Neutropenia after myeloablative conditioning regimens in cord blood transplant (CBT) recipients or following intensive chemotherapy for leukemia is associated with a high mortality risk from infectious complications necessitating interventions to reduce this neutropenic period.1,2 Several ex vivo expansion approaches have been identified that are capable of generating short-term hematopoietic repopulating cells derived from cord blood (CB) hematopoietic stem/progenitor cells (HSPC) that provide a myeloid bridge until hematopoietic reconstitution occurs.3–9 However, these have relied on real-time expansion of partially HLA-matched units with costs of the unit and manufacturing prohibitive for widespread use. Generation of greater HSPC per starting cell than currently achieved will be required to develop a cryopreserved product able to overcome HLA-matching barriers thereby making it readily and widely available.
Similar to models of leukemic cell self-renewal, we have undertaken a rational approach to maximize proliferation and inhibit differentiation to enhance generation of HSPC. We previously demonstrated that culture of CD34+ CB HSPC in the presence of Delta1Ext-IgG and growth factors results in a 16-fold increase in SCID repopulating cells (SRC).5 Likewise, Boitano et al. described an aryl hydrocarbon receptor (AhR) antagonist, Stem-Regenin1 (SR1), that results in a 17-fold increase in SRC.3 Here we demonstrate that SR1 combined with Delta1Ext-IgG generates a further 3-fold improvement in rapid repopulating cells (RRC) over either agent alone. This is mediated, at least in part, by the Notch target gene, HES1, consistent with the hypothesis that Delta1Ext-IgG is delaying differentiation in the presence of strong cytokine driven proliferative signals in SR1 cultures.
Human CB for research was obtained from normal deliveries under Swedish Medical Center Institutional Review Board (Seattle) approval after consent was obtained. Thawed and pooled CD34+ CB HSPC were cultured for 14–16 days (previously optimized5) with immobilized Delta1Ext-IgG or IgG (2.5 or 5 μg/ml) in StemSpan SFEM and cytokines (IL6, TPO, Flt-3 ligand, SCF (50 ng/ml)) and SR-1 (750nM).3,5 Quantitative PCR was performed on ABI PRISM 7700 (Applied Biosystems, NY, USA) with Taqman PCR Master Mix (Applied Biosystems, NY, USA) and primers for HES1 (Hs00172878_m1) and GUSB (Hs00939627_m1). Lentiviral wt-HES1 was a gift from Dr. Liyun Sang,10 and non-specific lentiviral control from Barbara Varnum-Finney. Sublethally irradiated (275 rad) NOD-SCID IL-2Rγ-null mice (NSG) approved for use by the Fred Hutchinson Cancer Research Center Institutional Animal Care and Use Committee were used for transplant. On average, TNC infused/mouse was 4.25 × 106 for Delta1ext-IgG, 1.88 × 107 for SR1, and 4.76 ×106 for the combination.
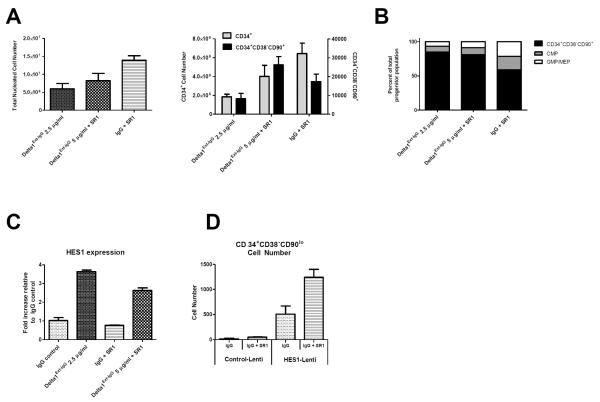
Addition of Delta1Ext-IgG (5μg/ml) to culture with SR1 significantly decreased generation of TNC as compared to SR1 alone (p<0.05, Figure 1A) with a trend towards decreased CD34+ cells (p=0.17, Figure 1A). However, greater numbers of the least mature CD34+CD38−CD90lo population11 were generated when Delta1Ext-IgG was present in cultures with SR1 compared to Delta1Ext-IgG alone (p=0.05) with a trend toward greater numbers compared to SR1 alone (p=0.14, Figure 1A). Analysis of CD34+CD38−CD90lo, common myeloid progenitors (CMP) and granulocyte-monocyte and megakaryocyte-erythrocyte (GMP/MEP) cells12 (Figure 1B) revealed the proportion of CD34+CD38−CD90lo cells in cultures with Delta1Ext-IgG and SR1 was equivalent to the proportion in cultures with Delta1Ext-IgG alone (p=0.33); however, both were significantly greater than in cultures with SR1 alone (p=0.04, p=0.02 respectively). In contrast, there was a relatively greater percent of more mature CMP and GMP/MEP cells in culture with SR1 alone as compared to Delta1Ext-IgG with or without SR1 (p=0.07, p=0.03, respectively), suggesting that Delta1Ext-IgG is further delaying myeloid differentiation resulting in enhanced generation of the least mature progenitors.
Figure 1. Delta1Ext-IgG delays differentiation of CB HSPC cultured with SR1 mediated at least in part through HES1 activation.
(A) CD34+ CB HSPC were cultured for 14 days in the presence of Delta1Ext-IgG (2.5 μg/ml), SR1 (750 nM), or the combination (SR1 750nM, Delta1Ext-IgG 5 μg/ml). Total nucleated cell (TNC) number was significantly decreased with Delta1Ext-IgG with or without SR1 as compared to SR1 alone (p<0.05, p= 0.004). CD34+ cell number generated was significantly decreased in culture with Delta1Ext-IgG as compared to SR1 alone (p=0.004) and approached significance when the combination was compared to SR1 alone (p=0.17). Greater numbers of CD34+CD38−CD90+ cells11 were generated when Delta1Ext-IgG was added to culture with SR1 as compared to culture with either Delta1Ext-IgG or SR1 alone (p=0.05, p=0.14). Results represent 5 independent experiments as mean +/− SEM. Statistical analysis was performed using 2-tailed, unpaired t-test.
(B) The proportion of CD34+CD38−CD90+ cells in culture with the combination as compared to Delta1Ext-IgG was equivalent (p=0.33) but both were significantly greater than seen in cultures containing SR1 (p=0.04 and p=0.02). Relatively greater percent of more mature common myeloid progenitors (CMP, CD34+CD38+CD123loCD45RA−)12, and granulocyte-monocyte/megakaryocyte-erythroid progenitors (GMP/MEP; CD34+CD38+CD123+CD45RA−/CD34+CD38+CD123−CD45RA−)11,12, populations were observed in culture with SR1 alone as compared to Delta1Ext-IgG or Delta1Ext-IgG and SR1 in combination (p=0.03, p=0.07). Results represent 5 independent experiments as mean +/− SEM. Statistical analysis was performed using 2-tailed, unpaired t-test.
(C) HES1 expression was assessed by quantitative PCR (qPCR) after cells were cultured for 6 hours on IgG, Delta1Ext-IgG, SR1, or Delta1Ext-IgG and SR1 in combination. HES1 expression was normalized to GUSB. HES1 expression was 3.5-fold higher in the Delta1Ext-IgG groups as compared to IgG control (p=0.0001) and 4.5-fold higher than SR1 alone (p<0.0001). HES1 expression when Delta1Ext-IgG was added to culture with SR1 was nearly 3.5-fold higher than in culture with SR1 alone (p=0.0002). Cells cultured with Delta1Ext-IgG alone had greater HES1 expression than cells cultured in the combination (p=0.0035). Results represent 3 independent experiments as mean +/− SEM. Statistical analysis was performed using 2-tailed, unpaired t-test.
(D) 1×104 CD34+ CB HSPC were cultured overnight in the presence of IgG (5 μg/ml) with or without SR1 (750nM) and transduced the following day with wt-HES1 or control with a multiplicity of infection of 25. On day 6 of culture, CB HSPC were sorted for GFP using BD FACSAria cell sorter. After 14 days in culture, GFP expressing cells were assessed for numbers of the least mature, CD34+CD38−CD90lo HSPC. There was a 2.5-fold increase in number of least mature HSPC generated when SR1 was added to culture with HES1 transduced HSPC (p=0.08). Culture of HES1 transduced cells in the presence of SR1 generated 25-fold more CD34+CD38−CD90lo HSPC than cells transduced with lenti-control (p=0.02). Results represent 2 independent experiments as mean +/− SEM. Statistical analysis was performed using 2-tailed, unpaired t-test.
We next determined whether induction of HES1 in cells cultured in the presence of Delta1Ext-IgG accounted, at least in part, for the ability of Delta1Ext-IgG to enhance SR1 induced effects on expansion of HSPC. HES1 expression was found to be 3.5-fold higher in cultures with Delta1Ext-IgG alone compared to IgG control and over 4.5-fold greater than SR1 alone (Figure 1C). When Delta1Ext-IgG was added to cultures with SR1, HES1 induction was nearly 3.5-fold greater than with SR1 alone, suggesting that Delta1Ext-IgG induces HES1 expression in cells cultured with SR1 and this increase may be responsible at least in part for the delay in differentiation and enhanced early precursor generation seen in these cultures. Interestingly, cells cultured with Delta1Ext-IgG alone had significantly greater HES1 expression as compared to those cultured in the combination (p=0.0035), possibly due to down-regulation of HES1 expression through AhR antagonism as AhR has been shown to up-regulate HES1 expression.13
Based on ex vivo studies of murine progenitors demonstrating that over-expression of Hes1 inhibits myeloid differentiation and enhances generation of multi-potent progenitor cells14, we determined whether HES1 overexpression in CB HSPC combined with SR1 might phenocopy effects of combining Delta1Ext-IgG with SR1. We found 5-fold higher HES1 expression in HES1-transduced cells cultured on IgG as compared to control-transduced cells and 2-fold higher expression for HES1 overexpressing cells as compared with control-infected cells in the presence of SR1 (data not shown). Importantly, culture of HES1-transduced cells in the presence of SR1 generated 25-fold more CD34+CD38−CD90lo cells than culture with cells transduced with lenti-control and SR1 (Figure 1D). To further illuminate the mechanism whereby HES1 expression affects culture with SR1, we attempted to abrogate HES1 expression through shRNA in CB HSPC cultured in the presence of Delta1Ext-IgG, SR1 or the combination; however, despite GFP expression consistent with successful transduction of shRNA constructs, HES1 expression was unchanged as compared to control cultures.
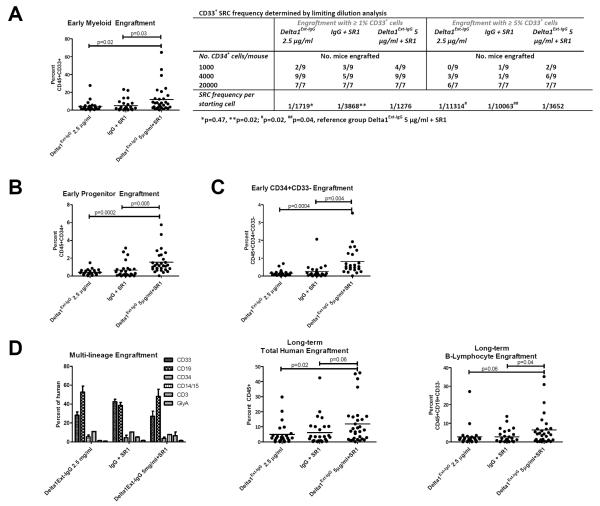
We next determined the functional significance of CB HSPC generated in the presence of Delta1Ext-IgG and/or SR1 by assessing their in vivo repopulating capability in immune deficient mice. We found significantly enhanced rapid myeloid reconstitution 2 weeks after transplantation from cells cultured with the combination in comparison with either factor alone (Figure 2A). Limiting dilution transplants revealed a significant nearly 3-fold increase in frequency of RRC able to reconstitute CD33+ myeloid cells in mice that received cells cultured in the presence of Delta1Ext-IgG and SR1 as compared to either alone based on robust, greater than 5% marrow repopulation that is nearly completely myeloid at this early time post-engraftment (Figure 2A). At a more limiting level of marrow repopulation (1%), we also found a significant 3-fold difference between cells cultured with SR1 alone and the combination. However, the 1½-fold difference found between cells cultured with Delta1Ext-IgG alone or in the presence of Delta1Ext-IgG and SR1 was not statistically significant, suggesting the difference in RRC frequency between SR1 and Delta1Ext-IgG at a more robust (5%) versus less robust (1%) level of marrow repopulation may reflect generation of less mature precursors with greater proliferative potential. Consistent with this hypothesis, the percent of CD34+ progenitor cells present in the marrow was also significantly greater due to transplantation of cells cultured with both Delta1Ext-IgG and SR1 compared with SR1 or Delta1Ext-IgG alone (Figure 2B). Further analysis revealed that a higher proportion of the CD34+ cells in the marrow reconstituted with cells cultured with SR1 and Delta1Ext-IgG were CD33− compared with those derived from cultures containing SR1 or Delta1Ext-IgG alone (Figure 2C) further suggesting the more primitive nature of the precursors generated by SR1 and Delta1 Ext-IgG.
Figure 2. Delta1Ext-IgG and SR1 in combination enhance generation of early progenitor and myeloid repopulating cells.
CD34+ selected CB HSPC were cultured for 16 days in the presence of Delta1Ext-IgG 2.5 μg/ml, IgG 2.5μg/ml with SR1 or the combination with Delta1Ext-IgG 5 μg/ml. The cultured progeny of 10,000 starting CD34+ cells were transplanted into NOD-SCID IL-2Rγ-null mice (NSG) and early and late repopulating capability assessed at 2 weeks by bone marrow aspirates and 12–14 weeks by bone marrow harvests. (A) Early myeloid (percent CD45+CD33+), (B) progenitor (percent CD45+CD34+), and (C) more immature progenitor (percent CD45+CD34+CD33−) were assessed. For limiting dilution transplant (A), mice were injected with the cultured progeny of 1000, 4000, or 20,000 CD34+ cells and bone marrow aspirates performed at 2 weeks. Two engraftment cut-offs were chosen based on percent engraftment observed in non-limiting transplantation studies. The frequency of SCID-repopulating cells (SRC) was determined by the method of maximum likelihood with L-CALC software (StemCell Technologies) from the proportions of engrafted recipients.
(D) All groups demonstrated multi-lineage engraftment with longer-term primary transplantation with enhanced total human and B-lymphoid (percent CD45+CD19+CD33−) engraftment in the combination group.
All p-values represent unpaired, two-tailed t-tests (GraphPad software) and are displayed on graphs with lines denoting comparisons. Results shown represent 4 independent experiments. Dots represent individual mice and line represents mean engraftment.
Of note, rapid repopulation by B-lymphoid cells (percent CD45+CD33−CD19+) was significantly enhanced by cells cultured in the presence of SR1 alone in comparison with cells cultured with Delta1Ext-IgG +/− SR1 (data not shown). This may be a result of Notch1-mediated inhibition of the differentiation of multi-potent precursors combined with enhanced generation of immature B-cells due to AhR inhibition as suggested by findings in AhR receptor-null mice.15
Evaluation of longer term repopulation at 12–14 weeks revealed multi-lineage engraftment in all groups (Figure 2D). While overall human engraftment was higher due to cells cultured with Delta1Ext-IgG and SR1 compared with SR1 or Delta1Ext-IgG alone (Figure 2D), this was primarily due to enhanced B-lymphoid engraftment (Figure 2D). It is not clear whether these lymphoid cells were newly derived or represented longer lived cells developed during earlier time points. While low-level CD3+ engraftment was present in all groups at this later time point, it constituted less than 0.6% of total marrow engraftment and was not significantly different between the groups (data not shown). However, it is not known whether differences in engraftment would have been discerned in the presence of human thymic explants in NSG mice. Following secondary transplantation, no differences were found between the groups (average CD45+ 0.22% in Delta1Ext-IgG, 0.11% in SR1, and 0.18% in the combination, data not shown) demonstrating all three culture conditions generate similar long-term engrafting cells.
Our data indicate that the addition of Delta1Ext-IgG to SR1 enhances the generation of more robust, rapid repopulating cells. Given the fixed collection volume and cellular yield obtained from an individual cord blood unit, the ability to generate greater numbers of HSPC from a given cord blood unit without increasing the utilization of culture media, cytokines or other costly materials further allows development of cost-effective HSPC expansion methodologies. Moreover, generation of greater numbers of HSPC than currently achieved has the potential to make use of the large inventory of cord blood units currently too small for clinical application. This will be critical when developing cellular therapy for treating neutropenia outside of the CBT setting.
Acknowledgments
Sources of Support: This work was supported by National Heart, Lung and Blood Institute grant U01HL100395 and National Institutes of Health Ruth L. Kirschstein National Research Service Award T32CA009351 (AD) and K12CA076930 (AD). CD is a Damon Runyon Clinical Investigator. Anthony Boitano and Michael Cooke (Genomics Institute of the Novartis Research Foundation, La Jolla, CA) provided SR1.
Footnotes
Conflicts of Interest: The Fred Hutchinson Cancer Research Center holds a patent on “methods for immortalizing cells” that covers the use of Notch ligand for expansion of hematopoietic stem cells. IDB is an inventor on this patent. AD, CB, CD have no conflicts of interest.
References
- 1.Canner J, Alonzo TA, Franklin J, Freyer DR, Gamis A, Gerbing RB, et al. Differences in outcomes of newly diagnosed acute myeloid leukemia for adolescent/young adult and younger patients: a report from the Children's Oncology Group. Cancer. 2013;119(23):4162–4169. doi: 10.1002/cncr.28342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunstein CG, Gutman JA, Weisdorf DJ, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116(22):4693–4699. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boitano AE, Wang J, Romeo R, Bouchez LC, Parker AE, Sutton SE, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329(5997):1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahlberg A, Delaney C, Bernstein ID. Ex vivo expansion of human hematopoietic stem and progenitor cells. Blood. 2011;117(23):6083–6090. doi: 10.1182/blood-2011-01-283606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16(2):232–236. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Lima M, McMannis J, Gee A, Komanduri K, Couriel D, Andersson BS, et al. Transplantation of ex vivo expanded cord blood cells using the copper chelator tetraethylenepentamine: a phase I/II clinical trial. Bone marrow transplant. 2008;41(9):771–778. doi: 10.1038/sj.bmt.1705979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Lima MR, McNiece I, Robinson SN, Munsell M, Eapen M, Horowitz M, et al. Cord blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012;367(24):2305–2315. doi: 10.1056/NEJMoa1207285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horowitz M, Stiff PJ, Chao NJ, Rizzieri D, Long G, Sullivan K, et al. Nicord® Expanded Hematopoietic Progenitor Cells (HPC) Are Capable of Outcompeting the Unmanipulated (UM) Cord Blood Unit and of Prolonged Myeloid and Lymphoid Engraftment Following Myeloablative Dual Umbilical Cord Blood (UCB) Transplantation. Biol Blood Marrow Transplant. 2013;19(2):S118. Abstract 18. [Google Scholar]
- 9.Wagner JE, Brunstein CG, McKenna D, Sumstad D, Maahs S, Boitano AE, et al. Safety and exploratory efficacy of ex-vivo expanded umbilical cord blood (UCB) hematopoietic stem and progenitor cells (HSPC) using cytokines and Stem-Regenin 1 (SR1): Interim results of a phase 1/2 dose escalation clinical study. Blood. 2013;122(21) Abstract 698. [Google Scholar]
- 10.Sang L, Coller HA, Roberts JM. Control of the reversibility of cellular quiescence by the transcriptional repressor HES1. Science. 2008;321(5892):1095–1100. doi: 10.1126/science.1155998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majeti R, Park CY, Weissman IL. Identification of a hierarchy of multipotent progenitors in human cord blood. Cell Stem Cell. 2007;1(6):635–645. doi: 10.1016/j.stem.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manz MG, Miyamoto T, Akashi K, Weissman IL. Prospective isolation of human clonogenic common myeloid progenitors. Proc Natl Acad Sci USA. 2002;99(18):11872–11877. doi: 10.1073/pnas.172384399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomsen JS, Kietz S, Ström A, Gustafsson J. HES-1, a novel target gene for the aryl hydrocarbon receptor. Mol Pharmacol. 2004;65(1):165–171. doi: 10.1124/mol.65.1.165. [DOI] [PubMed] [Google Scholar]
- 14.Kunisato A, Chiba S, Nakagami-Yamaguchi E, Kumano K, Saito T, Masuda S, et al. HES-1 preserves purified hematopoietic stem cells ex vivo and accumulates side population cells in vivo. Blood. 2003;101(5):1777–1783. doi: 10.1182/blood-2002-07-2051. [DOI] [PubMed] [Google Scholar]
- 15.Singh KP, Garrett RW, Casado FL, Gasiewicz TA. Aryl hydrocarbon-receptor null allele mice have hematopoietic stem/progenitor cells with abnormal characteristics and functions. Stem Cells Dev. 2011;20(5):769–784. doi: 10.1089/scd.2010.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]