Abstract
Patients who undergo autologous stem cell transplant (ASCT) for hematologic malignancies frequently have multiple comorbidities. The hematopoietic cell transplantation-specific comorbidity index (HCT-CI), a transplant-specific modification of the Charlson Comorbidity Index, can predict risk of readmission following allogeneic stem cell transplant. Its utility in the autologous setting is unknown. We evaluated 620 patients who underwent ASCT at the Ohio State University from 2007 to 2012 for lymphoma or multiple myeloma (MM) to identify factors associated with readmission. Univariable and multivariable logistic regression were used to estimate the odds of readmission within 30 days of discharge following ASCT. A Cox proportional hazards model was used to evaluate overall survival (OS). Sixty-four patients were readmitted within 30 days; the most common indications were fever and prolonged gastrointestinal toxicity. Multiple myeloma compared with lymphoma (OR 1.89, 95% CI 1.06–3.38, p=0.03), HCT-CI≥3 (OR 1.74, 95% CI 1.03–2.96, p=0.04), and length of hospitalization ≥28 days (OR 3.14, 95% CI 1.26–7.83, p=0.01) remained significantly associated with 30-day readmission in a multivariable model. While the model had excellent fit (p > 0.75), its ability to predict individual patients who would be readmitted was less than acceptable (ROC=0.64, 95% CI: 0.57–0.71). In a multivariable proportional hazards model, 30-day readmission (HR 1.81, 95% CI 1.04–3.18, p=0.04), length of hospitalization ≥28 days (HR 4.93, 95% CI 2.65–9.18, p<0.001), and chemorefractory disease (HR 3.08, 95% CI 1.74–5.43, p<0.001) were independently associated with inferior OS, but HCT-CI was not. Evaluation of other assessment tools may allow for better prediction of outcomes following ASCT.
INTRODUCTION
Early hospital readmissions have been identified as markers of poor health care quality,1–3 and to that end, the Centers for Medicare and Medicaid Services (CMS) began to penalize hospitals for higher standardized early all-cause readmission rates for heart failure, acute myocardial infarction, and pneumonia in 2012, with the expectation that these penalties will expand to include other diseases.4 The Charlson Comorbidity Index (CCI) was originally devised to predict 10-year mortality by classifying or weighting comorbid conditions.5 Since its publication, it has been shown to be useful in predicting readmission in multiple settings.6–8 In 2005, researchers at the Fred Hutchinson Cancer Research Center introduced a modification of the CCI, the hematopoietic cell transplant comorbidity index (HCT-CI), designed to be more applicable to the transplant population.9 While designed to predict risk of non-relapse mortality (NRM), a recent retrospective analysis of recipients of myeloablative allogeneic stem cell transplant (SCT) demonstrated that a higher HCT-CI was predictive of 30-day readmission, and that 30-day readmission was, in turn, an independent predictor of all-cause mortality.10 Likewise, donor type, stem cell source, conditioning regimen, and documented infection during index hospitalization have been linked with increased risk of readmission among allogeneic SCT recipients.11–13
While autologous stem cell transplant (ASCT) is potentially curative for patients with relapsed or refractory lymphoma and can extend survival in patients with multiple myeloma (MM), it is not without risk, and efforts have been made to better identify patients for whom the risks outweigh the potential benefits.14–17 Risk factors for readmission following ASCT have not been evaluated. The aim of this retrospective study was to evaluate readmissions within 30 days of discharge following ASCT for patients with MM, NHL, and Hodgkin’s lymphoma (HL) and to evaluate the ability of the HCT-CI and other variables of interest to identify patients at risk of adverse outcomes.
METHODS
Patients
A review of patient records for patients receiving their first ASCT for lymphoma or myeloma from January 2007 through April 2012 was performed after obtaining approval from the Ohio State University Institutional Review Board.
Transplantation Procedure and Supportive Care
All patients were treated in a HEPA-filtered inpatient bone marrow transplant unit and received fungal and herpes zoster prophylaxis as appropriate. Thawed autologous stem cells were infused through a central venous catheter on day 0 following conditioning chemotherapy. All patients received irradiated and leukoreduced blood products. Neutrophil engraftment was defined as the first of three successive days with an absolute neutrophil count ≥0.5×109/L after the post-transplant nadir, and platelet engraftment was defined as the first of three successive days with a platelet count ≥ 20×109/L without platelet transfusion.
Statistical Analysis
The primary objective of this retrospective study was to determine variables associated with hospital readmission within 30 days of discharge date following first ASCT in patients with MM or lymphoma. Variables evaluated included: HCT-CI, age, sex, race, histology (MM versus other lymphomas), Karnofsky performance status (KPS) at time of transplant (80 and lower versus 90/100), response at time of transplant (SD/PD vs. PR/CR), mobilization regimen, radiation received, cd34 cell dose received, time to ANC and platelet recovery, and occurrence of adverse events including mucositis, sepsis, atrial fibrillation, intubation, and dialysis during the hospital stay, as well as the duration of the index hospital admission. Logistic regression was used to investigate the impact of these variables on readmission. Univariable models were fit and any variable with a p-value < 0.20 using the likelihood ratio test was included in a full model. Variables with the largest p-value from the Wald test were removed sequentially until the only variables remaining in the final model had a p-value < 0.05. Odds ratios, 95% confidence intervals, and probabilities of 30-day readmission were estimated from the model. Goodness of fit was evaluated using the Hosmer-Lemeshow test or a deviance test comparing saturated and reduced models. Poorly fitting or highly influential observations were identified with diagnostic plots.
The area under the receiver-operator curve (ROC) was used to describe the model’s ability to discriminate between patients who would and would not be readmitted within 30 days, where a value of 1 represents perfect discrimination and a value of 0.5 suggests discrimination no better than random chance. A general rule for interpretation is that an ROC between 0.7 and 0.8 represents acceptable discrimination, between 0.8 and 0.9 is excellent, and higher than 0.9 is outstanding.18 A classification table comparing predicted outcome to actual readmission status within 30 days of discharge was generated using 0.10 as the predictive cut point. This level was chosen because it was close to the cutoff where curves for sensitivity and specificity crossed. Sensitivity, specificity, positive predictive value, negative predictive value, and total correct classification were assessed.
Secondary objectives included describing cumulative incidence of relapse (CIR, incidence of treatment-related mortality (TRM), and assessing the impact of 30-day readmission status and other variables on OS. Estimates of CIR and TRM were calculated by Gray’s method, where death without relapse was a competing risk for CIR and death due to disease was a competing risk for TRM.19 OS was modeled as a function of 30-day readmission status and other variables using a Cox proportional hazards model in a landmark analysis, where OS was calculated from 30 days following the date of discharge.20 Two patients with readmissions were excluded from this analysis, one who died before day 30 and one who had not been followed for at least 30 days. Proportional hazards modeling was conducted in a similar manner to the logistic regression modeling, using a limited backwards selection procedure. Hazard ratios and 95% confidence intervals are provided for variables that remained in the model with p<0.05. All tests were two-sided and statistical significance was declared at alpha=0.05
RESULTS
Patient Characteristics
Between January 2007 and April 2012, 620 patients underwent first ASCT for MM, NHL, or HL at the Ohio State University. Sixty-four patients (10%) were readmitted within 30 days of discharge from transplant hospitalization. Patient characteristics are listed in Table 1. Over half of the patients (58%) had MM and 42% had lymphoma. Median age was 57 years (range 18–76) and 60% of patients were male. The median KPS was 90 (range 50–100) and the median HCT-CI was 2 (range 0–9). The majority of patients had chemosensitive disease, as defined according to disease-specific criteria, prior to transplant (91%).
Table 1.
Characteristics of Patients Undergoing First Autologous Transplant
| Characteristic | All Patients N = 620 |
|---|---|
| Age—yr | |
| Median | 57 |
| Range | 18–76 |
| Age, years | |
| ≤ 60 | 378 (61) |
| > 60 | 242 (39) |
| Sex, n (%) | |
| Female | 249 (40) |
| Male | 371 (60) |
| Race, n (%) | |
| Caucasian | 544 (88) |
| Black | 70 (11) |
| Other | 6 (1) |
| Diagnosis, n (%) | |
| Multiple Myeloma | 361 (58) |
| Hodgkins Disease | 80 (13) |
| Diffuse Large B-Cell Lymphoma | 96 (15) |
| Follicular Lymphoma | 8 (1) |
| Mantle Cell Lymphoma | 55 (9) |
| Other Lymphoma | 20 (3) |
| Karnofsky Performance Status | |
| Median | 90 |
| Range | 50–100 |
| Karnofsky Performance Status, n (%) | |
| 50 | 1 (<1) |
| 60 | 4 (<1) |
| 70 | 38 (6) |
| 80 | 202 (33) |
| 90 | 273 (44) |
| 100 | 102 (16) |
| Transplant Comorbidity Index | |
| Median | 2 |
| Range | 0–10 |
| Transplant Comorbidity Index, n (%) | |
| 0 | 109 (18) |
| 1 | 80 (13) |
| 2 | 157 (25) |
| 3 | 132 (21) |
| 4 | 71 (11) |
| 5 | 42 (7) |
| 6 | 16 (3) |
| 7 | 7 (1) |
| 8 | 4 (<1) |
| 9 | 1 (<1) |
| 10 | 1 (<1) |
| Number of Prior Treatments | |
| Median | 1 |
| Range | 0–6 |
| Pre-Transplant Response Status, n (%) | |
| SD/PD | 58 (9) |
| CR/PR | 562 (91) |
| Conditioning, n (%) | |
| Melphalan | 360 (58) |
| BEAM | 241 (39) |
| BEC | 18 (3) |
| Other | 1 (0.2) |
| Mobilization, n (%) | |
| G-CSF | 293 (47) |
| Plerixafor | 196 (32) |
| E | 32 (5) |
| G-CSF+Chemo | 81 (13) |
| G-CSF+Plerixafor+Chemo | 18 (3) |
| Radiation Received, n (%) | |
| No | 485 (78) |
| Yes | 135 (22) |
| CD34 Dose | |
| Median (in millions of cells per kilogram) | 4.36 |
| Range | 1.27–53.68 |
| ANC Recovery Time, days | |
| Median | 10 |
| Range | 8–19 |
| Platelet Recovery Time, days | |
| Median | 18 |
| Range | 0–59 |
| Length of Stay, days | |
| Median | 17 |
| Range | 11–57 |
Abbreviations: EAR: Etoposide, cytarabine, and rituximab; ANC: Absolute neutrophil count
Transplant Characteristics
All patients with MM received pretransplant conditioning with melphalan 140 mg/m2 or 200 mg/m2, depending on renal function and age. With the exception of 18 patients with mantle cell lymphoma who received conditioning with BEC (busulfan, etoposide, and cyclophosphamide) and one patient with central nervous system (CNS) involvement who received thiotepa-based conditioning, all lymphoma patients received conditioning with BEAM (BCNU, etoposide, cytarabine, and melphalan). Transplant was complicated by mucositis in 33%, sepsis in 7%, development of atrial fibrillation in 8%, respiratory failure in 3%, and renal failure requiring dialysis in 1% of patients. The median time to neutrophil recovery was 10 days (range 8–19) and median time to platelet recovery was 18 days (range 0–59). The median duration of transplant admission was 17 days (range 11–57).
Readmissions and Risk Factors for 30-Day Readmission
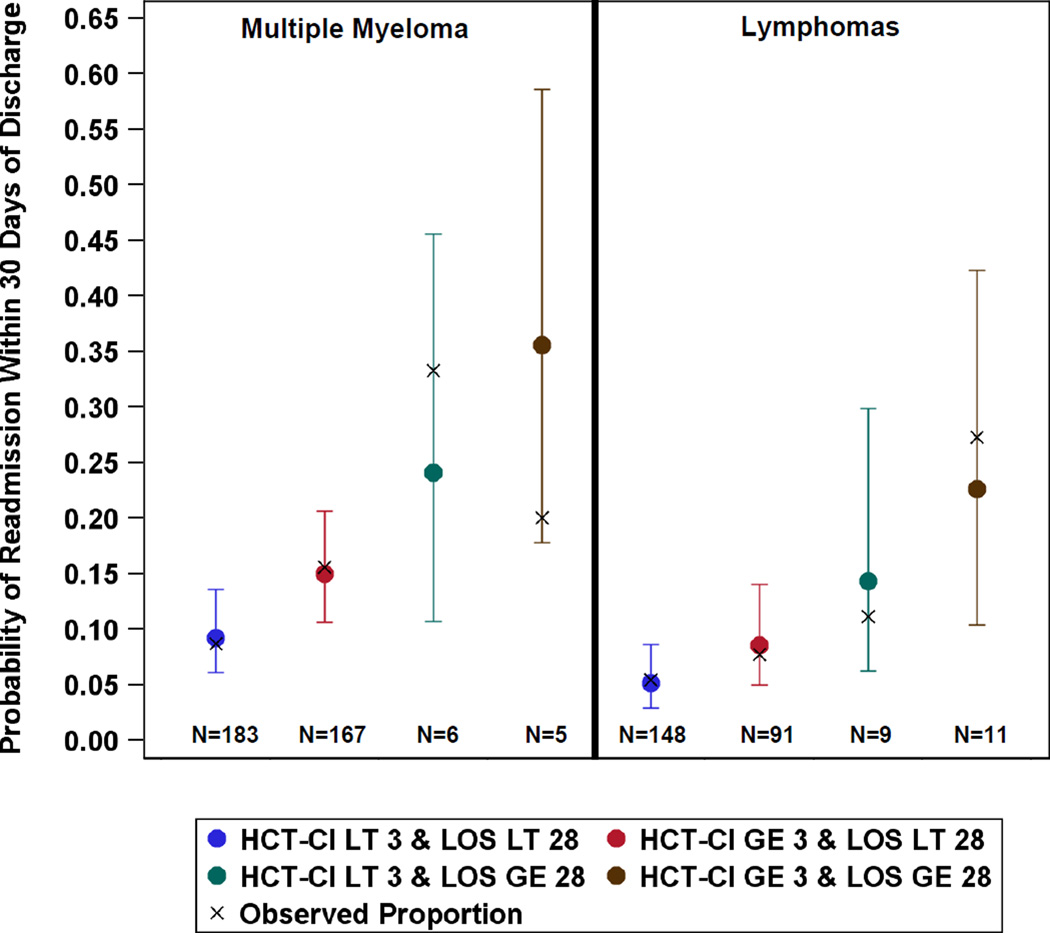
The reasons for 30-day readmission are listed in Table 2. By far, the most common reasons for readmission were fever without identification of a source and prolonged gastrointestinal toxicity associated with chemotherapy. Several variables were strongly or moderately associated with 30-day readmission (Table 3). On univariable analysis, significant risk factors for 30-day readmission included MM (p=.04), KPS of 80 or lower (p=0.04), increasing HCT-CI (p=0.004), and length of stay (LOS) of 28 or more days (p=0.03). Other variables, including older age (p=0.06), lower CD34 counts in the graft (p=0.18), chemorefractory disease (p=0.20), and development of atrial fibrillation (p=0.18), respiratory failure (p=0.07), or renal failure requiring dialysis (p=0.18) during hospital stay were moderately associated with higher probabilities of readmission. In a multivariable analysis, only disease histology, HCT-CI, and LOS remained significantly associated with 30-day readmission. Diagnostic plots showed that a few patients with longer LOS were highly influential. Thus, models were fit with disease histology, LOS grouped as less than 28 days versus at least 28 days, and categories of HCT-CI<3 versus HCT-CI > 3 (Table 4). For patients with the same disease histology and similar LOS, the odds of 30-day readmission was 1.74 times higher for those with HCT-CI of at least 3 compared to those with lower HCT-CI (95% CI: 1.03–2.96; p=0.04). Estimated probabilities of readmission for all combinations of the risk factors are shown in Figure 1. Patients with MM who had been hospitalized for 28 days or more and had HCT-CI scores of at least 3 had the highest predicted probability of readmission, whereas patients with lymphoma who had been hospitalized less than 28 days and had HCT-CI scores lower than 3 had the lowest predicted probability of readmission.
Table 2.
Reasons for Readmission within 30 Days of Discharge from Index Hospitalization
| Cause of readmission | Number |
|---|---|
| Fever, no source identified | 15 |
| Prolonged gastrointestinal toxicity after chemotherapy | 13 |
| Isolated hypotension | 4 |
| PE/DVT | 4 |
| Unknown | 4 |
| C. diff colitis | 2 |
| Dyspnea | 2 |
| Pneumonia | 2 |
| Relapse | 2 |
| UTI/Pyelonephritis | 2 |
| Bacteremia | 1 |
| Cellulitis | 1 |
| CHF exacerbation | 1 |
| Chills | 1 |
| Coronary artery disease | 1 |
| Gout | 1 |
| Hypotension and tachycardia | 1 |
| Respiratory failure (BCNU pneumonitis) | 1 |
| RSV pneumonitis | 1 |
| Seizure | 1 |
| Sinusitis | 1 |
| Stroke | 1 |
| Syncope | 1 |
| Viral esophagitis | 1 |
| Total | 64 |
Abbreviations: DVT: Deep venous thrombosis; C. diff: Clostridium difficile; UTI: Urinary tract infection; CHF: congestive heart failure; RSV: respiratory syncytial virus
Table 3.
Univariable Analysis of Potential Risk Factors for 30-Day Readmission
| Categoric Variable | Total Number |
Readmission within 30 Days |
% | P* |
| Age, years | 0.06 | |||
| ≤ 60 | 378 | 32 | 8 | |
| > 60 | 242 | 32 | 13 | |
| Disease Histology | 0.04 | |||
| Lymphoma | 259 | 19 | 7 | |
| Multiple Myeloma | 361 | 45 | 12 | |
| Karnofsky Performance Score | 0.04 | |||
| ≤ 80 | 245 | 33 | 13 | |
| 90/100 | 375 | 31 | 8 | |
| Pre-Transplant Response | 0.20 | |||
| PD/SD | 58 | 9 | 16 | |
| PR/CR | 562 | 55 | 10 | |
| Atrial fibrillation | 0.18 | |||
| No | 571 | 56 | 10 | |
| Yes | 49 | 8 | 16 | |
| Intubation | 0.07 | |||
| No | 599 | 59 | 10 | |
| Yes | 21 | 5 | 24 | |
| Dialysis | 0.18 | |||
| No | 613 | 62 | 10 | |
| Yes | 7 | 2 | 29 | |
| HCT-CI | 0.02 | |||
| < 3 | 346 | 27 | 8 | |
| ≤ 3 | 274 | 37 | 14 | |
| Admission Duration | 0.04 | |||
| < 28 days | 589 | 57 | 10 | |
| ≤ 28 days | 31 | 7 | 23 | |
| Continuous Variable |
Readmission Status at Day 30 |
Mean |
Standard Deviation |
P* |
| Age, years | Not Readmitted | 54 | 12 | 0.07 |
| Readmitted | 57 | 10 | ||
| HCT-CI | Not Readmitted | 2.3 | 1.7 | 0.004 |
| Readmitted | 3.0 | 1.9 | ||
| Admission duration, days | Not Readmitted | 18.4 | 4.8 | 0.03 |
| Readmitted | 19.9 | 6.8 | ||
Likelihood ratio test of univariable logistic regression models for those variables with p<0.20
Abbreviations: PD: progressive disease; SD: stable disease; CR: complete response; PR: partial response
Table 4.
Multivariable Logistic Regression Modeling the Probability of Readmission within 30 Days of Discharge
| Variable | Coeff. | SE | OR | 95% CI | P |
|---|---|---|---|---|---|
| HCT-CI, ≥3 vs. >3 | 0.556 | 0.270 | 1.74 | 1.03–2.96 | 0.04 |
| Multiple Myeloma vs. Lymphoma | 0.639 | 0.295 | 1.89 | 1.06–3.38 | 0.03 |
| Length of Stay, ≥28 vs. < 28 days | 1.145 | 0.466 | 3.14 | 1.26–7.83 | 0.01 |
| Constant | −2.932 |
Figure 1.
Estimated probabilities of readmission with 95% confidence intervals for all combinations of the three risk factors: disease histology (multiple myeloma versus lymphomas), length of hospital stay (LOS, less than (LT) versus greater than or equal to (GE) 28), and hematopoietic stem cell transplant comorbidity index (HCT-CI, less than (LT) versus greater than or equal to (GE) 3).
Although the model including disease histology, HCT-CI (<3 versus > 3) and LOS (<28 days versus > 28 days) had excellent fit (goodness-of-fit test, p > 0.75), with estimated probabilities of readmission corresponding well to observed probabilities, the ability of the model to predict individual patients who would be readmitted within 30 days of discharge was better than random chance but with less than acceptable performance (ROC=0.64, 95% CI: 0.57–0.71). Using the predictive cut point of 0.10, the predictive values for readmission and no readmission, sensitivity, and specificity were 17%, 93%, 52%, and 70%, respectively, for an overall correct classification rate of 68%. Further, the false positive rate was 83%, reflecting the fact that regardless of the combination of values for the three variables in the model, none separate out patients with a probability of readmission close to 100%. Taken together, this suggests that although we have identified variables that place patients at a higher risk of readmission, there are other variables that may better identify the patients who will and will not be readmitted.
Survival Following ASCT
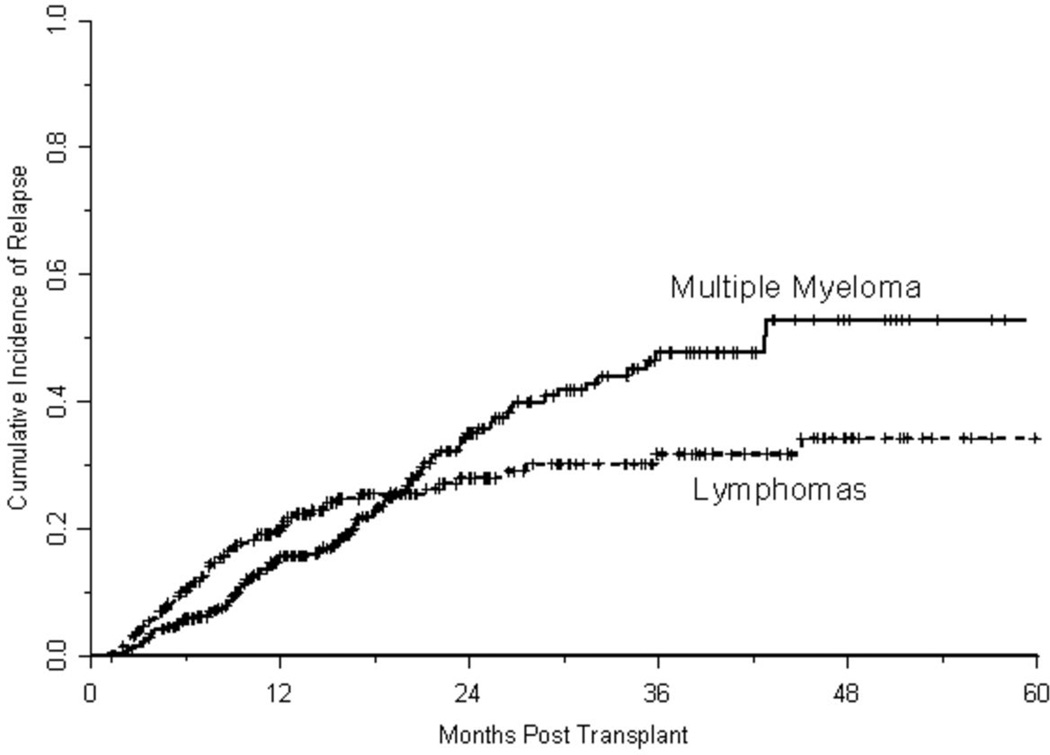
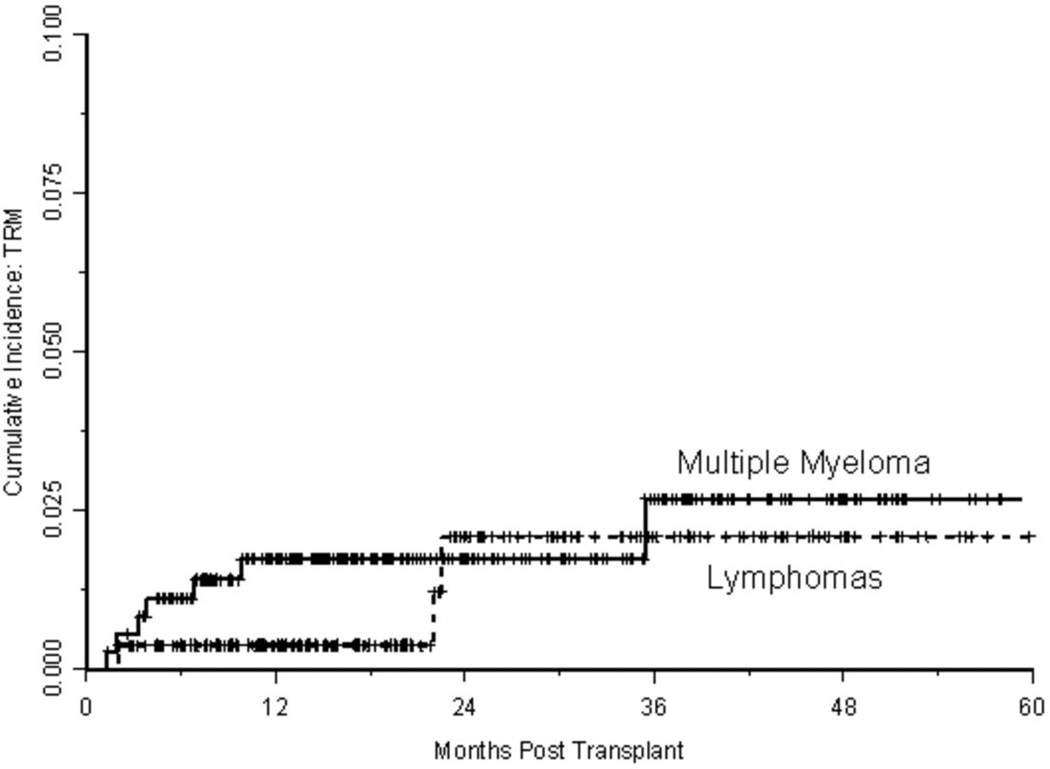
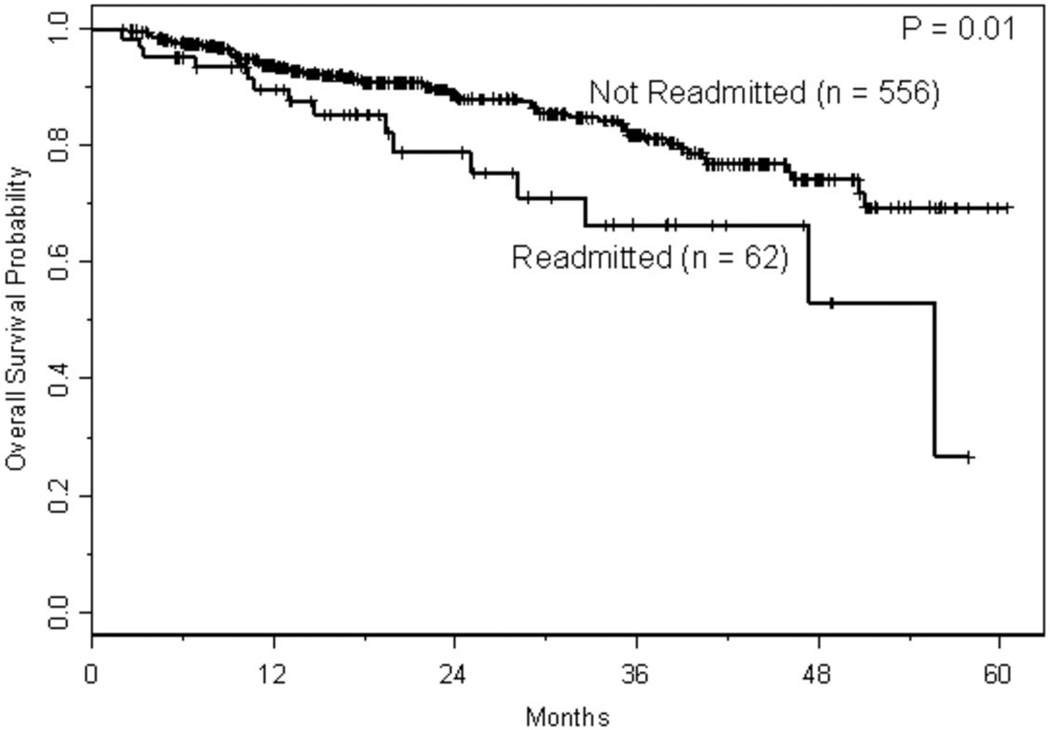
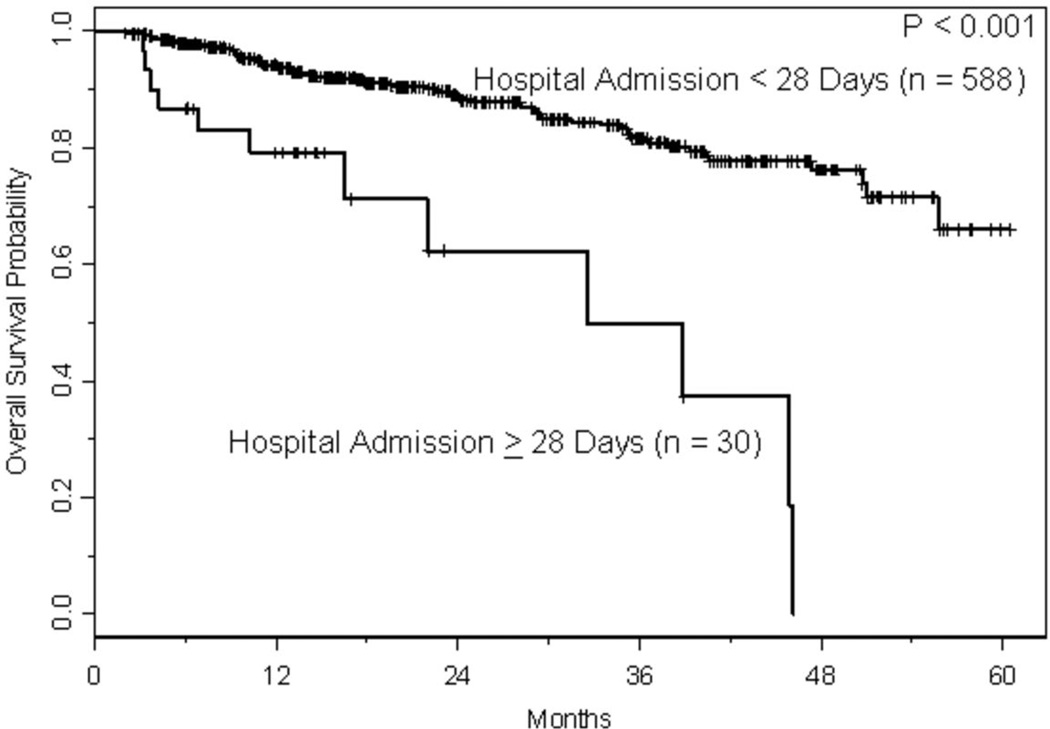
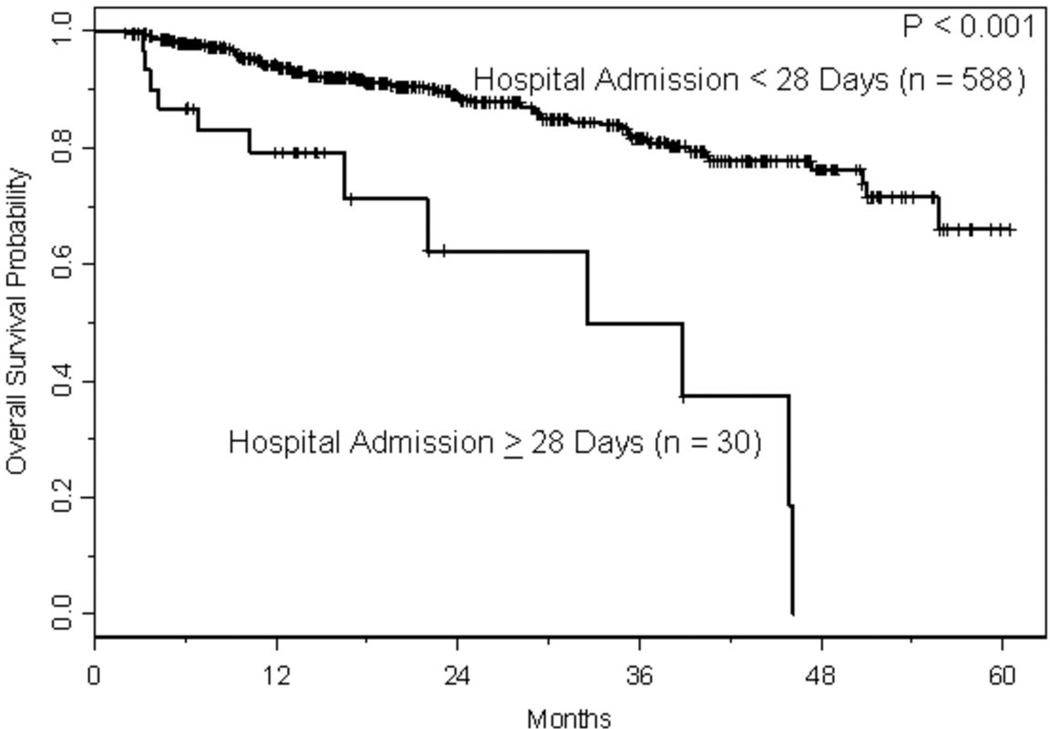
With a median follow-up of approximately 1.5 years, 166 relapses and 86 deaths have occurred, where 10 deaths were treatment-related. The estimated CIR at one and two years, respectively, was 17% (95% CI: 0.14–0.21) and 32% (95% CI: 0.27–0.37). As shown in Figure 2A, the risk of relapse was higher initially in patients with lymphoma, but had plateaued by 2 years, whereas the risk of relapse for those with MM was fairly constant with the CIR increasing steadily over the course of 3–4 years. The estimated TRM at one and two years, respectively, was 1.2% (95% CI: 0.003–0.021) and 1.9% (95% CI: 0.006–0.032). With only 10 TRM events, three had occurred in patients with lymphoma and seven in patients with MM. Two of the three events in patients with lymphomas occurred just prior to 2 years, whereas all but one of the events in patients with MM occurred within the first year following transplant (Figure 2B). Conditional on surviving 30 days following hospital discharge (n=618), survival estimates at one and two years were 92% (95% CI: 0.90–0.94) and 87% (95% CI: 0.83–0.90), respectively. Readmission within 30 days of discharge was associated with shorter survival (p=0.01; Figure 3A); among the variables predicting readmission status, only LOS discriminated between patients with longer versus shorter OS (p<0.001; Figure 3B), but disease histology and HCT-CI did not (p=0.30 and p=0.92, respectively). Not surprisingly, lack of a complete or partial response at time of transplant was significantly associated with OS (p<0.001; Figure 3C). Collectively, readmission by 30 days (HR 1.81, 95% CI 1.04–3.18, p=0.04), extended LOS (HR 4.93, 95% CI 2.65–9.18, p<0.001), and chemoresistance prior to ASCT (HR 3.08, 95% CI 1.74–5.43, p<0.001) predicted for inferior survival in a multivariable proportional hazards model for OS.
Figure 2.
a. Cumulative incidence of relapse curves based on disease histology.
b. Cumulative incidence of treatment-related mortality (TRM) curves based on disease histology.
Figure 3.
a. Kaplan-Meier curves for overall survival based on readmission within 30 days of discharge. Overall survival was calculated from a landmark time of 30 days following date of discharge until the date of death or last follow-up. Only patients who were still at risk 30 days following date of discharge were included.
b. Kaplan Meier curve for overall survival based on length of stay. Overall survival was calculated from a landmark time of 30 days following date of discharge until the date of death or last follow-up. Only patients who were still at risk 30 days following date of discharge were included.
c. Kaplan Meier curve for overall survival based on chemosensitivity prior to transplant. Overall survival was calculated from a landmark time of 30 days following date of discharge until the date of death or last follow-up. Only patients who were still at risk 30 days following date of discharge were included.
DISCUSSION
The role of ASCT is well established in patients with MM and relapsed/refractory diffuse large B cell lymphoma (DLBCL) and HL, but the role of HCT-CI for ASCT is less clear. A recent Center for International Blood and Marrow Transplant Research (CIBMTR) analysis of 11,652 patients who underwent ASCT showed that the three-year cumulative incidences of transplant-related mortality (TRM) according to HCT-CI scores of 0, 1–2 and 3 were 5%, 6% and 9%, respectively, (p<0.001) while corresponding 3-year OS probabilities were 79%, 73% and 70% (p<0.001), respectively, and this was not significantly different based on disease histology.14 Smaller, single-institution studies in MM and lymphoma have not been able to reproduce this.15,16 Our study was no different. Given the relatively low TRM among patients undergoing ASCT, a large sample is needed to detect an influence, so it is unsurprising that an ability to predict TRM using the HCT-CI has not been seen in single-institution studies. It also highlights the need for a tool that can provide more discriminative power in this setting.
Age has been traditionally used as an arbitrary exclusion criterion for transplant, and many clinical trials limit enrollment to patients under the age of 65, although the median age at diagnosis for most patients with hematologic malignancies ranges from 65 to 70 years. Several analyses of the influence of age on ASCT outcomes have identified no influence,18–21 although a retrospective analysis by the CIBMTR demonstrated that older patients with aggressive histologies of non-Hodgkin lymphoma (NHL) had inferior survivals.22 While the CCI score has been shown to influence OS among patients with NHL undergoing ASCT, patients over the age of 60 had toxicities and survivals comparable to younger patients. In that study, 100-day readmission rates were not significantly worse for patients over the age of 60, although the utility of the CCI in predicting readmission was not evaluated.17 Although in our study, age was moderately associated with 30-day readmission (p=0.06), patients over 60 years of age more often had longer admissions (p<0.0001), higher HCT-CI scores (p=0.02) and MM (p=0.13), three variables that better characterized readmission status than age alone. Once these variables were accounted for, age did not provide additional prognostic information (p>0.30).
In studies of Medicare patients, 30-day readmission rates range from 8–21%, with estimated annual costs of $17.4 billion.23,24 Readmission is increasingly being used as a surrogate measure for quality of care,25 and provisions of the Affordable Care Act now authorize lower payments from CMS to hospitals with high risk-standardized rates of readmission. Predictably, decreased OS in our cohort was associated with chemoresistance prior to transplant, and subsequently increased risk of relapse, on multivariable analysis. However, our data also indicated that readmission within 30 days and LOS of 28 or more days were independent predictors of decreased OS. In general, prolonged length of stay after ASCT is associated with complications that arise following conditioning, most often a result of chemotherapy-associated toxicity or as a result of the profound cytopenias seen with transplant. Following transplant, fever with negative blood cultures and prolonged gastrointestinal toxicity accounted for 44% of readmissions; the remainder of readmissions were from disparate causes, and based on the data presented, a reason for the relationship between 30-day readmission, LOS, and OS is not immediately apparent. We did not examine a potential relationship between 30-day readmission, LOS, and subsequent relapse rates.
Our analysis has demonstrated that an HCT-CI score of 3 or more is associated with increased odds of 30-day readmission following ASCT. This is in line with multiple studies demonstrating increased risk of readmission in patients with underlying chronic medical conditions. According to our model, the patients at highest risk for readmission are those with MM who have an HCT-CI of 3 or more and with an LOS of at least 28 days, although this represented fewer than 1% of patients in our study. A reasonable strategy to prevent readmission would be to target patients with one or more risk factor for earlier and more frequent follow-up after discharge, although, to date, no single intervention implemented alone has been consistently shown to reduce the rates of 30-day readmission.26
Based on model fit, our model estimates the probability of 30-day readmission following ASCT well, but it fails in determining who will and will not be readmitted, indicating that other strategies to identify at-risk patients need to be evaluated. Our study is limited by virtue of being a single-institution retrospective study with inherent institutional biases and would be strengthened by validation in a larger, multi-institution cohort, but it is thought-provoking. The HCT-CI can provide useful information regarding which patients are at higher risk for readmission following autologous transplant, but evaluation of other assessment tools, such as a comprehensive geriatric assessment,27,28 may better identify patients who could benefit from earlier rehabilitation intervention or more aggressive post-discharge care to prevent readmission or adverse outcomes associated with ASCT.
ACKNOWLEDGEMENTS
We thank Christina Bertram, Diane Scholl, and Becky Whittaker for their work as disease-specific transplant coordinators for multiple myeloma and lymphoma, as well as all of the staff involved in the care of our patients.
This work is supported by the National Cancer Institute (1K12 CA133250).
Footnotes
Financial Disclosure: The authors have no financial conflicts of interest to disclose.
References
- 1.Ashton CM, Kuykendall DH, Johnson ML, Wray NP, Wu L. The association between the quality of inpatient care and early readmission. Ann Intern Med. 1995;122:415–421. doi: 10.7326/0003-4819-122-6-199503150-00003. [DOI] [PubMed] [Google Scholar]
- 2.Ashton CM, Wray NP. A conceptual framework for the study of early readmission as an indicator of quality of care. Soc Sci Med. 1996;43:1533–1541. doi: 10.1016/s0277-9536(96)00049-4. [DOI] [PubMed] [Google Scholar]
- 3.Ashton CM, Del Junco DJ, Souchek J, Wray NP, Mansyur CL. The association between the quality of inpatient care and early readmission: a meta-analysis of the evidence. Med Care. 1997;35:1044–1059. doi: 10.1097/00005650-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Washington: U.S. Government Printing Office; 2010. United States., United States. Congress. House. Office of the Legislative Counsel, United States. Congress. House. Committee on Ways and Means., United States. Congress. House. Committee on Energy and Commerce., United States. Congress. House. Committee on Education and Labor. Compilation of Patient Protection and Affordable Care Act : as amended through November 1, 2010 including Patient Protection and Affordable Care Act health-related portions of the Health Care and Education Reconciliation Act of 2010. [Google Scholar]
- 5.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 6.Hasan O, Meltzer DO, Shaykevich SA, et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 25:211–219. doi: 10.1007/s11606-009-1196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capelastegui A, Espana Yandiola PP, Quintana JM, et al. Predictors of short-term rehospitalization following discharge of patients hospitalized with community-acquired pneumonia. Chest. 2009;136:1079–1085. doi: 10.1378/chest.08-2950. [DOI] [PubMed] [Google Scholar]
- 8.Chin MH, Goldman L. Correlates of early hospital readmission or death in patients with congestive heart failure. Am J Cardiol. 1997;79:1640–1644. doi: 10.1016/s0002-9149(97)00214-2. [DOI] [PubMed] [Google Scholar]
- 9.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bejanyan N, Bolwell BJ, Lazaryan A, et al. Risk factors for 30-day hospital readmission following myeloablative allogeneic hematopoietic cell transplantation (allo-HCT) Biol Blood Marrow Transplant. 2012;18:874–880. doi: 10.1016/j.bbmt.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 11.Jaing TH, Tsay PK, Yang CP, Hung IJ, Wen YC, Tseng CK. Evaluation of readmission in children receiving allogeneic hematopoietic stem cell transplantation: an institutional experience. Transplant Proc. 2008;40:3643–3645. doi: 10.1016/j.transproceed.2008.06.086. [DOI] [PubMed] [Google Scholar]
- 12.Rauenzahn S, Truong Q, Cumpston A, et al. Predictors and Impact of Thirty-Day Readmission on Patient Outcomes and Health Care Costs after Reduced-Toxicity Conditioning Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. doi: 10.1016/j.bbmt.2013.12.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dungarwalla M, Brennan J, Kulkarni S, et al. Duration of Initial Admission and Subsequent Readmission in the First 100 Days Following Reduced Intensity Conditioning Allogeneic Stem Cell Transplantation. ASH Annual Meeting Abstracts. 2007;110:1999. [Google Scholar]
- 14.Pasquini MC, Logan BR, Ho VT, et al. Comorbidity Index (CI) in Autologous Hematopoietic Cell Transplantation (HCT) for Malignant Diseases: Validation of the HCT-CI. ASH Annual Meeting Abstracts. 2012;120:814. [Google Scholar]
- 15.Lazaryan A, Bolwell B, Rybicki L, et al. The Role of Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI) in Autologous Stem Cell Transplantation for Multiple Myeloma. Biol Blood Marrow Transplant. 2012;18:S254. [Google Scholar]
- 16.Tamari R, Dahi P, Devlin S, et al. High Dose Therapy and Autologous Stem Cell Transplantation Results in Good Outcomes and Acceptable Toxicities in Elderly Patients with Non-Hodgkin's Lymphoma. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 19:S133. doi: 10.1016/j.bbmt.2014.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wildes TM, Augustin KM, Sempek D, et al. Comorbidities, not age, impact outcomes in autologous stem cell transplant for relapsed non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2008;14:840–846. doi: 10.1016/j.bbmt.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Hosmer DW, Lemeshow S. Applied logistic regression. ed 2nd. New York: Wiley; 2000. [Google Scholar]
- 19.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 20.Cox DR. Regression Models and Life-Tables. Journal of the Royal Statistical Society Series B-Statistical Methodology. 1972;34 187-+. [Google Scholar]
- 21.Jantunen E, Canals C, Attal M, et al. Autologous stem-cell transplantation in patients with mantle cell lymphoma beyond 65 years of age: a study from the European Group for Blood and Marrow Transplantation (EBMT) Ann Oncol. doi: 10.1093/annonc/mdr035. [DOI] [PubMed] [Google Scholar]
- 22.El Cheikh J, Kfoury E, Calmels B, et al. Age at transplantation and outcome after autologous stem cell transplantation in elderly patients with multiple myeloma. Hematol Oncol Stem Cell Ther. 4:30–36. doi: 10.5144/1658-3876.2011.30. [DOI] [PubMed] [Google Scholar]
- 23.Buadi FK, Micallef IN, Ansell SM, et al. Autologous hematopoietic stem cell transplantation for older patients with relapsed non-Hodgkin's lymphoma. Bone Marrow Transplant. 2006;37:1017–1022. doi: 10.1038/sj.bmt.1705371. [DOI] [PubMed] [Google Scholar]
- 24.Hosing C, Saliba RM, Okoroji GJ, et al. High-dose chemotherapy and autologous hematopoietic progenitor cell transplantation for non-Hodgkin's lymphoma in patients >65 years of age. Ann Oncol. 2008;19:1166–1171. doi: 10.1093/annonc/mdm608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazarus HM, Carreras J, Boudreau C, et al. Influence of age and histology on outcome in adult non-Hodgkin lymphoma patients undergoing autologous hematopoietic cell transplantation (HCT): a report from the Center For International Blood & Marrow Transplant Research (CIBMTR) Biol Blood Marrow Transplant. 2008;14:1323–1333. doi: 10.1016/j.bbmt.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker DW, Einstadter D, Husak SS, Cebul RD. Trends in postdischarge mortality and readmissions: has length of stay declined too far? Arch Intern Med. 2004;164:538–544. doi: 10.1001/archinte.164.5.538. [DOI] [PubMed] [Google Scholar]
- 27.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 28.Balla U, Malnick S, Schattner A. Early readmissions to the department of medicine as a screening tool for monitoring quality of care problems. Medicine (Baltimore) 2008;87:294–300. doi: 10.1097/MD.0b013e3181886f93. [DOI] [PubMed] [Google Scholar]
- 29.Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 155:520–528. doi: 10.7326/0003-4819-155-8-201110180-00008. [DOI] [PubMed] [Google Scholar]
- 30.Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hebert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet. 1999;353:205–206. doi: 10.1016/S0140-6736(98)04402-X. [DOI] [PubMed] [Google Scholar]
- 31.Speechley M, Tinetti M. Falls and injuries in frail and vigorous community elderly persons. J Am Geriatr Soc. 1991;39:46–52. doi: 10.1111/j.1532-5415.1991.tb05905.x. [DOI] [PubMed] [Google Scholar]
- 32.Campbell F, Vujanic GM. Bilateral femoral agenesis in femoral facial syndrome in a 19-week-old fetus. Am J Med Genet. 1997;72:315–318. doi: 10.1002/(sici)1096-8628(19971031)72:3<315::aid-ajmg12>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 33.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 34.Annweiler C, Bataille R, Ferriere N, Douillet D, Fantino B, Beauchet O. Plasma beta-2 microglobulin as a marker of frailty in older adults: a pilot study. J Gerontol A Biol Sci Med Sci. 66:1077–1079. doi: 10.1093/gerona/glr104. [DOI] [PubMed] [Google Scholar]
- 35.Hubbard RE, O'Mahony MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. 2009;13:3103–3109. doi: 10.1111/j.1582-4934.2009.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leng SX, Tian X, Matteini A, et al. IL-6-independent association of elevated serum neopterin levels with prevalent frailty in community-dwelling older adults. Age Ageing. 40:475–481. doi: 10.1093/ageing/afr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muffly LS, Boulukos M, Swanson K, et al. Pilot Study of Comprehensive Geriatric Assessment (CGA) in Allogeneic Transplant: CGA Captures a High Prevalence of Vulnerabilities in Older Transplant Recipients. Biol Blood Marrow Transplant. doi: 10.1016/j.bbmt.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Soubeyran P, Fonck M, Blanc-Bisson C, et al. Predictors of early death risk in older patients treated with first-line chemotherapy for cancer. J Clin Oncol. 2012;30:1829–1834. doi: 10.1200/JCO.2011.35.7442. [DOI] [PubMed] [Google Scholar]