Abstract
The discovery of the first nonsteroidal antiestrogen ethamoxytriphetol (MER25) in 1958, opened the door to a wide range of clinical applications. However, the finding that ethamoxytriphetol was a “morning after” pill in laboratory animals, energized the pharmaceutical industry to discover more potent derivatives. In the wake of the enormous impact of the introduction of the oral contraceptive worldwide, contraceptive research was a central focus in the early 1960’s. Numerous compounds were discovered eg: clomiphene, nafoxidine, and tamoxifen, but the fact that clinical studies showed no contraceptive actions, but, in fact, induced ovulation, dampened enthusiasm for clinical development. Only clomiphene moved forward to pioneer an application to induce ovulation in subfertile women. The fact that all the compounds were antiestrogenic made an application in patients to treat estrogen responsive breast cancer, an obvious choice. However, toxicities and poor projected commercial returns severely retarded clinical development for two decades. In the 1970’s a paradigm shift in the laboratory to advocate long term adjuvant tamoxifen treatment for early (non-metastatic) breast cancer changed medical care and dramatically increased survivorship. Tamoxifen pioneered that paradigm shift but it became the medicine of choice in a second paradigm shift for preventing breast cancer during the 1980’s and 1990’s. This was not surprising as it was the only medicine available and there was laboratory and clinical evidence for the eventual success of this application. Tamoxifen is the first medicine to be approved by the Food & Drug Administration (FDA) to reduce the risk of breast cancer in women at high risk. But it was the re-evaluation of the toxicology of tamoxifen in the 1980’s and the finding that there was both carcinogenic potential and a significant, but small, risk of endometrial cancer in postmenopausal women that led to a third paradigm shift to identify applications for selective estrogen receptor (ER) modulation. This idea was to establish a new group of medicines now called Selective ER Modulators (SERMs). Today there are 5 SERMs FDA approved (one other in Europe) for applications ranging from the reduction of breast cancer risk and osteoporosis to the reduction of menopausal hot flashes and improvements in dyspareunia and vaginal lubrication. This article charts the origins of the current path for progress in women’s health with SERMs.
Keywords: breast cancer, osteoporosis, women’s health, endometrial cancer
Introduction
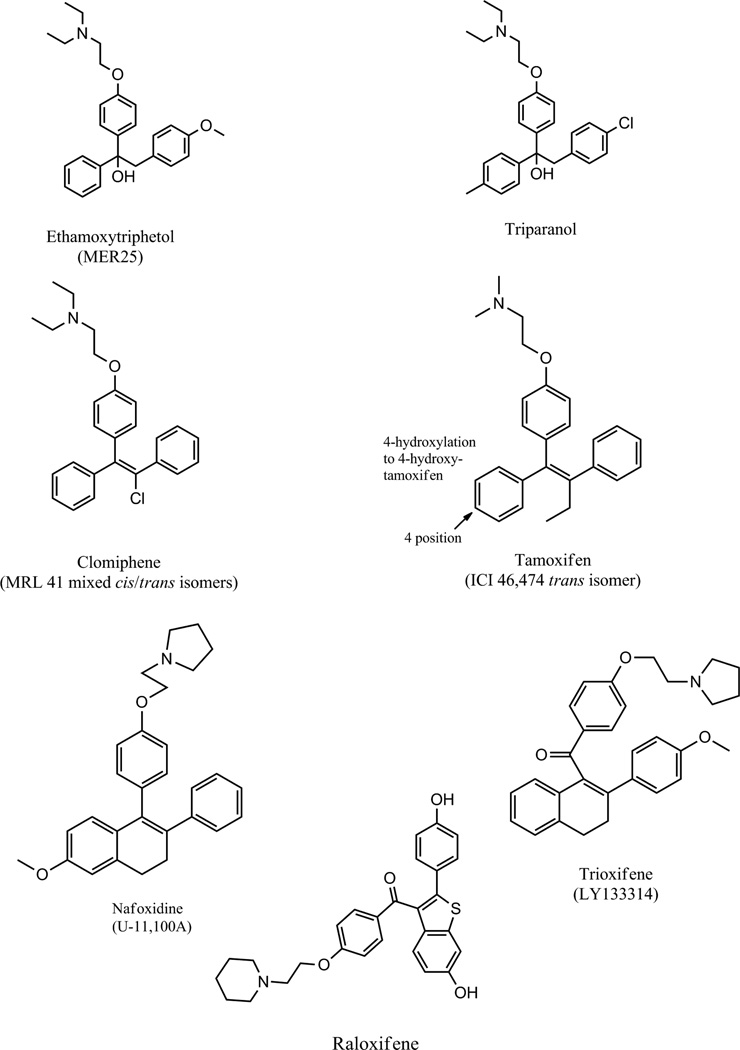
Today tamoxifen (Fig. 1) is part of the fabric of our society: almost everyone knows someone who is alive today because of their treatment with this antiestrogen used to prevent breast cancer recurrence. But this medicine is not only a pioneering breast cancer treatment and chemopreventive, but also a drug so thoroughly researched in the laboratory (I (VCJ) would always say this was to reveal “the Good, the Bad, and the Ugly”) that a whole new group of medicines, the Selective Estrogen Receptor Modulators (SERMs) was created to address specific tasks in therapeutics. This saga, that first started in 1958 (1) with the report of the first nonsteroidal antiestrogen MER25 (Fig. 1) will twist and turn as fashions and priorities in medical research changed. Many nonsteroidal antiestrogens were synthesized initially when it was thought that there was great potential for their use as “morning after pills” but this application was not to occur as the compounds guaranteed what they were designed to prevent in women! The long gestation period for nonsteroidal antiestrogens resulted in clomiphene (Fig. 1), the first medicine to induce ovulation in women (2) in the 1960’s, and then the orphan drug tamoxifen would be approved almost by chance, for the treatment of metastatic breast cancer in the 1970’s. Tamoxifen stood the test of time as a pioneering breast cancer treatment used ubiquitously to treat all stages of the disease, ductal carcinoma in situ, and as a pioneering chemopreventive. No other cancer drug has achieved this status. But with the description of SERMs in the 1980’s, a whole new era in women’s health was born. This is that story which will in the future encompass all members of the nuclear receptor superfamily.
Fig. 1.
Compounds described in the text.
Discovery of Nonsteroidal Antiestrogens
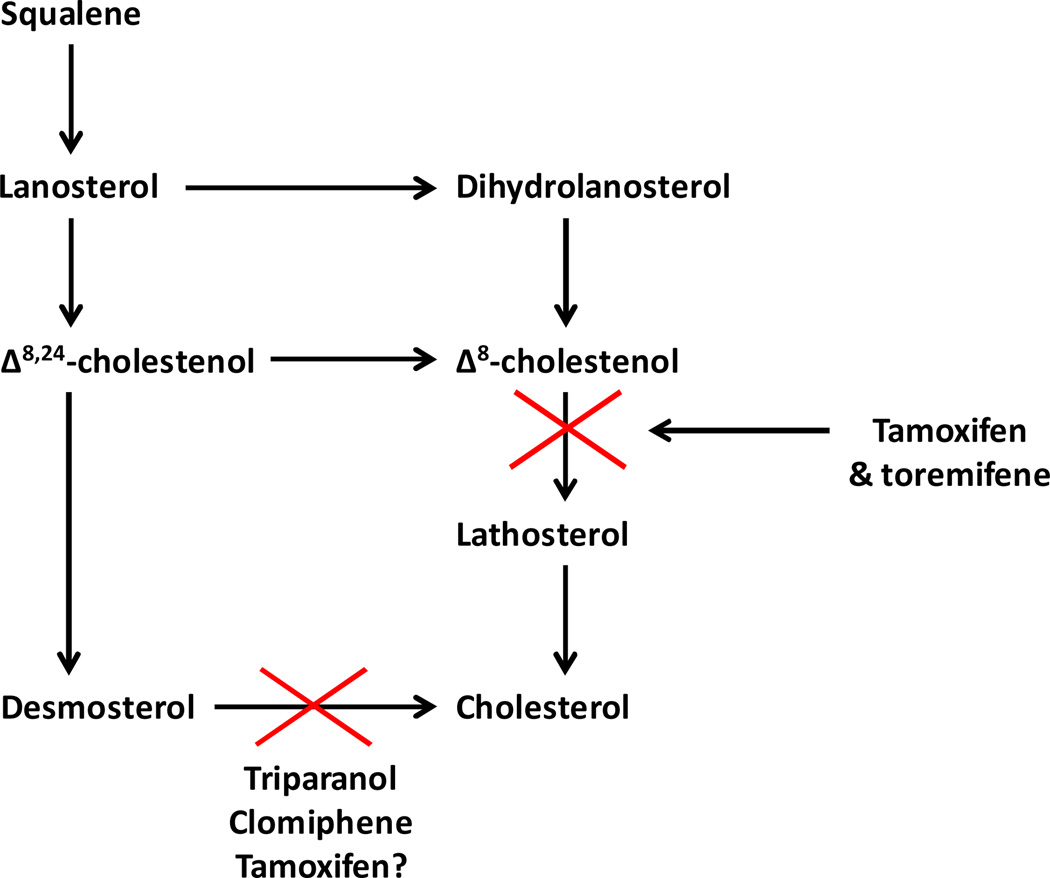
The discovery of the antiestrogenic properties of MER25 (ethamoxytriphetol) (Fig. 1) (1) was in part chance but an example of serendipity ie: an unanticipated advance in knowledge. Dr. Leonard Lerner was a young reproductive endocrinologist at the William S. Merrell Company in Cincinnati in the mid 1950’s, charged with the investigation of nonsteroidal estrogens for clinical applications. Lerner was glancing through compounds to be tested in the cardiovascular program and notice that one MER25 had a structure resembling triphenylethylene-like estrogens. He asked for the compound to test but unexpectedly he found no estrogen-like activity in any species tested (3). Instead he noted weak but consistent antiestrogenic action in all animal models (1). The compound was also structurally similar to triparanol (Fig. 1), a drug originally marketed by the Merrell Company to reduce circulating cholesterol levels, but was withdrawn because triparanol increases circulating desmosterol levels (Fig. 2) which was thought to be responsible for the rapid onset of cataracts in young women (4, 5).These observations were to be essential for the future drug development of nonsteroidal antiestrogens when they would eventually be required to be given for up to a decade (6).
Fig. 2.
The inhibition of cholesterol biosynthesis by triparanol, clomiphene, tamoxifen, and the β-chlorinated derivative of tamoxifen toremifene.
Clinical trials with MER25 were conducted (3) but not reported in the literature. By contrast, clinical trials with clomiphene (Fig. 1), a mixture of estrogenic and antiestrogenic geometric isomers of a triphenylethylene was tested extensively for the regulation of fertility but shown to induce ovulating in subfertile women (2) and showed modest activity as a breast cancer therapy (7). However, the breast cancer treatment option was discontinued as clomiphene produces an increase in desmosterol Fig. 2.
The Upjohn Company focused a huge synthetic effort on the antifertility properties of indene and naphthalene (8) derivatives thereby solving the issue of separating the isomers of triphenylethylenes, the landscape of which had actually been defensively patented by Merrell in the 1960’s. The Upjohn Company discovered a compound, U-11, 100A (Fig. 1) that would be unsuccessfully developed as a breast cancer drug (9). The compound, renamed nafoxidine was effective at controlling the growth of 30% of breast cancers for about a year but severe side effects such as photophobia precluded further clinical development. Nevertheless, nafoxidine was the structural basis for a new SERM lasofoxifene examined 20 years later (see last section). The fact that Merrell defensively patented triphenylethylenes as breast cancer drugs prevented patent security for tamoxifen in the United States until 1985 of their original patent submitted in the 1960’s! The defensive patenting of triphenylethylenes by Merrell was actually to turn out to be a stimulus for innovation in medicinal chemistry and after 1985 was to create a significant unanticipated financial windfall for ICI Pharmaceuticals Division that had now undergone a metamorphosis to Zeneca. In 1984, an NCI panel declared long term adjuvant tamoxifen therapy the antihormone treatment of choice for the treatment of ER positive breast cancer (10) and Zeneca now had 20 years patent protection. This provided profits to invest in chemoprevention and fund the development of a range of other leading and innovative antihormonal therapies: bicalutamide, anastrozole, and fulvestrent at Zeneca.
The patenting restrictions led Eli Lilly to explore chemistry described originally in India at the Central Drug Research Institute in Lucknow, India (11) to link the bulky antiestrogenic group by a ketone bridge to the ER ligand binding moiety (12). The result was trioxifene (Fig. 1) that was to fail against tamoxifen in clinical trials to treat breast cancer. Nevertheless, the structural advance gave the world the high affinity antiestrogens LY117036 (13) and LY156,728 (14) after it was discovered that tamoxifen was metabolically activated to the high affinity antiestrogen 4-hydroxytamoxifen (15, 16). During the late 1960’s and throughout the 1970’s tamoxifen was being developed glacially throughout the world (including the United States without patent protection) by ICI Pharmaceuticals Division. Why was that and how did the opportunity to change that, significantly advance women’s health?
The Tamoxifen Tale
During the early years of the 1960’s, Arthur Walpole, Mike Harper, and Dora Richardson were the key members of the Fertility Control program at ICI Pharmaceuticals Division, Alderley Park, near Macclesfield, Chesire. Walpole was the senior scientist and head of the program, Harper was the experimental reproductive endocrinologist and Richardson the synthetic organic chemist. The team was tasked with advancing the goal of discovering a safe and effective “post coital” contraceptive and the work on reproduction would be continued by Labhsetwar into the 1970’s (17–20) despite the fact that the fertility program was going nowhere. The principal achievements of the team was the discovery that the geometric isomers of a substituted triphenylethylene were estrogenic or antiestrogenic: the cis isomer ICI 47,699 was an estrogen (21) and the trans isomer ICI 46,474 was an antiestrogen with antifertility properties in the rat by preventing implantation that was found to be an estrogen dependent process (22, 23). Most importantly, for the future development of ICI 46,474, as a long term anticancer agent, the antiestrogen did not increase desmosterol levels in rats (22).
Although Walpole had an interest in cancer research (24) no studies were conducted at ICI Pharmaceuticals Division but Walpole did initiate clinical studies outside the company to demonstrate activity as an anticancer agent in metastatic breast cancer (25, 26) and like clomiphene, the induction of ovulation in subfertile women (27). However, in the spring of 1972, a meeting was held at ICI Pharmaceuticals Division to review all clinical progress with ICI 46,474 and the decision was subsequently made to terminate clinical development. Fortunately for me (VCJ), and probably for my future career, Arthur Walpole was the examiner of my PhD on “failed contraceptives” entitled: A Study of the Oestrogenic and Anti-oestrogenic Activities of Some Substituted Triphenylethylenes and Triphenylethanes. I passed my PhD examination and I was appointed as a faculty member at Leeds University in mid-1972 but was required to obtain my “Been to America” (BTA). My chairman in the Pharmacology Department Mike Barrett (formerly of ICI Pharmaceuticals) and Walpole recommended I spend two years at the Worcester Foundation (the home of the oral contraceptive), with Mike Harper who was now heading a research team to develop a once-a-month pill. Mike Harper had published all of the antifertility properties of ICI46,474 in the mid 1960’s (21–23) when he was at ICI Pharmaceuticals Division, Alderley Park.
When I (VCJ) got to the Foundation in September 1972, Harper had left to accept an appointment at the World Health Organization in Geneva and I was told I could do anything I wanted as long as some of it involved contraception. A phone call to Walpole at ICI Pharmaceuticals Division to discuss the idea that ICI 46,474 should be developed as a breast cancer drug, resulted in an unrestricted research grant to study the anticancer properties of ICI 46,474 in the laboratory, an appointment to be an ICI Americas consultant on the project and act as an advisor to them with clinical trial cooperative groups in America. What I did not know was that Walpole had tendered his resignation in 1972, but he agreed to remain at ICI Pharmaceuticals Division if ICI 46,474 was put on the market as an orphan drug. He suggested that funds be made available for me (VCJ) to discover the best strategy for the clinical use of ICI 46,474 as a breast cancer drug. ICI Americas/ICI Pharmaceuticals Division/and the Yorkshire Cancer Campaign would fund my (VCJ) laboratory first at the Worcester Foundation and then at Leeds University throughout the 1970’s. That decade resulted in publications to support three strategic applications of tamoxifen (formerly ICI 46, 474): target the ER in the tumor where tamoxifen and its metabolites block estrogen stimulated growth (15, 28), tamoxifen for the prevention of mammary carcinogenesis (29, 30) and the idea of long term adjuvant tamoxifen therapy would be the appropriate strategy to prevent tumor recurrence (31–33).
During the next 30 years, clinical studies established unequivocally that long term adjuvant tamoxifen therapy using 5 or more years of treatment produced major survival advantages for patients with as ER positive breast tumor (6, 34–36). However, it was the paradigm shift from treatment to chemoprevention during the 1980’s and 1990’s that opened up new opportunities in women’s health.
The chemoprevention of breast cancer in high risk women
The idea that breast cancer can be prevented is not new. In 1936, Professor Antoine Lacassagne (37) presented the following strategy at the Annual Meeting of the American Association for Cancer Research in Boston.
If one accepts the consideration of adenocarcinoma of the breast as the consequence of a special hereditary sensibility to the proliferative actions of oestrone, one is led to imagine a therapeutic preventative for subjects predisposed by their heredity to this cancer. It would consist – perhaps in the very near future when the knowledge and use of hormones will be better understood – in the suitable use of a hormone antagonistic or excretory, to prevent the stagnation of oestrone in the ducts of the breast.
However, at that time there were no “antiestrogenic” compounds and neither was there a target at which to aim. The compounds were to develop from the serendipitous discovery of MER25 (38). The main compounds were all discovered and developed on the evidence of a bioassay in vivo: the inhibition of postcoital implantation in rodents! The target was to be discovered using high specific activity tritiated estrogen in whole animal distribution studies with the tritiated estrogen binding in and being retained in estrogen target tissues ie: uterus, vagina, pituitary gland (39, 40). The ER was first identified as an extractable protein from immature rat uteri (41, 42). From there, translation to clinical applications in breast cancer flowed with the ER assay to determine estrogen dependent growth in breast tumors as a predictive test for ablative surgery in advanced disease (43) and then transformed into a target for antiestrogen action to treat breast cancer (44).
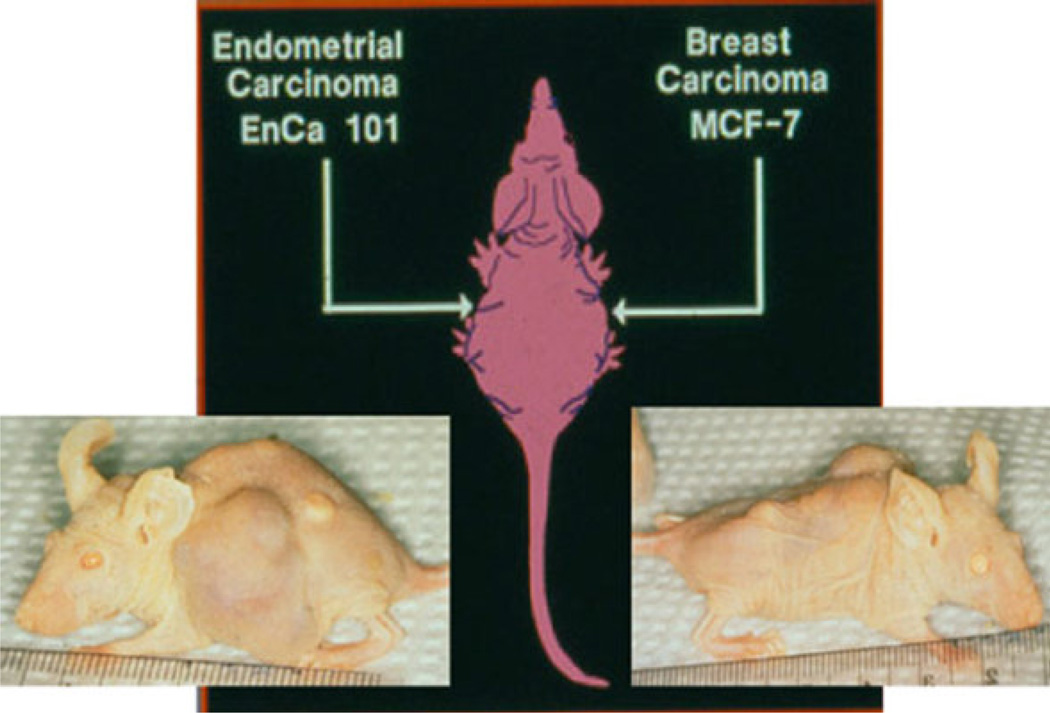
In 1986, Professor Trevor Powles took the initiative to be the first recruit a vanguard study of high risk women for what was to become the “Royal Marsden Study”. He based his plan on the fact that tamoxifen prevented rat mammary carcinogenesis (29, 30, 45) and adjuvant tamoxifen reduced the risk of contralateral breast cancer (46). His early results (47) proved provocative as there was maintained compliance vs. placebo but the spectre of carcinogenesis with tamoxifen was already apparent and this had to be addressed in any future trials. Nevertheless, substantial recruitment and compliance continued and a decrease in breast cancer incidence was noted at a 20 year follow-up (48). It was clear from studies in athymic mice with transplantable ER positive tumors, that a target site specificity with tamoxifen was occurring. Since Harper and Walpole’s (21, 22) first publications on ICI 46,474, there was known species specificity; tamoxifen was classified as an antiestrogen in the immature rat uterus but an estrogen in the ovariectomized mouse uterus and vagina. However, studies of metabolic differences did not prove the obvious – tamoxifen was an estrogen in the mouse because it is metabolized to an estrogen (49). Studies in the athymic mouse were to demonstrate that tamoxifen did not support the growth of ER+ MCF-7 tumors but stimulated the uterus to grow (50). It was stated “these studies strongly support the concept that the drug (tamoxifen) can selectively stimulate or inhibit events in target in tissues of different species without metabolic intervention”. “The drug-estrogen receptor complex is perceived as a stimulatory or inhibitory signal”(50). Subsequent studies in the high incidence mammary tumor strain of mice (C3HOUJ) demonstrated that tamoxifen prevented mammary tumor carcinogenesis and was superior to oophorectomy (51). The original prediction by Lacassagne was correct (37). Nevertheless, the “breakthrough” experiment that had major ramifications for clinical medicine and patient care was the finding that athymic mice bitransplanted with an ER positive breast tumor (MCF-7) and an ER positive endometrial cancer (EnCa 101) would exhibit “antiestrogenic” actions on the breast tumor to stop growth but “estrogenic” action in the endometrial cancer to promote growth (52) (Fig. 3). These data were presented to the clinical community (53, 54) with the concern “a large cohort of patients under long term tamoxifen therapy (>5 years) needs to be monitored for the occurrence of tamoxifen-stimulated endometrial tumors” (52).
Fig. 3.
The pioneering bitransplantation study by Gottardis (72) with an ER-positive breast tumor (MCF-7) implanted in one axilla and an ER-positive endometrial tumor (EnCa 101) in the other axilla. Tamoxifen blocks estrogen-stimulated growth of the breast tumor (right), but tamoxifen encourages the growth of the endometrial tumor (left). These data were transmitted immediately to the clinical community (53, 54), confirmed in clinical trials (55, 56) to change clinical practice.
Retrospective analysis of clinical trials data confirmed there was a low but significant increase in endometrial cancer incidence in postmenopausal women receiving long term adjuvant tamoxifen treatment (55, 56). Not only was this finding important for patient care in general practice but also this knowledge was essential to ensure safety for the trials that were planned to test the worth of tamoxifen to prevent breast cancer in high risk women (57–59). However, the surprise was the toxicological finding that high dose tamoxifen treatment for the life-time of a rat would initiate hepatocellular carcinoma (60–62). Fortunately these data did not translate to clinical practice. The Oxford Overview of clinical trials did not show an increase in hepatocellular carcinoma in patients receiving adjuvant therapy but it is clear that if tamoxifen had been tested for carcinogeneity in 1973 when the first animal studies for adjuvant therapy and chemoprevention were started, tamoxifen would not have been developed by the pharmaceutical industry (63). Hundreds of thousands of women would have died and the aromatase inhibitors would have been abandoned as these new antihormonal agents were only developed because the strategy of long term adjuvant tamoxifen was shown to be successful financially (63)!
This is not the place to review the results of the tamoxifen trials of chemoprevention. Suffice to say they were successful overall (48, 57–59, 64, 65) and tamoxifen was approved by the FDA in 1998 as the pioneer for the reduction of breast cancer incidence in pre and postmenopausal women with a high risk.
What is important to stress is the fact that a more transparent understanding of tamoxifen’s pharmacology and long term safety was needed in the 80’s if tamoxifen was to advance in the 90’s for broad clinical testing as a chemopreventive. The question was straight forward: “if tamoxifen is classified as an antiestrogen but estrogen is necessary to maintain bone density and (as was thought at the time) to decrease the risk of coronary heart disease, what advantage would there be in preventing half a dozen breast cancers per 1000 women per year if 300 women developed osteoporosis and there were more women dying of heart attacks?” Unexpectedly, a series of laboratory studies was to provide reassurances that tamoxifen was not “just an antiestrogen” but it was selectively estrogenic and antiestrogenic in different estrogen target tissues around a woman’s body. Most importantly, the laboratory finding all translated to successful clinical trials and a new paradigm was conceived with the creation of a new group of medicines – the Selective Estrogen Receptor Modulators or SERMs.
Nonsteroidal antiestrogens were “born” but SERMs were “conceived”
Nonsteroidal antiestrogens had initially been developed and failed in their primary application as “morning after” pills but in the 1960’s and 70’s both clomiphene and tamoxifen succeed in a secondary application. The fact that subfertile women could now induce ovulation and successfully give birth to children was a pioneering advance but not, at that time, a significant market. Another secondary application was the treatment of metastatic breast cancer, but this too was an insignificant market for a palliative drug such as tamoxifen. By contrast, what happened over 30 years was the confirmation that long term adjuvant tamoxifen therapy was the best strategy for clinical trials (66) and would be found to save perhaps millions of lives. The FDA approval of tamoxifen for chemoprevention in 1998 would now result in another blockbuster drug resurrected through the development of the new and novel strategy (38) of using a SERM (raloxifene) to prevent multiple diseases in women.
Serendipity took control with an initial investigation of the effects of tamoxifen and a failed breast cancer drug keoxifene on ovarectomized rat bone loss (67). The findings were not anticipated; what was anticipated was that these two nonsteroidal antiestrogens would increase bone loss. What was found was that the opposite occurred and that ovarectomized rats treated with the antiestrogen plus estrogen had no bone loss. By contrast, the antiestrogens blocked estrogen induced increases in uterine weight (67). There was target site specificity for nonsteroidal antiestrogens. This was not unlike the estrogen-like effects of tamoxifen in the athymic mouse uterus vs the prevention of estrogen stimulated growth of an implanted breast tumor (50) or the stimulation of endometrial cancer growth against the inhibition of growth of a breast tumor implanted in the same athymic mouse (52). All results had been observed at the same time in our Tamoxifen Team laboratory in Wisconsin – it was a principle! This was the preliminary data used to fund and advance subsequently successful clinical trials (68–70). With this knowledge, and the fact that tamoxifen caused a decrease in circulating cholesterol in rats (Fig. 2) (22) which, incidentally, caused ICI Pharmaceuticals Division to place a “hypocholesterolanemic” indication in their patent application 20 years earlier (71), it was now possible to consider a new approach to preventing breast cancer by developing multifunctional medicines for women’s health. This was a prescient concept because the carcinogenic problems with tamoxifen, once they surfaced, (55, 61) would not go away and would preclude broad applications for the medicine in women’s health. The new concept (38) was stated simply and directly based on laboratory data ie: before the publication of the results of ongoing clinical trials at the time with tamoxifen or initiation of new trials with other SERMs, as a roadmap for the pharmaceutical industry to follow.
Is this the end of the possible applications for antioestrogens? Certainly not. We have obtained valuable clinical information about this group of drugs that can be applied in other disease states. Research does not travel in straight lines and observations in one field of science often become major discoveries in another. Important clues have been garnered about the effects of tamoxifen on bone and lipids so it is possible that derivatives could find targeted applications to retard osteoporosis or atherosclerosis. The ubiquitous application of novel compounds to prevent diseases associated with the progressive changes after menopause may, as a side effect, significantly retard the development of breast cancer. The target population would be post-menopausal women in general, thereby avoiding the requirement to select a high risk group to prevent breast cancer.
Raloxifene (Fig. 1) was the result. It was actually the obvious choice (the compound had been tested clinically previously and was a failed breast cancer treatment under its former name keoxifene) as it was now known to preserve bone density in the laboratory (67), prevent carcinogen induced mammary cancer in rats (72), be less uterotrophic then tamoxifen in rats (14, 73, 74) and inhibit tamoxifen stimulated endometrial cancer growth (75). These findings were subsequently confirmed by others in the laboratory (76), in clinical trials for osteoporosis (77, 78) and trials to evaluate the reduction of risk in breast cancer in high risk postmenopausal women (79, 80). Raloxifene is now FDA approved for the treatment and prevention of osteoporosis and the chemoprevention of breast cancer in postmenopausal high risk women. The SERMs had travelled from concept (38, 81) to a clinically proven “cluster”, of medicines: tamoxifen (and the related compound toremifene – a safer SERM in rats (62) but used to treat breast cancer(82)) and raloxifene that succeeded despite their original development plan as a breast cancer drug which failed. It has taken about 15 years of clinical gestation since tamoxifen (breast cancer risk reduction) and raloxifene (prevention of osteoporosis) were FDA approved for use in women at risk for disease but there has been a recent flurry of SERM approvals that deserve special comment. The new SERMs are innovative reinventions of early molecules in medicinal chemistry as the science has become more sophisticated and novel target for improvements in women’s health more imaginative.
New developments
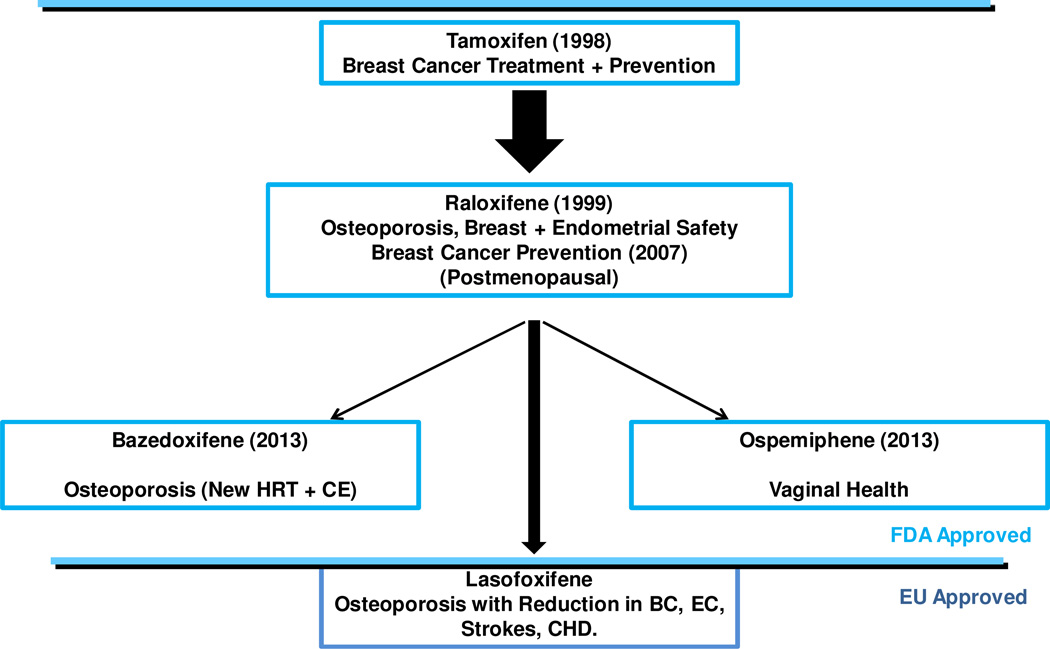
Current progress in the FDA approvals of the new SERMs bazedoxifene (Fig. 4) for the prevention of osteoporosis and (in combination with conjugated estrogen) for the amelioration of postmenopausal hot flashes and ospemiphene (Fig. 4) for the improvement of atrophic vaginal symptoms and vaginal lubrication has been presented earlier (83, 84). The summary of FDA approved SERMs to enhance and cement the market are illustrated in Fig. 4 but the figure also includes lasofoxifene that was approved in the European Union but with no plans for launching the product for the treatment and prevention of osteoporosis. Approval has lapsed. Despite this deficit, the medicine is worthy of comment because of the advance in pharmacology as a multifunctional medicine in women health.
Fig. 4.
The approvals of individual selective oestrogens receptor modulators (SERMs) in the United States of America through the evaluation system of the Food and Drug Administration (FDA). Approvals were specifically for indications at the highest level of toxicologic safety for women without disease but as a new hormone replacement therapy with conjugated estrogen (HRT+CE) to prevent disease ie: chemoprevention of osteoporosis, breast cancer (BC), menopausal symptoms or dyspareunia. One SERM, lasofoxifene, was approved for use in the European Union (EU) but was never launched or marketed despite the fact that clinical trials demonstrated a reduction in breast cancer (BC), osteoporosis fracture, strokes, endometrial cancer (EC) and coronary heart disease (CHD) (89).
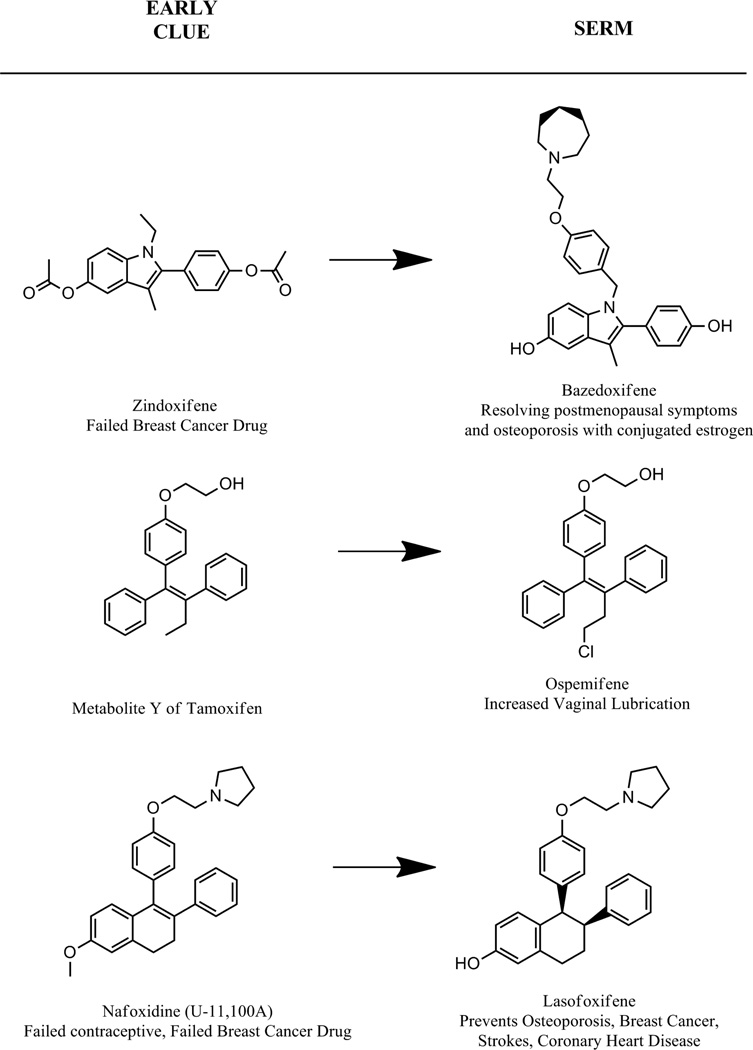
Bazedoxifene, ospemifene, and lasofoxifene each are compounds derived from prior pharmalogical knowledge (Fig. 5). The principal structural feature of basedoxifene that binds to the ER is a potential metabolite of a failed breast cancer drug called zindoxifene that was found to actually be an estrogen (85). The core ligand was married to a predictable bulky antiestrogenic side chain to create the new SERM bazedoxifene (86). Ospemiphene is a known metabolite of the SERM toremifene (82) that was studied in detail twenty years ago when tamoxifen was found to have the potential to be carcinogenic in rat liver at high doses (62). It seems that tamoxifen is hydroxylated in the α position on the ethyl substitution at the ethylene bond and this is the metabolite that caused adduct formation in the rat liver DNA. Toremifene has a β chlorine so α hydroxylation does not occur (62) and it is a safer SERM in rat liver. However, this metabolic transformation has no toxicological relevance in patients. The “antiestrogenic” side chain of ospemiphene is a glycol formed by the deamination of the dealkylated toremifene side chain. This metabolic transformation was first noted for tamoxifen (87, 88) in patient sera and the metabolite, metabolite Y was confirmed as a weakly antiestrogenic compound with partial estrogen-like actions (88).
Fig. 5.
Origins of current selective ER modulators from earlier nonsteroidal antiestrogens. The discovery that the metabolite of tamoxifen, 4-hydroxytamoxifen (Fig. 1) has a very high binding affinity for the ER (15) acted as a catalyst for the design of the majority of future SERMs. The raloxifene drug development “odyssey” throughout the 1980’s (97) is a replay of the tamoxifen tale (71). During the 70’s (71), interestingly enough the work was done in the same laboratory but on different continents! The repurposing (92) and repatenting (97) of a failed breast cancer drug (keoxifene) resulted in raloxifene (Fig. 1), the same SERM, to establish a principle in translational research. Bazedoxifene is an adaptation of an estrogenic metabolite from a failed breast cancer drug Zindoxifene (85). Ospemifene is a known metabolite of the breast cancer drug toremifene. The metabolite of toremifene was found because an analogous metabolite Y was discovered for tamoxifen in the early 1980’s (88). Lasofoxifene has its origins with failed antifertility agent discovered in the early 1960’s U-11, 100A (8). The compound renamed nafoxidine was tested as a drug for the treatment of breast cancer but again failed because of serious side effects (9).
We have met the origins of lasofoxifene earlier. It is the compound U-11,100A or nafoxidine (Fig. 1), discovered at the Upjohn research laboratories in their search for antifertility agents (8) but developed as a potential breast cancer drug that failed because of severe toxicities (9). Lasofoxifene (Fig. 5) is a miracle of medicinal chemistry. With demethylation of nafoxidine, the resulting molecule has high affinity for the ER but as a result, the molecule also has rapid clearance because of phase II metabolism and increased excretion. This principle was first illustrated by 4-hydroxytamoxifen (15, 16, 32) and noted in raloxifene analogs (74). However, reduction of the lone double bond in the non-aromatic ring of nafoxidine results in a possibility of two diasterioisomers. One isomer is used that is protected from conjugation and phase II metabolism. As a result lasofoxifene is used at a daily dose of 0.5mg for the treatment and prevention of osteoporosis (89). This contrasts with raloxifene used at a 60mg daily dose either for the treatment and prevention of osteoporosis or the prevention of breast cancer (77, 79, 90).
Once the SERM concept was conceived (38, 81) clinical development advanced effectively with raloxifene as the molecule was known to be free from endometrial cancer in animals and did not produce rat liver carcinogenesis. The drug would be safely used for the prevention of osteoporosis in otherwise healthy women! However, despite the fact raloxifene reduces cholesterol levels in rats (76), there was no evidence of any benefit by a reduction of coronary heart disease in high risk women (91). However, tamoxifen and raloxifene were both two “repurposed” drugs (92) and there was still a long way to go to discover the “ideal SERM” as a multifunctional medicine (Fig 6). Lasofoxifene, the nafoxidine derivative, was to produce a few surprises. The PEARL trial of lasofoxifene in postmenopausal women at risk for osteoporosis used 0.25mg and 0.5mg daily doses against a placebo control to determine the prevention of osteoporosis (89). Fractures were decreased, and breast cancer incidence was also reduced (93). The surprise was a decrease in coronary heart disease and also a decrease in strokes (89). The incidence of endometrial cancer was not increased but there was an estrogen-like increase in deep vein thrombosis. Lasofoxifene has demonstrated that medicinal chemistry and a commitment to large well organized clinical trial can provide much valuable information about the potential of selective modulation of the nuclear receptor superfamily.
Fig. 6.
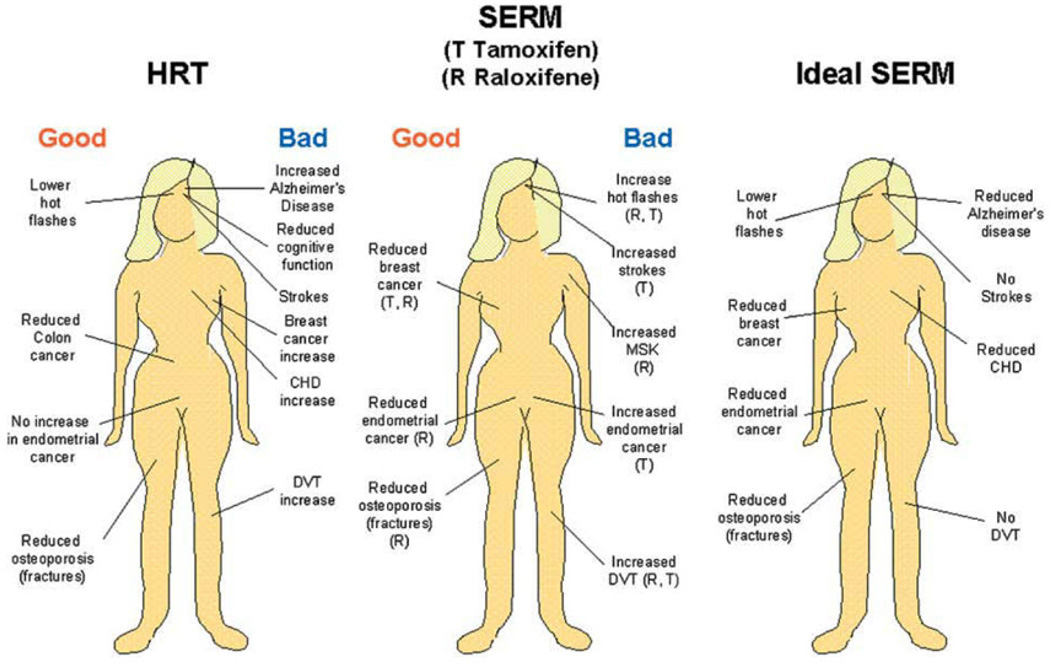
Progress toward an ideal SERM. The overall good or bad aspects of administering hormone replacement therapy (HRT) to postmenopausal women compared with the observed site-specific actions of the SERMs tamoxifen and raloxifene. The known beneficial or negative actions of SERMs have opened the door for drug discovery to create the ideal SERM or targeted SERMs to either improve quality of life or prevent diseases associated with aging in women. This figure is published with permission from Elsevier. Jordan, V.C. Selective estrogen receptor modulation: Concept and consequences in cancer. Cancer Cell, 2004 Mar; 5(3): 207–213.
If there is a message from the past 40 years of drug discovery, it is that a failure in one application can be a discovery in another (38). There were a lot of “failures” but translational research was advanced to benefit women’s health. In the closing years of the 19th century, the French author Jules Verne wrote: whatever one man is capable of conceiving, other men are capable of achieving. The SERMs were conceived (38) based on a cluster of interlocking experiments conducted by the Tamoxifen Team at the University of Wisconsin Comprehensive Cancer Center (1980–1993) (49–52, 67, 72–74, 85, 87, 88, 94). A more detailed survey of SERMs and their origins for women’s health can be found elsewhere (95, 96). Today, this particular special issue of STEROIDS provides opportunities for the next generation of men and women medical scientists to achieve success in their professional careers with the discovery of new modulating medicines in human health targeting the nuclear receptor superfamily.
Acknowledgments
Acknowledgements/Grants
This work (VCJ) was supported by the Department of Defense Breast Program under Award number W81XWH-06-1-0590 Center of Excellence; the Susan G Komen For The Cure Foundation under Award number SAC100009; GHUCCTS CTSA (Grant # UL1RR031975) and the Lombardi Comprehensive Cancer Center Support Grant (CCSG) Core Grant NIH P30 CA051008. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. Additionally, the views and opinions of the author(s) do not reflect those of the US Army or the Department of Defense.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lerner LJ, Holthaus FJ, Jr, Thompson CR. A non-steroidal estrogen antiagonist 1-(p-2-diethylaminoethoxyphenyl)-1-phenyl-2-p-methoxyphenyl ethanol. Endocrinology. 1958 Sep;63(3):295–318. doi: 10.1210/endo-63-3-295. [DOI] [PubMed] [Google Scholar]
- 2.Greenblatt RB, Barfield WE, Jungck EC, Ray AW. Induction of ovulation with MRL/41. Preliminary report. JAMA. 1961 Oct 14;178:101–104. doi: 10.1001/jama.1961.03040410001001. [DOI] [PubMed] [Google Scholar]
- 3.Lerner LJ. The first nonsteroidal antioestrogen - MER25. In: Sutherland RL, Jordan VC, editors. Non-Steroidal Antioestrogens: Molecular Pharmacology and Antitumour Activity. Sydney and New York: Academic Press; 1981. pp. 1–16. [Google Scholar]
- 4.Avigan J, Steinberg D, Vroman HE, Thompson MJ, Mosettig E. Studies of cholesterol biosynthesis. I. The identification of desmosterol in serum and tissues of animals and man treated with MER-29. J Biol Chem. 1960 Nov;235:3123–3126. [PubMed] [Google Scholar]
- 5.Laughlin RC, Carey TF. Cataracts in patients treated with triparanol. JAMA. 1962 Jul 28;181:339–340. doi: 10.1001/jama.1962.03050300059020a. [DOI] [PubMed] [Google Scholar]
- 6.Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013 Mar 9;381(9869):805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herbst AL, Griffiths CT, Kistner RW. Clomiphene Citrate (NSC-35770) in Disseminated Mammary Carcinoma. Cancer Chemother Rep. 1964 Dec;43:39–41. [PubMed] [Google Scholar]
- 8.Lednicer D, Lyster SC, Aspergren BD, Duncan GW. Mammalian antifertility agents. 3. 1-Aryl–2-phenyl-1,2,3,4-tetrahydro-1-naphthols, 1-aryl-2-phenyl-3,4-dihydronaphthalenes, and their derivatives. J Med Chem. 1966 Mar;9(2):172–176. doi: 10.1021/jm00320a002. [DOI] [PubMed] [Google Scholar]
- 9.Legha SS, Slavik M, Carter SK. Nafoxidine--an antiestrogen for the treatment of breast cancer. Cancer. 1976 Oct;38(4):1535–1541. doi: 10.1002/1097-0142(197610)38:4<1535::aid-cncr2820380415>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 10.Consensus development conference. Report: Adjuvant chemotherapy of breast cancer. JAMA. 1985 Oct 18;254(15):3461–3463. [PubMed] [Google Scholar]
- 11.Iyer RN, Gopalachari R, Kamboj VP, Kar AB. Anti-implantation effect of 2,3-diphenylacrylophenones. Indian J Exp Biol. 1967 Jul;5(3):169–170. [PubMed] [Google Scholar]
- 12.Jones CD, Suarez T, Massey EH, Black LJ, Tinsley FC. Synthesis and antiestrogenic activity of [3,4-dihydro-2-(4-methoxyphenyl)-1-naphthalenyl][4-[2-(1-pyrrolidinyl)ethoxy]-phenyl]methanone, methanesulfonic acid salt. J Med Chem. 1979 Aug;22(8):962–966. doi: 10.1021/jm00194a015. [DOI] [PubMed] [Google Scholar]
- 13.Black LJ, Goode RL. Uterine bioassay of tamoxifen, trioxifene and a new estrogen antagonist (LY117018) in rats and mice. Life Sci. 1980 Apr 28;26(17):1453–1458. doi: 10.1016/0024-3205(80)90049-1. [DOI] [PubMed] [Google Scholar]
- 14.Black LJ, Jones CD, Falcone JF. Antagonism of estrogen action with a new benzothiophene derived antiestrogen. Life Sci. 1983 Feb 28;32(9):1031–1036. doi: 10.1016/0024-3205(83)90935-9. [DOI] [PubMed] [Google Scholar]
- 15.Jordan VC, Collins MM, Rowsby L, Prestwich G. A monohydroxylated metabolite of tamoxifen with potent antioestrogenic activity. J Endocrinol. 1977 Nov;75(2):305–316. doi: 10.1677/joe.0.0750305. [DOI] [PubMed] [Google Scholar]
- 16.Allen KE, Clark ER, Jordan VC. Evidence for the metabolic activation of non-steroidal antioestrogens: a study of structure-activity relationships. Br J Pharmacol. 1980;71(1):83–91. doi: 10.1111/j.1476-5381.1980.tb10912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Labhsetwar AP. Role of oestrogen in spontaneous ovulation demonstrated by use of an antagonist of oestrogen, ICI 46,474. Nature. 1970 Jan 3;225(5227):80–81. doi: 10.1038/225080a0. [DOI] [PubMed] [Google Scholar]
- 18.Labhsetwar AP. Role of estrogens in ovulation: A study using the estrogen-antagonist, ICI. 46,474. Endocrinology. 1970 Sep;87(3):542–551. doi: 10.1210/endo-87-3-542. [DOI] [PubMed] [Google Scholar]
- 19.Labhsetwar AP. Effects of an antioestrogen on the corpus luteum of rabbits and rats. J Reprod Fertil. 1971 May;25(2):295–297. doi: 10.1530/jrf.0.0250295. [DOI] [PubMed] [Google Scholar]
- 20.Labhsetwar AP. Role of estrogens in spontaneous ovulation: evidence for the positive feedback in hamsters. Endocrinology. 1972 Apr;90(4):941–946. doi: 10.1210/endo-90-4-941. [DOI] [PubMed] [Google Scholar]
- 21.Harper MJ, Walpole AL. Contrasting endocrine activities of cis and trans isomers in a series of substituted triphenylethylenes. Nature. 1966 Oct 1;212(5057):87. doi: 10.1038/212087a0. [DOI] [PubMed] [Google Scholar]
- 22.Harper MJ, Walpole AL. A new derivative of triphenylethylene: effect on implantation and mode of action in rats. J Reprod Fertil. 1967 Feb;13(1):101–119. doi: 10.1530/jrf.0.0130101. [DOI] [PubMed] [Google Scholar]
- 23.Harper MJ, Walpole AL. Mode of action of ICI. 46,474 in preventing implantation in rats. J Endocrinol. 1967 Jan;37(1):83–92. doi: 10.1677/joe.0.0370083. [DOI] [PubMed] [Google Scholar]
- 24.Jordan VC. The development of tamoxifen for breast cancer therapy: a tribute to the late Arthur L. Walpole. Breast Cancer Res Treat. 1988 Jul;11(3):197–209. doi: 10.1007/BF01807278. [DOI] [PubMed] [Google Scholar]
- 25.Cole MP, Jones CT, Todd ID. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br J Cancer. 1971 Jun;25(2):270–275. doi: 10.1038/bjc.1971.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ward HW. Anti-oestrogen therapy for breast cancer: a trial of tamoxifen at two dose levels. Br Med J. 1973 Jan 6;1(5844):13–14. doi: 10.1136/bmj.1.5844.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klopper A, Hall M. New synthetic agent for the induction of ovulation: preliminary trials in women. Br Med J. 1971 Jan 16;1(5741):152–154. doi: 10.1136/bmj.1.5741.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jordan VC, Koerner S. Tamoxifen (ICI 46,474) and the human carcinoma 8S oestrogen receptor. Eur J Cancer. 1975 Mar;11(3):205–206. doi: 10.1016/0014-2964(75)90119-x. [DOI] [PubMed] [Google Scholar]
- 29.Jordan VC. Antitumour activity of the antioestrogen ICI 46,474 (tamoxifen) in the dimethyl benzanthracene (DMBA)-induced rat mammary carcinoma model. J Steroid Biochem. 1974;5:354. [Google Scholar]
- 30.Jordan VC. Effect of tamoxifen (ICI 46,474) on initiation and growth of DMBA-induced rat mammary carcinomata. Eur J Cancer. 1976 Jun;12(6):419–424. doi: 10.1016/0014-2964(76)90030-x. [DOI] [PubMed] [Google Scholar]
- 31.Jordan VC. Use of the DMBA-induced rat mammary carcinoma system for the evaluation of tamoxifen as a potential adjuvant therapy. Reviews on Endocrine-related Cancer. 1978 Oct;(supplement):49–55. [Google Scholar]
- 32.Jordan VC, Allen KE. Evaluation of the antitumour activity of the non-steroidal antioestrogen monohydroxytamoxifen in the DMBA-induced rat mammary carcinoma model. Eur J Cancer. 1980 Feb;16(2):239–251. doi: 10.1016/0014-2964(80)90156-5. [DOI] [PubMed] [Google Scholar]
- 33.Jordan VC, Dix CJ, Allen KE. The effectiveness of long term tamoxifen treatment in a laboratory model for adjuvant hormone therapy of breast cancer. In: Salmon S, Jones S, editors. Adjuvant Therapy of Cancer II. New York: Grune & Stratton Inc.; 1979. pp. 19–26. [Google Scholar]
- 34.Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998 May 16;351(9114):1451–1467. [PubMed] [Google Scholar]
- 35.Early Breast Cancer Trialists' Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005 May 14–20;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 36.Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011 Aug 27;378(9793):771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lacassagne A. Hormonal Pathogenesis of Adenocarcinoma of the Breast. The American Journal of Cancer. 1936;27(2):217–228. [Google Scholar]
- 38.Lerner LJ, Jordan VC. Development of antiestrogens and their use in breast cancer: eighth Cain memorial award lecture. Cancer Res. 1990 Jul 15;50(14):4177–4189. [PubMed] [Google Scholar]
- 39.Glascock RF, Hoekstra WG. Selective accumulation of tritium-labelled hexoestrol by the reproductive organs of immature female goats and sheep. Biochem J. 1959 Aug;72:673–682. doi: 10.1042/bj0720673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen EV, Jacobson HI. Basic guides to the mechanism of estrogen action. Recent Progress in Hormone Research. 1962;18:387–414. [Google Scholar]
- 41.Toft D, Gorski J. A receptor molecule for estrogens: isolation from the rat uterus and preliminary characterization. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1574–1581. doi: 10.1073/pnas.55.6.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toft D, Shyamala G, Gorski J. A receptor molecule for estrogens: studies using a cell-free system. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1740–1743. doi: 10.1073/pnas.57.6.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGuire WL, Carbone PP, Vollmer EP, editors. Estrogen Receptors in Human Breast Cancer. Raven Press; 1975. [Google Scholar]
- 44.Jensen EV, Jordan VC. The estrogen receptor: a model for molecular medicine. Clin Cancer Res. 2003 Jun;9(6):1980–1969. [PubMed] [Google Scholar]
- 45.Jordan VC, Naylor KE, Dix CJ, Prestwich G. Anti-oestrogen action in experimental breast cancer. Recent Results Cancer Res. 1980;71:30–44. doi: 10.1007/978-3-642-81406-8_8. [DOI] [PubMed] [Google Scholar]
- 46.Cuzick J, Baum M. Tamoxifen and contralateral breast cancer. Lancet. 1985 Aug 3;2(8449):282. doi: 10.1016/s0140-6736(85)90338-1. [DOI] [PubMed] [Google Scholar]
- 47.Powles TJ, Hardy JR, Ashley SE, Farrington GM, Cosgrove D, Davey JB, et al. A pilot trial to evaluate the acute toxicity and feasibility of tamoxifen for prevention of breast cancer. Br J Cancer. 1989 Jul;60(1):126–131. doi: 10.1038/bjc.1989.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Powles TJ, Ashley S, Tidy A, Smith IE, Dowsett M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007 Feb 21;99(4):283–290. doi: 10.1093/jnci/djk050. [DOI] [PubMed] [Google Scholar]
- 49.Lyman SD, Jordan VC. Metabolism of tamoxifen and its uterotrophic activity. Biochem Pharmacol. 1985 Aug 1;34(15):2787–2794. doi: 10.1016/0006-2952(85)90580-5. [DOI] [PubMed] [Google Scholar]
- 50.Jordan VC, Robinson SP. Species-specific pharmacology of antiestrogens: role of metabolism. Fed Proc. 1987 Apr;46(5):1870–1874. [PubMed] [Google Scholar]
- 51.Jordan VC, Lababidi MK, Langan-Fahey S. Suppression of mouse mammary tumorigenesis by long-term tamoxifen therapy. J Natl Cancer Inst. 1991 Apr 3;83(7):492–496. doi: 10.1093/jnci/83.7.492. [DOI] [PubMed] [Google Scholar]
- 52.Gottardis MM, Robinson SP, Satyaswaroop PG, Jordan VC. Contrasting actions of tamoxifen on endometrial and breast tumor growth in the athymic mouse. Cancer Res. 1988 Feb 15;48(4):812–815. [PubMed] [Google Scholar]
- 53.Hardell L. Tamoxifen as risk factor for carcinoma of corpus uteri. Lancet. 1988 Sep 3;2(8610):563. doi: 10.1016/s0140-6736(88)92675-x. [DOI] [PubMed] [Google Scholar]
- 54.Jordan VC. Tamoxifen and endometrial cancer. Lancet. 1988 Oct 29;2(8618):1019. doi: 10.1016/s0140-6736(88)90765-9. [DOI] [PubMed] [Google Scholar]
- 55.Fornander T, Rutqvist LE, Cedermark B, Glas U, Mattsson A, Silfversward C, et al. Adjuvant tamoxifen in early breast cancer: occurrence of new primary cancers. Lancet. 1989 Jan 21;1(8630):117–120. doi: 10.1016/s0140-6736(89)91141-0. [DOI] [PubMed] [Google Scholar]
- 56.Fisher B, Costantino JP, Redmond CK, Fisher ER, Wickerham DL, Cronin WM. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst. 1994 Apr 6;86(7):527–537. doi: 10.1093/jnci/86.7.527. [DOI] [PubMed] [Google Scholar]
- 57.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998 Sep 16;90(18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 58.Powles T, Eeles R, Ashley S, Easton D, Chang J, Dowsett M, et al. Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomised chemoprevention trial. Lancet. 1998 Jul 11;352(9122):98–101. doi: 10.1016/S0140-6736(98)85012-5. [DOI] [PubMed] [Google Scholar]
- 59.Cuzick J, Forbes J, Edwards R, Baum M, Cawthorn S, Coates A, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet. 2002 Sep 14;360(9336):817–824. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 60.Williams GM, Iatropoulos MJ, Djordjevic MV, Kaltenberg OP. The triphenylethylene drug tamoxifen is a strong liver carcinogen in the rat. Carcinogenesis. 1993 Feb;14(2):315–317. doi: 10.1093/carcin/14.2.315. [DOI] [PubMed] [Google Scholar]
- 61.Greaves P, Goonetilleke R, Nunn G, Topham J, Orton T. Two-year carcinogenicity study of tamoxifen in Alderley Park Wistar-derived rats. Cancer Res. 1993 Sep 1;53(17):3919–3924. [PubMed] [Google Scholar]
- 62.Hard GC, Iatropoulos MJ, Jordan K, Radi L, Kaltenberg OP, Imondi AR, et al. Major difference in the hepatocarcinogenicity and DNA adduct forming ability between toremifene and tamoxifen in female Crl:CD(BR) rats. Cancer Res. 1993 Oct 1;53(19):4534–4541. [PubMed] [Google Scholar]
- 63.Jordan VC. What if tamoxifen (ICI 46,474) had been found to produce rat liver tumors in 1973? A personal perspective. Ann Oncol. 1995 Jan;6(1):29–34. doi: 10.1093/oxfordjournals.annonc.a059035. [DOI] [PubMed] [Google Scholar]
- 64.Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005 Nov 16;97(22):1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 65.Cuzick J, Forbes JF, Sestak I, Cawthorn S, Hamed H, Holli K, et al. Long-term results of tamoxifen prophylaxis for breast cancer--96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007 Feb 21;99(4):272–282. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 66.Jordan C. Tamoxifen as the first targeted long term adjuvant therapy for breast cancer. Endocr Relat Cancer. 2014 Mar 21; doi: 10.1530/ERC-14-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jordan VC, Phelps E, Lindgren JU. Effects of anti-estrogens on bone in castrated and intact female rats. Breast Cancer Res Treat. 1987 Oct;10(1):31–35. doi: 10.1007/BF01806132. [DOI] [PubMed] [Google Scholar]
- 68.Love RR, Newcomb PA, Wiebe DA, Surawicz TS, Jordan VC, Carbone PP, et al. Effects of tamoxifen therapy on lipid and lipoprotein levels in postmenopausal patients with node-negative breast cancer. J Natl Cancer Inst. 1990 Aug 15;82(16):1327–1332. doi: 10.1093/jnci/82.16.1327. [DOI] [PubMed] [Google Scholar]
- 69.Love RR, Wiebe DA, Newcomb PA, Cameron L, Leventhal H, Jordan VC, et al. Effects of tamoxifen on cardiovascular risk factors in postmenopausal women. Ann Intern Med. 1991 Dec 1;115(11):860–864. doi: 10.7326/0003-4819-115-11-860. [DOI] [PubMed] [Google Scholar]
- 70.Love RR, Mazess RB, Barden HS, Epstein S, Newcomb PA, Jordan VC, et al. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med. 1992 Mar 26;326(13):852–856. doi: 10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- 71.Jordan VC. Tamoxifen: a most unlikely pioneering medicine. Nat Rev Drug Discov. 2003 Mar;2(3):205–213. doi: 10.1038/nrd1031. [DOI] [PubMed] [Google Scholar]
- 72.Gottardis MM, Jordan VC. Antitumor actions of keoxifene and tamoxifen in the N-nitrosomethylurea-induced rat mammary carcinoma model. Cancer Res. 1987 Aug 1;47(15):4020–4024. [PubMed] [Google Scholar]
- 73.Jordan VC, Gosden B. Differential antiestrogen action in the immature rat uterus: a comparison of hydroxylated antiestrogens with high affinity for the estrogen receptor. J Steroid Biochem. 1983 Sep;19(3):1249–1258. doi: 10.1016/0022-4731(83)90147-4. [DOI] [PubMed] [Google Scholar]
- 74.Jordan VC, Gosden B. Inhibition of the uterotropic activity of estrogens and antiestrogens by the short acting antiestrogen LY117018. Endocrinology. 1983 Aug;113(2):463–468. doi: 10.1210/endo-113-2-463. [DOI] [PubMed] [Google Scholar]
- 75.Gottardis MM, Ricchio ME, Satyaswaroop PG, Jordan VC. Effect of steroidal and nonsteroidal antiestrogens on the growth of a tamoxifen-stimulated human endometrial carcinoma (EnCa101) in athymic mice. Cancer Res. 1990 Jun 1;50(11):3189–3192. [PubMed] [Google Scholar]
- 76.Black LJ, Sato M, Rowley ER, Magee DE, Bekele A, Williams DC, et al. Raloxifene (LY139481 HCI) prevents bone loss and reduces serum cholesterol without causing uterine hypertrophy in ovariectomized rats. J Clin Invest. 1994 Jan;93(1):63–69. doi: 10.1172/JCI116985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999 Jun 16;281(23):2189–2197. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- 78.Martino S, Cauley JA, Barrett-Connor E, Powles TJ, Mershon J, Disch D, et al. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004 Dec 1;96(23):1751–1761. doi: 10.1093/jnci/djh319. [DOI] [PubMed] [Google Scholar]
- 79.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006 Jun 21;295(23):2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 80.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res. 2010 Jun;3(6):696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jordan VC. Chemosuppression of breast cancer with tamoxifen: laboratory evidence and future clinical investigations. Cancer Invest. 1988;6(5):589–595. doi: 10.3109/07357908809082124. [DOI] [PubMed] [Google Scholar]
- 82.Wiseman LR, Goa KL. Toremifene. A review of its pharmacological properties and clinical efficacy in the management of advanced breast cancer. Drugs. 1997 Jul;54(1):141–160. doi: 10.2165/00003495-199754010-00014. [DOI] [PubMed] [Google Scholar]
- 83.Maximov PY, Lee TM, Jordan VC. The discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice. Curr Clin Pharmacol. 2013 May;8(2):135–155. doi: 10.2174/1574884711308020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jordan VC. A(nother) scientific strategy to prevent breast cancer in post-menopausal women by enhancing estrogen-induced apoptosis? Menopause. doi: 10.1097/GME.0000000000000220. (ePub). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Robinson SP, Koch R, Jordan VC. In vitro estrogenic actions in rat and human cells of hydroxylated derivatives of D16726 (zindoxifene), an agent with known antimammary cancer activity in vivo. Cancer Res. 1988 Feb 15;48(4):784–787. [PubMed] [Google Scholar]
- 86.Miller CP, Collini MD, Tran BD, Harris HA, Kharode YP, Marzolf JT, et al. Design, synthesis, and preclinical characterization of novel, highly selective indole estrogens. J Med Chem. 2001 May 24;44(11):1654–1657. doi: 10.1021/jm010086m. [DOI] [PubMed] [Google Scholar]
- 87.Bain RR, Jordan VC. Identification of a new metabolite of tamoxifen in patient serum during breast cancer therapy. Biochem Pharmacol. 1983 Jan 15;32(2):373–375. doi: 10.1016/0006-2952(83)90571-3. [DOI] [PubMed] [Google Scholar]
- 88.Jordan VC, Bain RR, Brown RR, Gosden B, Santos MA. Determination and pharmacology of a new hydroxylated metabolite of tamoxifen observed in patient sera during therapy for advanced breast cancer. Cancer Res. 1983 Mar;43(3):1446–1450. [PubMed] [Google Scholar]
- 89.Cummings SR, Ensrud K, Delmas PD, LaCroix AZ, Vukicevic S, Reid DM, et al. Lasofoxifene in postmenopausal women with osteoporosis. N Engl J Med. 2010 Feb 25;362(8):686–696. doi: 10.1056/NEJMoa0808692. [DOI] [PubMed] [Google Scholar]
- 90.Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999 Aug 18;282(7):637–45. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 91.Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006 Jul 13;355(2):125–137. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- 92.Jordan VC. Repurposing failed pharmaceuticals as the first targeted medicines for the treatment and prevention of breast cancer. Hamdan Medical Journal. In Press. [Google Scholar]
- 93.LaCroix AZ, Powles T, Osborne CK, Wolter K, Thompson JR, Thompson DD, et al. Breast cancer incidence in the randomized PEARL trial of lasofoxifene in postmenopausal osteoporotic women. J Natl Cancer Inst. 2010 Nov 17;102(22):1706–1715. doi: 10.1093/jnci/djq415. [DOI] [PubMed] [Google Scholar]
- 94.Gottardis MM, Jiang SY, Jeng MH, Jordan VC. Inhibition of tamoxifen-stimulated growth of an MCF-7 tumor variant in athymic mice by novel steroidal antiestrogens. Cancer Res. 1989 Aug 1;49(15):4090–4093. [PubMed] [Google Scholar]
- 95.Maximov PY, McDaniel RE, Jordan VC. Milestones in Drug Therapy. Springer; Basel: 2013. Tamoxifen: Pioneering Medicine in Breast Cancer. [Google Scholar]
- 96.Jordan VC, editor. Estrogen Action, Selective Estrogen Receptor Modulators and Women's Health: Progress and Promise. London: Imperial College Press; 2013. [Google Scholar]
- 97.Lewis JS, Jordan VC. Case Histories: Raloxifene. In: Taylor J, Triggle D, editors. Comprehensive Medicinal Chemistry II. Oxford: Elsevier Limited; 2006. pp. 103–121. [Google Scholar]