurement precludes combining data. In Study 1, there was a 3-h time gap between breakfast and the lunch test meal whereas in Study 2, a pre-load was served at 3 h and the lunch test meal was administered 1 h later. Data are presented as means ± SEM. Significance was considered as P< 0.05.
Pearson correlations were performed to assess the relationship between hormone concentration at 180 min after breakfast and food intake at the ad libitum lunch test meal as well as change in hormone/metabolite concentration at 180 min from fasting and food intake at the ad libitum lunch in Study 1. Pearson correlations were also performed to assess the relationship between the difference in hormone concentrations at 180 min between MCT and LCT and the difference in food intake at the ad libitum lunch in Study 1. Study 2 participants were not included in these analyses because they received a pre-load after the 180 min blood draw. Correlations were performed with both test oils combined and separately by test oil.
Results
Twenty men were recruited (Study 1, n = 13; Study 2, n = 7) and 17 completed (Study 1, n = 10; Study 2, n = 7, Table 2) the study. Two participants dropped out after screening because of scheduling conflicts, another dropped out in the middle of the first test day after feeling faint and nauseated following consumption of the MCT-containing breakfast. Another participant (Study 2) reported diarrhea following the MCT test day, but remained in the study. No other side effects were noted.
Table 2.
Characteristics of study participants.
| Characteristics | All Participants | Study 1 | Study 2 |
|---|---|---|---|
| Age (y) | 39.4 ± 1.8 | 39.2 ± 2.7 | 39.6 ± 2.1 |
| Body Weight (kg) | 88.9 ± 2.3 | 87.1 ± 1.7 | 91.9 ± 5.1 |
| Body Mass Index (kg/m2) | 28.2 ±0.3 | 28.1 ±0.6 | 28.4 ±0.5 |
| Height (m) | 1.77 ± 0.02 | 1.76 ± 0.01 | 1.79 ± 0.04 |
| Systolic Blood Pressure (mmHg) | 122 ± 2 | 117 ± 2 | 127 ± 3 |
| Diastolic Blood Pressure (mmHg) | 80 ± 1 | 79 ± 2 | 82 ± 2 |
| Ethnicity (C, AA, H, O) | 5, 8, 2, 2 | 3, 7, 0, 0 | 2, 1, 2, 2 |
Results are means ± SEM, n=17. C = Caucasian, AA = African Americans, O = Other.
Food intake
Food intake at the ad libitum test lunch did not differ by oil type in Study 1 (MCT, 2 548.0 ± 459.6 kJ vs. LCT, 2 773.2 ± 531.6 kJ, P=0.41). In Study 2, when participants received a 711.6-kJ pre-load 1 h before lunch, food intake at lunch was significantly lower during the MCT test day (MCT, 2 227.0 ± 616.2 kJ vs. LCT, 3 369.3 ± 769.0 kJ, P<0.050).
Hormones and metabolites: Absolute change
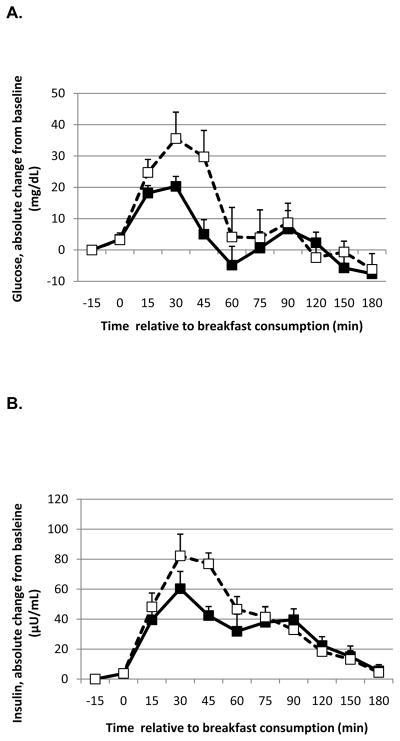
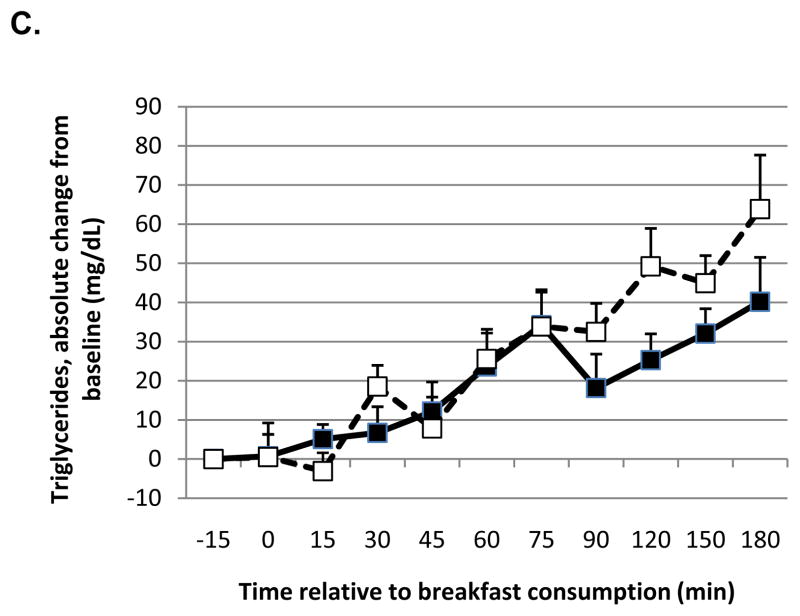
In the combined analyses, there was a significant effect of time on change in glucose concentrations (P=0.025) and a trend for an effect of oil with a lower rise in glucose with MCT consumption compared to LCT (P=0.066) that was significant in Study 2 alone (P=0.0017; Figure 1A). Concurrent with these results, the change in insulin was not affected by oil in the combined analyses (P=0.99) but there was a trend for a lower rise in insulin (effect of oil P=0.13; time P=0.017) when data from Study 2 were analyzed separately (Figure 1B). There was a significant time x oil interaction on TG concentrations (P=0.0046) with a lower rise in TG (Figure 1C) with MCT consumption compared to LCT (Study 2).
Figure 1.
Absolute change from baseline in glucose (A), insulin (B) and triglycerides (C) in response to a meal containing MCT oil (black squares) and LCT oil (open squares) in Study 2. Blood samples were obtained after consumption of a liquid meal containing 20 g of either MCT oil or corn oil (LCT). Data were analyzed using linear mixed model, controlling for body weight. The meal was provided immediately before the time 0 blood draw. There was a significant effect of oil type on glucose (P = 0.0017) and a trend for insulin (P = 0.13). There was a significant effect of time on insulin (P = 0.017) and a time x oil interaction on triglycerides (P = 0.0046). Data represent means ± SEM, n = 7.
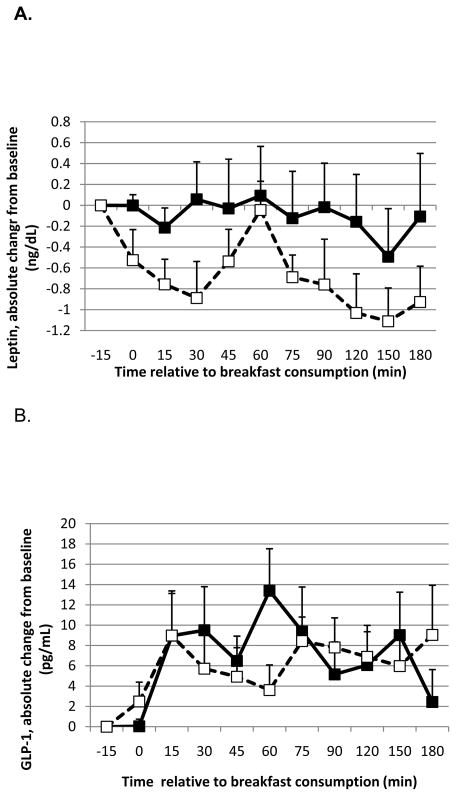
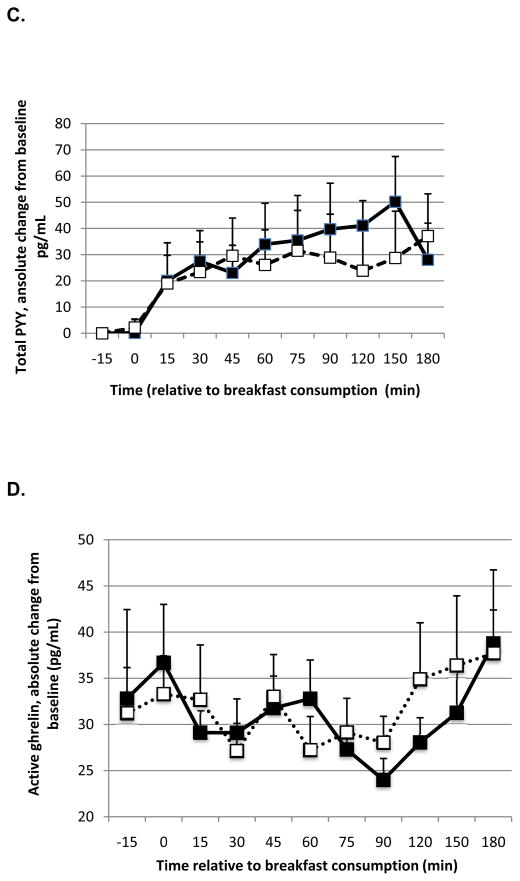
In the combined analyses, leptin levels after the MCT-containing breakfast increased to a greater extent compared to LCT consumption (effect of oil P=0.036; time P=0.62). Active ghrelin concentration after the MCT-containing breakfast was reduced to a lesser extent compared to LCT (effect of oil P=0.0031; time P=0.10), but the effect of oil type on the change in total ghrelin, albeit in the same direction, was not significant (effect of oil P=0.21; time P=0.20). When the analyses were restricted to Study 2, the effect of oil type on changes in leptin was stronger (effect of oil P<0.001; time P=0.038; Figure 2A), ie. leptin remained higher after MCT consumption relative to LCT, whereas the change in active ghrelin was no longer significant (effect of oil P=0.28; time P=0.95).
Figure 2.
Absolute change from baseline for leptin (A), GLP-1 (B), total PYY (C), and active ghrelin (D) in response to a meal containing MCT oil (black squares) and LCT oil (open squares) in Study 2. Data were analyzed using linear mixed model, controlling for body weight. The meal was provided immediately before the time 0 blood draw. There was a significant effect of oil type and time on leptin (P < 0.001 and 0.038, respectively), PYY (P = 0.030 and < 0.0001, respectively) and active ghrelin (P=0.0031; trend for time P=0.10, respectively). There was no effect of oil type on GLP-1 (P = 0.40) but a trend for an effect of time (P = 0.071). Data represent means ± SEM, n = 7.
There was a significant effect of oil (P=0.017) and time (P<0.0001) on total PYY with a greater rise in total PYY post-prandially with MCT oil than LCT (Study 2 alone: effect of oil P=0.030; time P<0.0001; Figure 2B). GLP-1 was not affected by oil type in the combined analysis (P=0.39) or in Study 2 alone (P=0.40; Figure 2C), although there was a significant effect of time in the combined analysis (P=0.0097) but not Study 2 alone (P=0.071).
Online supplementary material provides information on percent change in hormone concentrations from baseline.
Correlation between hormones and food intake at the ad libitum lunch
Food intake at the ad libitum lunch was negatively correlated with leptin concentrations at time 180 min when both test oils were combined (r=−0.46, P=0.037). However, food intake at the ad libitum lunch test meal was positively correlated with GLP-1 (r=0.81, P<0.0001), PYY (r=0.52, P=0.018), and percent change in PYY from fasting (r=0.56, P=0.010). There was a trend for food intake to be correlated with percent change in insulin concentrations (r=0.44, P=0.053). Those correlations indicate that those with lower leptin and higher GLP-1, PYY and change in PYY and insulin from fasting had greater intakes at the ad libitum meal.
When correlations were run separately by oil type, only GLP-1 concentrations were correlated with food intake after the MCT-rich breakfast (r=0.89, P=0.0005), indicating that higher GLP-1 concentrations pre-meal were associated with greater food intake. Intake at the ad libitum lunch following the LCT breakfast was correlated with GLP-1 (r=0.74, P=0.037), percent change in insulin (r=0.66, P=0.038), and percent change in PYY (r=0.78, P=0.0073). Percent change in leptin tended to be inversely correlated with food intake (r=−0.62, P=0.058): a rise in leptin was associated with lower intakes at the ad libitum lunch test meal.
The difference in food intake between MCT and LCT was negatively correlated with the difference in total ghrelin between MCT and LCT immediately before lunch (r=−0.85, P=0.0017). There was also a trend for a positive correlation between the difference in food intake and the difference in insulin concentrations with MCT and LCT breakfast meals (r=0.60, P=0.069). Also, the difference in food intake between MCT and LCT tended to be correlated with the difference in the area under the curve for glucose (r=0.581, P=0.078) and insulin (r=0.59, P=0.073). There was no significant correlation for the difference in leptin, GLP-1, PYY, or ghrelin area under the curve.
Discussion
This report provides results of studies examining the effects of MCT vs. LCT consumption on food intake and a wide range of hormones involved in food intake control, and metabolic risk factors. No previous study has examined the effects of MCT consumption on leptin, ghrelin, PYY, and GLP-1, specifically. We show that food intake is lower after an MCT-rich pre-load compared to an LCT-rich pre-load and that leptin and PYY levels remained higher after MCT consumption compared to LCT. These results suggest that MCT consumption may trigger the release of satiety signals more effectively than LCT. However, correlations between hormone levels and food intake were not in the direction expected. Moreover, GLP-1 concentrations were not affected by the type of oil consumed at breakfast and active ghrelin was reduced to a lesser extent, in the combined analysis, with MCT consumption. In line with these data, MCT is known to be a good substrate for the conversion of ghrelin to active ghrelin, without necessarily affecting total ghrelin concentrations (ref. 34). However, why MCT, which trigger ghrelin acylation via ghrelin O-acyltransferase, a seemingly orexigenic process (ref. 35), would also be associated with weight loss and increased reduction in adiposity (ref. 27) warrants further investigation. We also found that MCT consumption leads to lower post-prandial glucose and TG than LCT consumption.
That differences in appetite-regulating hormones were not related to differences in food intake is puzzling. In fact, the correlation between differences in ghrelin levels and differences in food intake between MCT and LCT was in the opposite direction than expected, as were correlations of food intake with GLP-1 and PYY. Gibbons et al. (ref. 26) have assessed the effects of various macronutrients on ghrelin, GLP-1, and PYY levels and reported significant associations between changes in circulating levels of GLP-1 and ghrelin and food intake at an ad libitum meal, despite no difference in food intake between test diets. Based on this, we would have expected the greater rise in PYY after MCT consumption relative to LCT to be related to lower food intake at the ad libitum meal. On the other hand, van der Klaauw et al. (ref. 36) also found significant differences in PYY and GLP-1 in response to meals varying in protein content with no differences in food intake at a test meal. Other studies examining the role of protein on appetite-regulation have assessed hormone concentrations. Leidy et al. (ref. 37) found lower ghrelin and higher leptin after consumption of a high protein breakfast compared to skipping breakfast but there was no difference when compared to the normal protein breakfast; food intake was not assessed in that study. Ratliff et al. (ref. 31) also found that an egg breakfast led to lower glucose, insulin and ghrelin area under the curve over 3 h post consumption relative to a breakfast consisting of bagel and cream cheese but found no effect of breakfast type on leptin, GLP-1, and PYY. It is important to note, however, that our study was not meant to establish a mechanism of action by which MCT modulation of appetite-regulating hormones could affect satiety and food intake. Prior to conducting this study, the effects of MCT consumption on circulating levels of ghrelin, PYY and GLP-1 were relatively unknown and this study provides some basic information on the effects of MCT on those hormones. Future studies are needed to determine whether changes in these hormones mediate the effects of MCT consumption on food intake.
From the results obtained in this study, we posit that changes in gut hormones might not be the primary modulators of food intake control following MCT consumption. Thermal and oxidative pathways may be a more likely mechanism. In fact, prior research by our group and others has shown that MCT can enhance thermogenesis relative to LCT (ref. 11, 12, 13, 16, 21, 38) and increases in thermic effect of food have been correlated with enhanced satiety (ref. 39). Although the association between substrate oxidation rate and energy intake has not been consistently observed (ref. 40), others have proposed that fatty acid oxidation rate could influence appetite and subsequent food intake (ref. 41,42). It is therefore plausible that the thermogenic- and fat oxidation-enhancing effects of MCT could be involved in appetite regulation, leading to lower energy intakes. This remains to be addressed directly.
The effects of MCT on food intake may be acute, rather than long-acting. In the present study, there was no effect of MCT vs LCT at breakfast on food intake 3 h later (Study 1) whereas food intake 1 h after a pre-load (Study 2) was reduced. It is unfortunate that no blood samples were obtained after the pre-load and prior to the ad libitum lunch test meal in Study 2 to assess correlations between hormones and food intake. Follow-up studies would be needed to perform this measurement and also to test the effects of hormone antagonists on food intake after MCT and LCT-rich breakfasts. This would truly help uncover a mechanism linking hormonal responses to MCT consumption and food intake. For example, PYY, a hormone previously demonstrated to induce satiation when infused, and described as being secreted in proportion to macronutrient intake (ref. 43), was higher after MCT than LCT consumption. Further studies with blockers of PYY receptors in conjunction with MCT consumption would be needed to test whether an increase in PYY could be one mechanism by which MCT induces satiation.
Data from Study 2 demonstrate a lower rise in TG with MCT consumption relative to LCT after the test breakfast. This corroborates data from Maki et al. (ref. 44) who found a lower TG incremental area under the curve over 8 h after consumption of a milkshake containing 30 g of MCT compared to LCT (mix of high oleic safflower oil, canola oil, soy oil, and safflower oil). However, contrary to our results, that study reported higher median blood glucose levels at 2 h after MCT compared to LCT consumption (ref. 44). The difference in blood sampling protocol between our two studies may explain the different results: Maki et al. (ref. 44) sampled blood every 2 h for 8 h following the breakfast meal whereas we had frequent samples starting immediately after the meal. By not sampling blood before 2 h post-prandially, the glucose peak may have been missed. This is evident from our 120 min data point, which would have prompted different conclusions in the present study as well. In addition, Broussolle et al. (ref. 45) noted more pronounced reductions in plasma glucose with a 1:1 intravenous infusion of coconut oil, a rich source of MCT, to soy oil compared to saline or soy oil infusion alone in healthy normal weight men. Future studies should examine the long-term effects of MCT consumption on glucose and lipid profiles.
To date, only two studies have examined the effects of MCT on satiety-modulating hormones such as cholecystokinin (CCK) (ref. 46,47). It has been well established that the regulation of CCK release following consumption of fat is chain length-dependent, with long chain fatty acids exerting a greater effect than short and medium chain fatty acids (ref. 46, 48). An analysis of CCK was not included in the present study and this could be considered a weakness. However, based on prior knowledge of the regulation of CCK release, LCT would have elicited a larger release of CCK than MCT. Measurement of apolipoprotein A-IV, which is secreted in response to dietary fats (ref. 49), may have also been relevant.
Other weaknesses of the present study include its small sample size, the difference in the format of the breakfast meals and lack of provision of a pre-load before the ad libitum lunch test meal in Study 1. Also, although we have exhaustive objective information on food intake and its hormonal controls, we did not take subjective measures of appetite and satiety, which would have provided additional information. Our study may have been under-powered to detect some differences between test oils but this was a pilot study and the data provided can be used as the basis for a larger study in the future. Based on the exploratory nature of this study, a small sample size was warranted to avoid wasting resources and unduly performing research on healthy participants. None of the hypothesis tests should be taken as definitive but rather as indicative of potential effects, subject to confirmation.
Our study has several strengths. We used a crossover design and enrolled only overweight men, reducing the variability of our results. On the other hand, this prevents extrapolation to women or normal weight individuals. Our study included purified MCT oil and provided identical breakfast test meals within each study. Additionally, we obtained frequent blood samples over a 3 h post-prandial period and analyzed a variety of hormones and metabolites that could be involved in the appetite-regulating effect of foods.
The results from the present study suggest that fats differing in fatty acid chain length and saturation level differentially affect the secretion of metabolites and hormones that regulate food intake. However, those differences were not correlated with differences in food intake. These results prompt further research in the mechanism by which MCT consumption could modulate food intake to lead to improved weight management. This report further provides evidence that acute intakes of up to 20 g of MCT do not adversely affect glucose and TG concentrations. The long-term safety, and potentially beneficial, effects of MCT consumption on metabolic risk factors should be examined further.
Supplementary Material
Acknowledgments
We would like to thank all participants for their involvement in this study and Xinyue Tong and Lilly Nhan for their assistance in the conduct of the study for study 1 participants. MPSO, BM, HRK and BL designed the research; MPSO, BM, and MO conducted research; MPSO, BM, and AR analyzed data; MPSO, BM, HRK, BL, and AR wrote the paper; MPSO had primary responsibility for final content. All authors read and approved the final manuscript.
This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1 TR000040, formerly the National Center for Research Resources, Grant Number UL1 RR024156, the New York Obesity Nutrition Research Center Grant Number P30-DK26687. MCT oil was provided by Stepan Company. This trial was registered on Clinicaltrials.gov, identifier NCT01952977.
Footnotes
Supplementary information is available at the journal’s website.
Conflict of interest
Dr. St-Onge is on the Advisory Board of Free life LLC. None of the other authors have any conflicts to disclose.
Literature cited
- 1.Institute of Medicine. Opportunities in the nutrition and food sciences--a new institute of medicine report. Nutr Rev. 1994;52(3):106–9. doi: 10.1111/j.1753-4887.1994.tb01400.x. [DOI] [PubMed] [Google Scholar]
- 2.St-Onge MP. Dietary fats, teas, dairy, and nuts: potential functional foods for weight control? Am J Clin Nutr. 2005;81(1):7–15. doi: 10.1093/ajcn/81.1.7. [DOI] [PubMed] [Google Scholar]
- 3.St-Onge MP, Jones PJ. Greater rise in fat oxidation with medium-chain triglyceride consumption relative to long-chain triglyceride is associated with lower initial body weight and greater loss of subcutaneous adipose tissue. Int J Obes Relat Metab Disord. 2003;27(12):1565–71. doi: 10.1038/sj.ijo.0802467. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 5.Seimon RV, Wooster T, Otto B, Golding M, Day L, Little TJ, et al. The droplet size of intraduodenal fat emulsions influences antropyloroduodenal motility, hormone release, and appetite in healthy males. Am J Clin Nutr. 2009;89(6):1729–36. doi: 10.3945/ajcn.2009.27518. [DOI] [PubMed] [Google Scholar]
- 6.Babayan VK. Medium chain triglycerides and structured lipids. Lipids. 1987;22(6):417–20. doi: 10.1007/BF02537271. [DOI] [PubMed] [Google Scholar]
- 7.Bach AC, Babayan VK. Medium-chain triglycerides: an update. Am J Clin Nutr. 1982;36 (5):950–62. doi: 10.1093/ajcn/36.5.950. [DOI] [PubMed] [Google Scholar]
- 8.St-Onge M-P, Bosarge A. Weight-loss diet that includes consumption of medium-chain triacylglycerol oil leads to a greater rate of weight and fat mass loss than does olive oil. The American Journal of Clinical Nutrition. 2008;87(3):621–626. doi: 10.1093/ajcn/87.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuji H, Kasai M, Takeuchi H, Nakamura M, Okazaki M, Kondo K. Dietary Medium-Chain Triacylglycerols Suppress Accumulation of Body Fat in a Double-Blind, Controlled Trial in Healthy Men and Women. The Journal of Nutrition. 2001;131(11):2853–2859. doi: 10.1093/jn/131.11.2853. [DOI] [PubMed] [Google Scholar]
- 10.Hill JO, Peters JC, Reed GW, Schlundt DG, Sharp T, Greene HL. Nutrient balance in humans: effects of diet composition. Am J Clin Nutr. 1991;54(1):10–7. doi: 10.1093/ajcn/54.1.10. [DOI] [PubMed] [Google Scholar]
- 11.Dulloo AG, Fathi M, Mensi N, Girardier L. Twenty-four-hour energy expenditure and urinary catecholamines of humans consuming low-to-moderate amounts of medium-chain triglycerides: a dose-response study in a human respiratory chamber. Eur J Clin Nutr. 1996;50(3):152–8. [PubMed] [Google Scholar]
- 12.Scalfi L, Coltorti A, Contaldo F. Postprandial thermogenesis in lean and obese subjects after meals supplemented with medium-chain and long-chain triglycerides. The American Journal of Clinical Nutrition. 1991;53(5):1130–1133. doi: 10.1093/ajcn/53.5.1130. [DOI] [PubMed] [Google Scholar]
- 13.Seaton T, Welle S, Warenko M, Campbell R. Thermic effect of medium-chain and long-chain triglycerides in man. The American Journal of Clinical Nutrition. 1986;44(5):630–634. doi: 10.1093/ajcn/44.5.630. [DOI] [PubMed] [Google Scholar]
- 14.Hill JO, Peters JC, Yang D, Sharp T, Kaler M, Abumrad NN, et al. Thermogenesis in humans during overfeeding with medium-chain triglycerides. Metabolism. 1989;38(7):641–8. doi: 10.1016/0026-0495(89)90101-7. [DOI] [PubMed] [Google Scholar]
- 15.White MD, Papamandjaris AA, Jones PJ. Enhanced postprandial energy expenditure with medium-chain fatty acid feeding is attenuated after 14 d in premenopausal women. The American Journal of Clinical Nutrition. 1999;69(5):883–889. doi: 10.1093/ajcn/69.5.883. [DOI] [PubMed] [Google Scholar]
- 16.St-Onge MP, Bourque C, Jones PJ, Ross R, Parsons WE. Medium- versus long-chain triglycerides for 27 days increases fat oxidation and energy expenditure without resulting in changes in body composition in overweight women. Int J Obes Relat Metab Disord. 2003;27(1):95–102. doi: 10.1038/sj.ijo.0802169. [DOI] [PubMed] [Google Scholar]
- 17.Bray GA, Lee M, Bray TL. Weight gain of rats fed medium-chain triglycerides is less than rats fed long-chain triglycerides. Int J Obes. 1980;4(1):27–32. [PubMed] [Google Scholar]
- 18.Furuse M, Choi YH, Mabayo RT, Okumura J. Feeding behavior in rats fed diets containing medium chain triglyceride. Physiol Behav. 1992;52(4):815–7. doi: 10.1016/0031-9384(92)90419-3. [DOI] [PubMed] [Google Scholar]
- 19.Stubbs RJ, Harbron CG. Covert manipulation of the ratio of medium- to long-chain triglycerides in isoenergetically dense diets: effect on food intake in ad libitum feeding men. Int J Obes Relat Metab Disord. 1996;20(5):435–44. [PubMed] [Google Scholar]
- 20.Van Wymelbeke V, Himaya A, Louis-Sylvestre J, Fantino M. Influence of medium-chain and long-chain triacylglycerols on the control of food intake in men. Am J Clin Nutr. 1998;68(2):226–34. doi: 10.1093/ajcn/68.2.226. [DOI] [PubMed] [Google Scholar]
- 21.St-Onge MP, Ross R, Parsons WD, Jones PJ. Medium-chain triglycerides increase energy expenditure and decrease adiposity in overweight men. Obes Res. 2003;11(3):395–402. doi: 10.1038/oby.2003.53. [DOI] [PubMed] [Google Scholar]
- 22.Poppitt SD, Strik CM, MacGibbon AK, McArdle BH, Budgett SC, McGill AT. Fatty acid chain length, postprandial satiety and food intake in lean men. Physiol Behav. 2010;101(1):161–7. doi: 10.1016/j.physbeh.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 23.Matzinger D, Degen L, Drewe J, Meuli J, Duebendorfer R, Ruckstuhl N, et al. The role of long chain fatty acids in regulating food intake and cholecystokinin release in humans. Gut. 2000;46(5):688–693. doi: 10.1136/gut.46.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drewe J, Gadient A, Rovati LC, Beglinger C. Role of circulating cholecystokinin in control of fat-induced inhibition of food intake in humans. Gastroenterology. 1992;102(5):1654–9. doi: 10.1016/0016-5085(92)91726-k. [DOI] [PubMed] [Google Scholar]
- 25.Maas MI, Hopman WP, Katan MB, Jansen JB. Release of peptide YY and inhibition of gastric acid secretion by long-chain and medium-chain triglycerides but not by sucrose polyester in men. Eur J Clin Invest. 1998;28(2):123–30. doi: 10.1046/j.1365-2362.1998.00255.x. [DOI] [PubMed] [Google Scholar]
- 26.Gibbons C, Caudwell P, Finlayson G, Webb DL, Hellstrom PM, Naslund E, et al. Comparison of postprandial profiles of ghrelin, active GLP-1, and total PYY to meals varying in fat and carbohydrate and their association with hunger and the phases of satiety. J Clin Endocrinol Metab. 2013;98(5):E847–55. doi: 10.1210/jc.2012-3835. [DOI] [PubMed] [Google Scholar]
- 27.St-Onge MP, Bosarge A. Weight-loss diet that includes consumption of medium-chain triacylglycerol oil leads to a greater rate of weight and fat mass loss than does olive oil. Am J Clin Nutr. 2008;87(3):621–6. doi: 10.1093/ajcn/87.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kant AK, Graubard BI. Secular trends in patterns of self-reported food consumption of adult Americans: NHANES 1971–1975 to NHANES 1999–2002. Am J Clin Nutr. 2006;84(5):1215–23. doi: 10.1093/ajcn/84.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, et al. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell metabolism. 2006;4(3):223–33. doi: 10.1016/j.cmet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Holt S, Brand J, Soveny C, Hansky J. Relationship of satiety to postprandial glycaemic, insulin and cholecystokinin responses. Appetite. 1992;18(2):129–41. doi: 10.1016/0195-6663(92)90190-h. [DOI] [PubMed] [Google Scholar]
- 31.Ratliff J, Leite JO, de Ogburn R, Puglisi MJ, VanHeest J, Fernandez ML. Consuming eggs for breakfast influences plasma glucose and ghrelin, while reducing energy intake during the next 24 hours in adult men. Nutr Res. 2010;30(2):96–103. doi: 10.1016/j.nutres.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Anderson GH, Tecimer SN, Shah D, Zafar TA. Protein source, quantity, and time of consumption determine the effect of proteins on short-term food intake in young men. J Nutr. 2004;134(11):3011–5. doi: 10.1093/jn/134.11.3011. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Thornton JC, Bari S, Williamson B, Gallagher D, Heymsfield SB, et al. Comparisons of waist circumferences measured at 4 sites. Am J Clin Nutr. 2003;77(2):379–84. doi: 10.1093/ajcn/77.2.379. [DOI] [PubMed] [Google Scholar]
- 34.Nishi Y, Hiejima H, Hosoda H, Kaiya H, Mori K, Fukue Y, et al. Ingested medium-chain fatty acids are directly utilized for the acyl modification of ghrelin. Endocrinology. 2005;146(5):2255–64. doi: 10.1210/en.2004-0695. [DOI] [PubMed] [Google Scholar]
- 35.Kirchner H, Gutierrez JA, Solenberg PJ, Pfluger PT, Czyzyk TA, Willency JA, et al. GOAT links dietary lipids with the endocrine control of energy balance. Nat Med. 2009;15(7):741–5. doi: 10.1038/nm.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Klaauw AA, Keogh JM, Henning E, Trowse VM, Dhillo WS, Ghatei MA, et al. High protein intake stimulates postprandial GLP1 and PYY release. Obesity (Silver Spring) 2013;21(8):1602–7. doi: 10.1002/oby.20154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leidy HJ, Ortinau LC, Douglas SM, Hoertel HA. Beneficial effects of a higher-protein breakfast on the appetitive, hormonal, and neural signals controlling energy intake regulation in overweight/obese, “breakfast-skipping,” late-adolescent girls. Am J Clin Nutr. 2013;97(4):677–88. doi: 10.3945/ajcn.112.053116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flatt JP, Ravussin E, Acheson KJ, Jequier E. Effects of dietary fat on postprandial substrate oxidation and on carbohydrate and fat balances. J Clin Invest. 1985;76(3):1019–24. doi: 10.1172/JCI112054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westerterp-Plantenga MS, Wijckmans-Duijsens NE, Verboeket-van de Venne WP, De Graaf K, Weststrate JA, Van Het Hof KH. Diet-induced thermogenesis and satiety in humans after full-fat and reduced-fat meals. Physiol Behav. 1997;61(2):343–9. doi: 10.1016/s0031-9384(96)00444-1. [DOI] [PubMed] [Google Scholar]
- 40.Brondel L, Landais L, Romer MA, Holley A, Penicaud L. Substrate oxidation influences liking, wanting, macronutrient selection, and consumption of food in humans. Am J Clin Nutr. 2011;94(3):775–83. doi: 10.3945/ajcn.111.017319. [DOI] [PubMed] [Google Scholar]
- 41.Langhans W. The enterocyte as an energy flow sensor in the control of eating. Forum of nutrition. 2010;63:75–84. doi: 10.1159/000264395. [DOI] [PubMed] [Google Scholar]
- 42.Astrup A. The relevance of increased fat oxidation for body-weight management: metabolic inflexibility in the predisposition to weight gain. Obes Rev. 2011;12(10):859–65. doi: 10.1111/j.1467-789X.2011.00894.x. [DOI] [PubMed] [Google Scholar]
- 43.Feinle-Bisset C, Patterson M, Ghatei MA, Bloom SR, Horowitz M. Fat digestion is required for suppression of ghrelin and stimulation of peptide YY and pancreatic polypeptide secretion by intraduodenal lipid. Am J Physiol Endocrinol Metab. 2005;289(6):E948–53. doi: 10.1152/ajpendo.00220.2005. [DOI] [PubMed] [Google Scholar]
- 44.Maki KC, Mustad V, Dicklin MR, Geohas J. Postprandial metabolism with 1,3-diacylglycerol oil versus equivalent intakes of long-chain and medium-chain triacylglycerol oils. Nutrition. 2009;25(6):627–33. doi: 10.1016/j.nut.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 45.Broussolle C, Beylot M, Chassard D, Beaufrere B. Effects of different lipid substrates on glucose metabolism in normal postabsorptive humans. Metabolism. 1992;41(12):1276–83. doi: 10.1016/0026-0495(92)90096-s. [DOI] [PubMed] [Google Scholar]
- 46.Vu MK, Verkijk M, Muller ESM, Biemond I, Lamers CBHW, Masclee AAM. Medium chain triglycerides activate distal but not proximal gut hormones. Clinical Nutrition. 1999;18(6):359–363. doi: 10.1016/s0261-5614(99)80016-8. [DOI] [PubMed] [Google Scholar]
- 47.Feltrin KL, Little TJ, Meyer JH, Horowitz M, Smout AJ, Wishart J, et al. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Am J Physiol Regul Integr Comp Physiol. 2004;287(3):R524–33. doi: 10.1152/ajpregu.00039.2004. [DOI] [PubMed] [Google Scholar]
- 48.Maggio CA, Koopmans HS. Satiety effects of intragastric meals containing triglycerides with different chain lengths. Am J Physiol. 1987;252(6 Pt 2):R1106–13. doi: 10.1152/ajpregu.1987.252.6.R1106. [DOI] [PubMed] [Google Scholar]
- 49.D’Alessio D. Intestinal hormones and regulation of satiety: the case for CCK, GLP-1, PYY, and Apo A-IV. JPEN Journal of parenteral and enteral nutrition. 2008;32(5):567–8. doi: 10.1177/0148607108322401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.