Abstract
Importance
According to evidence-based, expert recommendations, long-term users of chloroquine (CQ) or hydroxychloroquine (HCQ) should undergo regular visits to eye-care providers and diagnostic testing to check for maculopathy.
Objective
To determine whether patients with rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE) taking CQ or HCQ are regularly visiting eye-care providers and being screened for maculopathy.
Setting, Design and Participants
Patients with RA or SLE who were continuously enrolled in a particular managed-care network for ≥5 years during 2001-2011 were studied. Patients' amount of CQ/HCQ use in the 5 years since initial RA/SLE diagnosis was calculated, along with their number of eye-care visits and diagnostic tests for maculopathy. Those at high risk for maculopathy were identified. Visits to eye providers and diagnostic testing for maculopathy were assessed for each enrollee over the study period. Logistic regression was performed to assess potential factors associated with regular eye-care-provider visits (≥3 in 5 years) among CQ/HCQ users, including those at greatest risk for maculopathy.
Main Outcome Measures
Among CQ/HCQ users and those at high risk for toxic maculopathy, the proportions with regular eye-care visits and diagnostic testing, and the likelihood of regular eye-care visits (odds ratios [ORs] with 95% confidence intervals [CI]).
Results
Among 18,051 beneficiaries with RA or SLE, 6,339 (35.1%) had ≥1 record of HCQ/CQ use and 1,409 (7.8%) used HCQ/CQ for ≥4 years. Among those at high risk for maculopathy, 27.9% lacked regular eye-provider visits, 6.1% had no visits to eye providers, and 34.5% had no diagnostic testing for maculopathy during the 5-year period. Among high-risk patients, each additional month of HCQ/CQ use was associated with a 2.0%-increased likelihood of regular eye care (adjusted OR=1.02, CI=1.01-1.03). High-risk patients whose SLE/RA were managed by rheumatologists had a 77%-increased likelihood of regular eye care (adjusted OR=1.77, CI=1.27-2.47), relative to other patients.
Conclusions and Relevance
In this insured population, many patients at high risk for HCQ/CQ-associated maculopathy are not undergoing routine monitoring for this serious side effect. Future studies should explore factors contributing to suboptimal adherence to expert guidelines and the potential impact on patients' vision-related outcomes.
Hydroxychloroquine (HCQ) and chloroquine (CQ) are commonly used medications for rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and other connective tissue disorders.1-5 More than 1.5 million persons have these conditions,6 and many of them receive HCQ as the initial treatment.7-10 Although HCQ is safer than CQ, both medications can cause irreversible retinal toxicity. Early in the course of retinal toxicity, patients are often asymptomatic but exhibit abnormalities on fundus examination or diagnostic testing. Since no effective treatment exists for HCQ/CQ-induced retinopathy, early identification of this side effect and prompt discontinuation of therapy are essential.11-13 Periodic monitoring with ocular examinations and diagnostic testing is therefore crucial.
Although no gold-standard test can identify HCQ/CQ-associated retinopathy, American Academy of Ophthalmology (AAO)–published guidelines in 2002 recommend that, first, all patients using these agents should have a baseline ocular examination within 1 year after HCQ/CQ therapy commences. In addition, annual retinopathy screening is recommended for anyone with >5 years' exposure to HCQ/CQ or those at high risk for toxic maculopathy—including persons who are ≥60 years; have renal or hepatic disease, obesity, or concomitant retinal disease; use >6.5 mg/kg of ideal body weight daily of HCQ; or use >3.0 mg/kg daily of CQ.14 Retinal toxicity screening involves a dilated eye examination, Amsler grid or Humphrey 10-2 visual field (VF) testing (Carl Zeiss Meditec, Dublin, CA), optional color-vision testing, fundus photography, fluorescein angiography (FA), or multifocal electroretinography (mfERG).
Revised recommendations, issued in 2011, additionally defined high-risk patients as anyone with cumulative dosing >1000 g of HCQ or >600 g of CQ. The updated guidelines no longer recommended Amsler grid testing, fundus photography, color-vision testing, or FA for screening; instead, use of spectral-domain optical coherence tomography (SD-OCT), mfERG, or fundus autofluorescence (FAF) was encouraged. Still recommended was annual screening of high-risk patients.15
Retinal toxicity rates from HCQ/CQ have been documented.13,16-19 However, to our knowledge, no prior study has investigated adherence to the AAO guidelines on ophthalmologic care for HCQ/CQ users. We assessed whether patients with RA/SLE taking HCQ/CQ had regular eye-care-provider visits and testing to check for maculopathy, as the guidelines recommend.
Methods
Data Source
The Clinformatics database (OptumInsight, Eden Prairie, MN) contains de-identified records of all beneficiaries in a nationwide managed-care network. We accessed data on all eye-care recipients—patients with ≥1 International Classification of Diseases20 (ICD-9-CM) codes for any eye-related diagnosis (360-379.9) or Current Procedural Terminology21 (CPT-4) code for any eye-related visit, or diagnostic or therapeutic procedure (65091-68899, 92002-92499); or any ophthalmologist- or optometrist-submitted claim—in 2001-2011. The database includes claims for all ocular and nonocular medical conditions, records of all outpatient medications prescribed, and sociodemographic information (age, sex, race, education, income) for the enrollees.22-24
Sample Selection
All persons with continuous plan enrollment for ≥5 years after their initial RA/SLE diagnosis were identified based on ICD-9-CM billing codes (SLE-710.0, RA-714.0). To help address concerns about miscoding, we also required ≥1 confirmatory RA/SLE diagnosis made on a different date. Noncontinuously enrolled beneficiaries were excluded, as were enrollees with <5 years of postdiagnosis follow-up.
Chloroquine/Hydroxychloroquine Use
We reviewed outpatient pharmacy records to identify HCQ/CQ users on the basis of American Hospital Formulary Service drug-class codes for Aralen Hydrochloride, Aralen Phosphate, Chloroquine Phosphate, Hydroxychloroquine Sulfate, and Plaquenil. We calculated the number of days of use of these medications in the 5-year period after patients' first-documented RA/SLE diagnosis for which HCQ/CQ was prescribed. Patients' drug exposure in the 5-year period was categorized as none, <12, 12-23, 24-47, or ≥48 months.
Eye-Care Visits
We determined the proportion of patients with RA/SLE who visited an eye-care provider (ophthalmologist/optometrist) in each of the 5 years after initial diagnosis. Visits were identified by using CPT codes. (etable1) Regular eye care meant annual visits in ≥3 of the 5 years.
Diagnostic Testing
We used billing codes to assess utilization of perimetry (CPT 92081-3), mfERG (92275), FA (92235), and fundus photography (92250). We also assessed OCT utilization (92134, 92135), which was not specifically recommended in the 2002 guidelines but became widely used in evaluating macular disease over the past decade and was included in the 2011 guidelines.15
High-Risk Patients
On the basis of the 2002 guidelines, high risk for HCQ/CQ-associated maculopathy meant the patient was aged ≥60 years, or used HCQ/CQ for ≥1 of the first 5 years after initial diagnosis and had concomitant renal, hepatic, or retinal disease (non-neovascular/neovascular age-related macular degeneration). (etable1)
Greatest Chloroquine/Hydroxychloroquine Users
We also assessed eye-services utilization for patients with the most HCQ/CQ exposure, identifying persons prescribed these medications for ≥4 of the 5 years examined.
New Chloroquine/Hydroxychloroquine Users
In another analysis, we identified patients with SLE/RA who had no recorded HCQ/CQ use in their first 3 years in the plan who subsequently began using HCQ/CQ. Among these new users, the proportion undergoing a baseline examination in the first year of therapy was calculated.
Chloroquine/Hydroxychloroquine Use for ≥5 Years
Since according to the AAO guidelines, all patients with ≥5 years' HCQ/CQ exposure should receive annual examinations, irrespective of other risk factors present, we also analyzed, separately, patients with continuous HCQ/CQ use for ≥5 years to assess eye visits and diagnostic testing in years 6-7.
Analyses
Statistical analysis was performed using SAS 9.3 (SAS Institute, Cary, NC). Visits, diagnostic testing, and HCQ/CQ use were summarized by using means and standard deviations (SDs) for continuous variables and frequencies and percentages for categorical variables.
We compared the frequency of eye visits and maculopathy-related diagnostic testing between patients with RA/SLE who did and did not have the most HCQ/CQ exposure and between those with and without high maculopathy risk by conducting chi-square, Fisher-exact, and two-sample t-tests.
In the first three of four logistic regression models, we evaluated (1) the potential impact of each additional month's HCQ/CQ use on the likelihood of receiving regular eye care among all patients with RA/SLE, (2) the odds of regular eye care among the patients at high risk for HCQ/CQ-associated maculopathy, and (3) potential factors associated with regular eye care among the highest HCQ/CQ users. The fourth model calculated the odds of regular eye care among an aggregated sample of the greatest HCQ/CQ users and those with high maculopathy risk. Covariates for all models included age, sex, race, education, income, diabetes with/without complications, hypertension with/without complications, depression, care by a rheumatologist (≥2 visits during the study), and long-term (≥3 months) oral corticosteroid use. Complicated disease meant the patient had diabetes/hypertension-related end-organ damage (e.g., nephropathy). (eTable1) Regression analyses generated odds ratios (ORs) with 95% confidence intervals (CIs). For all analyses, p<0.05 was considered statistically significant. This study was exempt from requiring University of Michigan Institutional Review Board approval.
Results
18,051 enrollees met the inclusion criteria. (eFigure1) 13,102 patients (72.6%) had RA, 3,385 (18.8%) had SLE, and 1,564 (8.7%) had ≥2 records of both conditions. Participants' mean (±SD) age at enrollment was 51.6±12.1 years. Among the participants, 78.2% were female (n=14,114); 83.5% (n=13,850) were white, 5.3% (n=883) black, and 8.1% (n=1,342) Latino. (Table 1) Seventy-two percent of participants (n=12,964) received care from a rheumatologist.
Table 1. Characteristics of Study Population.
| Chloroquine / Hydroxychloroquine Use** | |||||
|---|---|---|---|---|---|
| Yes | No | Overall | p-value | ||
| Total | 6,339 (35.1) | 11,712 (64.9) | 18,051 (100.0) | ||
| Rheumatologic Condition (Nmiss = 0) | SLE | 1,768 (27.9) | 1,617 (13.8) | 3,385 (18.8) | < 0.0001‡ |
| Rheumatoid Arthritis | 3,570 (56.3) | 9,532 (81.4) | 13,102 (72.6) | ||
| Both | 1,001 (15.8) | 563 (4.8) | 1,564 (8.7) | ||
| Sex (Nmiss = 0) | Male | 1,004 (15.8) | 2,933 (25.0) | 3,937 (21.8) | < 0.0001‡ |
| Female | 5,335 (84.2) | 8,779 (75.0) | 14,114 (78.2) | ||
| Race (Nmiss = 1,459) | White | 4,910 (84.3) | 8,940 (83.0) | 13,850 (83.5) | 0.003‡ |
| Black | 325 (5.6) | 558 (5.2) | 883 (5.3) | ||
| Latino | 406 (7.0) | 936 (8.7) | 1,342 (8.1) | ||
| Asian | 131 (2.2) | 233 (2.2) | 364 (2.2) | ||
| Other | 55 (0.9) | 98 (0.9) | 153 (0.9) | ||
| Education (Nmiss = 509) | Less than High School | 86 (1.4) | 174 (1.5) | 260 (1.5) | 0.0118‡ |
| High School Diploma | 2,057 (33.3) | 3,906 (34.4) | 5,963 (34.0) | ||
| Some College | 2,500 (40.5) | 4,721 (41.5) | 7,221 (41.2) | ||
| College Diploma | 1,523 (24.7) | 2,549 (22.4) | 4,072 (23.2) | ||
| Advanced Degree | 12 (0.2) | 14 (0.1) | 26 (0.1) | ||
| Household Income (Nmiss = 1,126) | < $30,000 | 468 (7.9) | 944 (8.6) | 1,412 (8.3) | 0.0230‡ |
| $30,000 – $60,000 | 1,966 (33.1) | 3,796 (34.6) | 5,762 (34.0) | ||
| $60,000 – $100,000 | 2,139 (36.0) | 3,912 (35.6) | 6,051 (35.8) | ||
| $100,000 – $125,000 | 759 (12.8) | 1,291 (11.8) | 2,050 (12.1) | ||
| > $125,000 | 613 (10.3) | 1037 (9.4) | 1,650 (9.7) | ||
| Medication Use (Nmiss = 0) | CQ use only | 36 (0.6) | 0 (0.0) | 36 (0.2) | < 0.0001‡ |
| HCQ use only | 6,248 (98.6) | 0 (0.0) | 6,248 (34.6) | ||
| Both medications | 55 (0.9) | 0 (0.0) | 55 (0.3) | ||
| Neither | 0 (0.0) | 11,712 (100.0) | 11,712 (64.9) | ||
| Medication Use Duration (Nmiss = 0) | No Medication Use | 0 (0.0) | 11,712 (100.0) | 11,712 (64.9) | < 0.0001‡ |
| < 12 Months | 2,150 (33.9) | 0 (0.0) | 2,150 (33.9) | ||
| 12-23 Months | 914 (14.4) | 0 (0.0) | 914 (14.4) | ||
| 24-47 Months | 1866 (29.4) | 0 (0.0) | 1866 (29.4) | ||
| ≥ 48 Months | 1409 (22.2) | 0 (0.0) | 1409 (22.2) | ||
| Eye Visits (Nmiss = 0) | No eye visits | 906 (14.3) | 3,308 (28.2) | 4,214 (23.3) | < 0.0001‡ |
| One Year | 1,135 (17.9) | 2,811 (24.0) | 3,946 (21.9) | ||
| Two years | 1,041 (16.4) | 1,774 (15.1) | 2,815 (15.6) | ||
| Three years | 906 (14.3) | 1,494 (12.8) | 2,400 (13.3) | ||
| Four years | 1,034 (16.3) | 1,133 (9.7) | 2,167 (12.0) | ||
| Five years | 1,317 (20.8) | 1,192 (10.2) | 2,509 (13.9) | ||
| Depression | No | 5,360 (84.6) | 10,009 (85.5) | 15,369 (85.1) | 0.1‡ |
| Yes | 979 (15.4) | 1,703 (14.5) | 2,682 (14.9) | ||
| Hypertension | None | 2,241 (35.4) | 3,250 (27.7) | 5,491 (30.4) | < 0.0001‡ |
| Uncomplicated | 3,121 (49.2) | 6,238 (53.3) | 9,359 (51.8) | ||
| Complicated | 977 (15.4) | 2,224 (19.0) | 3,201 (17.7) | ||
| Diabetes mellitus | None | 4,782 (75.4) | 7,683 (65.6) | 12,465 (69.1) | < 0.0001‡ |
| Uncomplicated | 1,079 (17.0) | 2,848 (24.3) | 3,927 (21.8) | ||
| Complicated | 478 (7.5) | 1,181 (10.1) | 1,659 (9.2) | ||
| Age at Enrollment (years) | 49.67 (11.43) | 52.70 (12.33) | 51.63 (12.11) | < 0.0001Δ | |
| Time in Plan (years) | 7.90 (1.82) | 8.08 (1.82) | 8.01 (1.82) | < 0.0001Δ | |
| Medication Use (days) | 824.10 (610.20) | ----- | 824.10 (610.20) † | ----- | |
| Eye Visits | 5.06 (5.40) | 3.82 (6.17) | 4.25 (5.94) | < 0.0001Δ | |
| Charlson Comorbidity Index* | 3.78 (2.92) | 4.25 (3.11) | 4.08 (3.06) | < 0.0001Δ | |
Chloroquine/hydroxychloroquine use in the first five years of enrollment. Complicated diabetes=diabetes with end organ damage, complicated hypertension=hypertension with end organ damage, SLE = Systemic Lupus Erythematosis, CQ = chloroquine; HCQ = hydroxychloroquine
Excludes diabetes.
Only evaluated for the 6,339 individuals taking medication.
Chi-square test.
Two-sample t-test. Miss=Missing.
Chloroquine/Hydroxychloroquine Use
6,339 patients (35.1%) had ≥1 record of HCQ/CQ use in the first 5 years after initial RA/SLE diagnosis. Thirty-six beneficiaries (0.6%) received CQ prescriptions only, 6,248 (98.6%) HCQ only, and 55 received ≥1 prescription of both medications. Participants' mean (±SD) duration of drug exposure was 289.4±534.3 days. 11,712 patients (64.9%) took neither medication in the 5-year study period; 4,930 patients (27.3%) received prescriptions for <4 years. 1,409 patients (7.8%) used CQ or HCQ for ≥4 years; these patients were considered the greatest medication users.
Eye-Provider Visits
Among the participants, 4,214 (23.3%) saw no eye-care provider in the 5-year period. Each year, 67.6-72.5% of participants visited an eye-care provider. Among the 11,712 participants who were consistent nonusers of HCQ/CQ, the proportions with eye-care-provider visits in 0,1,2,3,≥4 of the 5 years were 28.2%, 24.0%, 15.1%, 12.8%, and 19.9%, respectively, whereas the proportions among those with any HCQ/CQ use were 14.3%, 17.9%, 16.4%, 14.3%, and 37.1%, respectively. (Table 2) Of the enrollees at high risk for maculopathy, 6.1% had no eye-care-provider visits in the 5-year period. 66.3-71.9% of patients each year received specialty eye care. The proportions with visits in 1,2,3,≥4 of the years were 9.2%, 12.6%, 15.0%, and 57.0%, respectively. (Table 3)
Table 2. Eye Visits and Diagnostic Testing Based on Amount of Medication Use.
| Variable | Chloroquine / Hydroxychloroquine Use | ||||||
|---|---|---|---|---|---|---|---|
| Any use | No use | p-value | Greatest users†† | All others* | p-value | ||
| Total | 6,339 (35.1) | 11,712 (64.9) | 1,409 (7.8) | 16,642 (92.2) | |||
| Years with Eye Visits | 0 | 906 (14.3) | 3,308 (28.2) | < 0.0001 | 106 (7.5) | 4,108 (76.7) | < 0.0001 |
| 1 | 1,135 (17.9) | 2,811 (24.0) | 123 (8.7) | 3,823 (78.1) | |||
| 2 | 1,041 (16.4) | 1,774 (15.1) | 149 (10.6) | 2,666 (84.4) | |||
| 3 | 906 (14.3) | 1,494 (12.8) | 202 (14.3) | 2,198 (86.7) | |||
| 4 + | 2,351 (37.1) | 2,325 (19.9) | 829 (58.8) | 3,847 (74.1) | |||
| Regular Eye Visits | 3,257 (51.4) | 3,819 (32.6) | <0.0001 | 1,031 (73.2) | 6,045 (36.3) | <0.0001 | |
| Years with Diagnostic Tests | 0 | 3,245 (51.2) | 9,884 (84.4) | < 0.0001 | 508 (36.1) | 12,621 (75.8) | < 0.0001 |
| 1 | 1,192 (18.8) | 986 (8.4) | 254 (18.0) | 1,924 (11.6) | |||
| 2 | 735 (11.6) | 378 (3.2) | 193 (13.7) | 920 (5.5) | |||
| 3 | 495 (7.8) | 211 (1.8) | 164 (11.6) | 542 (3.3) | |||
| 4 + | 672 (10.6) | 253 (2.2) | 290 (20.6) | 635 (3.8) | |||
| Diagnostic Tests | 3,094 (48.8) | 1,828 (15.6) | <0.0001 | 901 (63.9) | 4,021 (24.2) | <0.0001 | |
| Visual Field Testing | 3,005 (47.4) | 1529 (13.1) | <0.0001 | 882 (62.6) | 3,652 (21.9) | <0.0001 | |
| OCT | 588 (9.3) | 965 (8.2) | <0.0001 | 138 (9.8) | 1,415 (8.5) | 0.10 | |
| ERG | 14 (0.2) | 9 (0.1) | 0.01 | 1 (0.1) | 22 (0.1) | 0.55 | |
Includes HCQ/CQ users with continuous use of one or both of these medications for at least 4 of the 5-year study period.
Represent all HCQ/CQ users except highest users. ERG = electroretinogram, OCT = optical coherence tomography, HCQ = hydroxychloroquine, CQ = chloroquine. All p-values represent results of Chi-square tests.
Table 3. Eye Visits and Diagnostic Testing Among Low and High Risk Patients.
| Variable | Risk | ||||
|---|---|---|---|---|---|
| High risk† | Low risk | p-value | Overall | ||
| Total | 1,450 (8.0) | 16,601 (92.0) | 18,051 | ||
| Years with Eye Visits | 0 | 89 (6.1) | 4,125 (24.8) | <0.0001 | 4,214 (23.3) |
| 1 | 134 (9.2) | 3,812 (23.0) | 3,946 (21.9) | ||
| 2 | 182 (12.6) | 2,633 (15.9) | 2,815 (15.6) | ||
| 3 | 218 (15.0) | 2,182 (13.1) | 2,400 (13.3) | ||
| 4 + | 827 (57.0) | 3,849 (23.2) | 4,676 (25.9) | ||
| Regular Eye Visits | 1,045 (72.1) | 6,031 (36.3) | <0.0001 | 7,076 (39.2) | |
| Years with Diagnostic Tests | 0 | 500 (34.5) | 12,629 (76.1) | < 0.0001 | 13,129 (72.7) |
| 1 | 266 (18.3) | 1,912 (11.5) | 2178 (12.1) | ||
| 2 | 235 (16.2) | 878 (5.3) | 1113 (6.2) | ||
| 3 | 184 (12.7) | 522 (3.1) | 706 (3.9) | ||
| 4 + | 265 (18.3) | 660 (4.0) | 925 (5.1) | ||
| Diagnostic Testing | 950 (65.5) | 3,972 (23.9) | <0.0001 | 4,922 (27.3) | |
| Visual Field Testing | 917 (63.2) | 3,617 (21.8) | <0.0001 | 4,534 (25.1) | |
| OCT | 233 (16.1) | 1,320 (8.0) | <0.0001 | 1,553 (8.6) | |
| ERG | 7 (0.5) | 16 (0.1) | <0.0001 | 23 (0.1) | |
Includes patients using HCQ/CQ for at least one year during the first five years of enrollment, who are over the age of 60, have concomitant renal or hepatic disease, or retinal disease. ERG = electroretinogram, OCT = optical coherence tomography, HCQ = hydroxychloroquine, CQ = chloroquine. All p-values represent results of Chi-square tests.
Among the highest HCQ/CQ users, 7.5% saw no eye-care provider in the 5-year period. The proportion receiving eye-provider care in a given year was 67.6-72.5%. The proportions with visits in 1,2,3,≥4 of the years were 8.7%, 10.6%, 14.3%, and 58.8%, respectively. (Table 2)
Among the 959 patients taking HCQ/CQ continuously for >5 years, an optometrist or ophthalmologist was seen by 753 persons (78.5%) in year 6 or 7 while the remaining 21.5% did not visit an eye provider in years 6 or 7. (eTable2)
Among the 1,110 new HCQ/CQ users, 497 (44.8%) had a baseline eye-care-provider visit in the first 12 months after initiating therapy with these medications.
Diagnostic Testing
Roughly 75% of participants underwent no diagnostic tests for maculopathy in the 5 years; approximately 10-13% of patients underwent testing in any given year.
Of the participants with consistent nonuse of HCQ/CQ, the proportions undergoing diagnostic testing in 0,1,2,3,≥4 of the 5 years were 84.4%, 8.4%, 3.2%, 1.8%, and 2.2%, respectively, compared with 51.2%, 18.8%, 11.6%, 7.8%, and 10.6%, respectively, among patients with any HCQ/CQ exposure.
Of patients at high risk for maculopathy, 65.5% had ≥1 form of diagnostic testing in 5 years; 63.2% had VF testing, and 16.1% had OCT. (Table 3) Among patients with the highest HCQ/CQ consumption, 36.1% underwent no diagnostic testing for maculopathy in 5 years. The proportions undergoing any diagnostic testing in 1,2,3,≥4 of the years were 18.0%, 13.7%, 11.6%, and 20.6%, respectively. (Table 2)
Among the patients with >5 years of HCQ/CQ use, 458 (47.8%) had ≥1 VF test and 9.5% had OCT in year 6 or 7. (eTable 2)
Factors Associated with Eye-Provider Visits
After adjustment for potential confounders, HCQ/CQ users had a 3.5%-increased odds of undergoing an eye-care visit for each additional month of use (adjusted OR=1.04, CI=1.03-1.04), compared with nonusers. Other factors associated with increased odds of eye-care visits include female sex (adjusted OR=1.48, CI=1.36-1.62), older age (adjusted OR=1.05, CI=1.04-1.05), higher education level, and comorbid diabetes with complications (adjusted OR=1.97, CI=1.75-2.23) or hypertension with complications (adjusted OR=1.21, CI=1.08-1.36) (p<0.004 for all comparisons). Black patients had a 19%-decreased odds of eye-care-provider visits (adjusted OR=0.81, CI=0.69-0.96), relative to whites. Patients receiving care by rheumatologists had a 29%-increased odds of eye-care visits (adjusted OR=1.29, CI=1.19-1.40). (Figure 1)
Figure 1. Odds of seeking regular eye care in all patients.
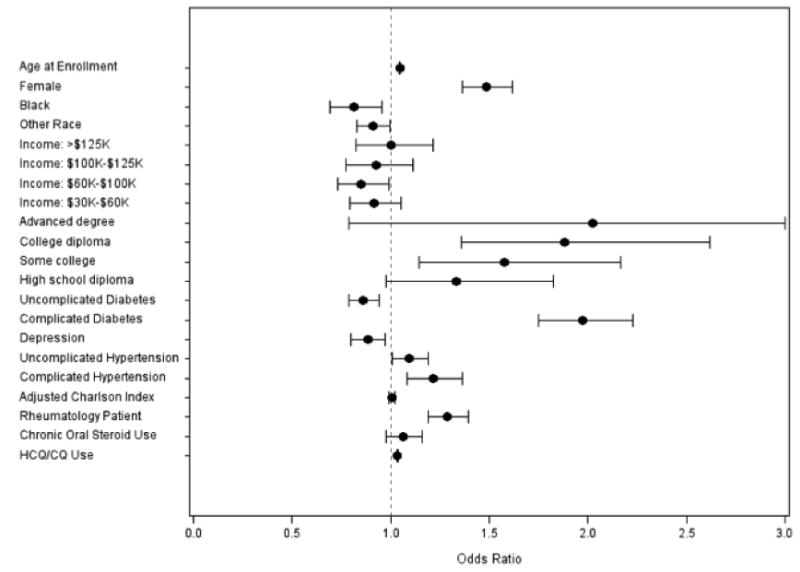
Regular eye care is defined as visits in ≥3 of the 5 years of follow-up. Reference groups: male sex, white race, income <$30,000, education level<high school, no diabetes, no hypertension, no depression, and seeing a rheumatologist 1 or fewer times in the first 5 years. Complicated diabetes=diabetes with end organ damage, complicated hypertension=hypertension with end organ damage, adjusted Charlson Index=Charlson Comorbidity Index with diabetes omitted, rheumatology patient=enrollees seen by a rheumatologist ≥2 times during the 5 years, chronic oral steroid use=prescribed oral steroids for ≥3 months, HCQ=hydroxychloroquine, CQ=chloroquine.
For patients at high risk for maculopathy, each additional month's HCQ/CQ use was associated with 2%-increased odds of an eye-care visit (adjusted OR=1.02, CI=1.01–1.03). Among the high-risk patients, older age (adjusted OR=1.02, CI=1.01-1.04) and receipt of a rheumatologist's care (adjusted OR=1.77, CI=1.27–2.47) were associated with increased odds of eye-care-provider visits. (Figure 2)
Figure 2. Odds of seeking regular eye care in high-risk patients.
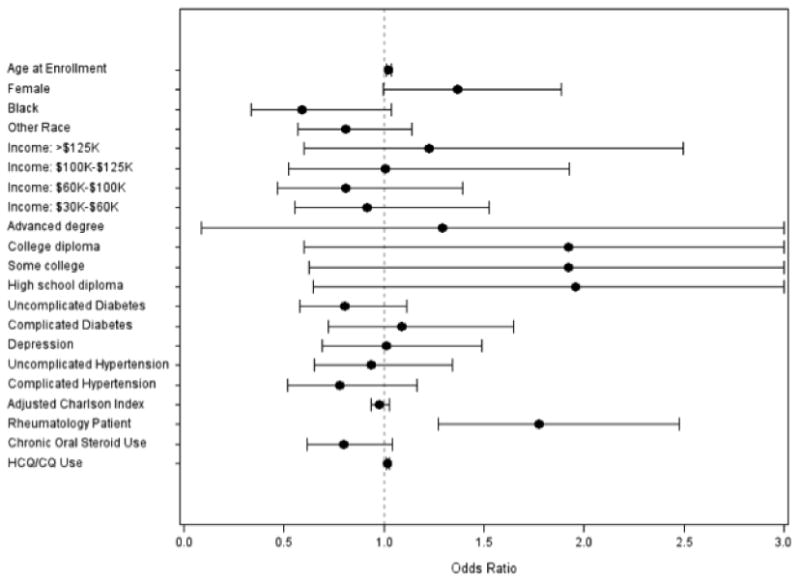
High-risk patients are defined as individuals using HCQ/CQ for at least one year during the first five years of enrollment and have concomitant renal or hepatic disease, retinal disease, or are age ≥ 60 years. Regular eye care is defined as visits to eye providers in ≥3 of the 5 years. Reference groups: male sex, white race, income <$30,000, education level<high school, no diabetes, no hypertension, no depression, and seeing a rheumatologist 1 or fewer times during the 5 years of follow-up. Complicated diabetes=diabetes with end organ damage, complicated hypertension=hypertension with end organ damage, adjusted Charlson Index=Charlson Comorbidity Index with diabetes omitted, rheumatology patient=enrollees with ≥2 visits to rheumatologists during the 5 years, chronic oral steroid use=being on oral steroids for ≥3 months during the 5 years, HCQ=hydroxychloroquine, CQ=chloroquine.
Among the greatest HCQ/CQ users, female sex and older age were associated with elevated odds of eye-care visits (p<0.05 for both). The odds of eye-care visits were increased by 58% among rheumatology-care recipients (adjusted OR=1.58, CI=1.12-2.25) but decreased by 56% among black patients (adjusted OR=0.44, CI=0.21-0.88). (eFigure2)
The findings of an additional regression model assessing factors associated with receipt of regular eye care among the highest CQ/HCQ users or the patients at high risk for toxic retinopathy are similar to those of the other models. (eTable 3)
Discussion
More than half the 6,339 patients with RA/SLE who took HCQ/CQ (51.4%) had regular eye-care-provider visits and nearly one-fifth (18.4%) underwent diagnostic testing for maculopathy, as AAO guidelines recommend. However, among patients at greatest risk for toxic maculopathy, more than one-quarter (27.9%) received no regular eye care; moreover, among the high-risk patients, 6.1% had no eye-care visits and 34.5% had no diagnostic testing for maculopathy during the 5-year period examined.
Although all enrollees had health insurance, and thus theoretically had access to services, we identified many long-term HCQ/CQ users and those at high risk for toxic maculopathy who were not routinely examined by eye-care providers or given diagnostic tests to detect maculopathy. Likewise, among new HCQ/CQ users, fewer than 50% had an ocular examination in their first year of therapy. Possible explanations for these findings are that HCQ/CQ-prescribing providers may be unfamiliar with ocular side effects of long-term use or the patient characteristics associated with high risk for macular toxicity, or they may not appreciate the usefulness of pre-emptive screening, before vision is lost. Alternatively, patients may be nonadherent to providers' recommendations to seek eye care or not understand the importance of maculopathy screening. Furthermore, communication between the prescribing clinicians and eye-care providers may be poor, and some eye-care providers may lack access to the appropriate diagnostic equipment.
Patients receiving rheumatological care had increased odds of eye-care visits. Rheumatologists may have more experience prescribing HCQ/CQ and thus be more familiar with the drugs' side effects and the need for eye-provider referral. In addition, uveitis is common in patients with autoimmune conditions.25,26 These patients often require long-term treatment for the ocular diseases associated with these conditions, often leading to close rheumatologist-ophthalmologist collaboration. Another possibility is that patients of rheumatologists may have relatively more-severe disease, requiring higher HCQ/CQ doses for longer durations. In 2001, Fraenkel and Felson reported that a majority of surveyed rheumatologists highly valued routine retinopathy screening of HCQ/CQ users.27 Our findings support these practice patterns.
Other factors associated with eye-care-provider visits include female sex, older age, higher education level, and complicated diabetes or hypertension. Visual impairment is known to worsen with increasing age, making older persons more inclined to seek eye care. According to published data, one in six U.S. adults aged ≥70 years is visually impaired.28 Women have higher risks for most major eye diseases, making them more likely to utilize eye-care services.29-32 We also found that better-educated patients had higher odds of eye-care utilization, consistent with previous studies.32-36 Similar to other studies,32-34,37-38 black patients had decreased odds of eye-care visits, relative to whites, although blacks often have more visual impairment.28,39,40 Such disparities are particularly disconcerting because among the greatest HCQ/CQ users, black patients had substantially reduced odds of eye-care visits.
Mavrikakis and coworkers13 first reported toxic retinopathy after nonoverdose HCQ therapy, raising concerns about long-term use. Of 1,207 HCQ users, Levy and colleagues17 reported one patient with definite toxicity and five with probable toxicity. In the largest published series, by Wolfe and Marmor, 18 involving 3,995 patients, the toxicity rate exceeded 1% among patients with 5-7 years of HCQ/CQ use. Among 67 patients with cumulative doses >1250 g of HCQ, 50% developed abnormalities detected on mfERG associated with toxic maculopathy.19 As the toxicity risk increases, screening becomes more important.
We used the 2002 guidelines in our analysis because they were in place for much of the study period (2001-2011). The updated, 2011 version reflected the development of more-sensitive diagnostic tests and recognized that the toxicity risk is higher than previously realized.14,15
Screening for toxic maculopathy could be improved by developing automated alert systems and incorporating them in electronic health records to identify high-risk patients. In addition, toolsets calculating patients' cumulative HCQ/CQ dosage taken per ideal body weight could be developed and used in primary-care settings, reminding providers to refer high-risk patients for eye-care-provider screening. Implementing pay-for-performance programs to incentivize physicians to improve screening rates may also help. Moreover, a practice improvement module on HCQ/CQ screening could be offered to improve ophthalmologists' rates of diagnostic testing. Educating non–eye-care providers about ocular side effects of HCQ/CQ and better identifying patients at high risk for toxic maculopathy are also important. Programs to assist non–eye-care providers at the point of care may also be helpful. Furthermore, pharmacists may have a role in educating HCQ/CQ users.
Co-management is an integral part of our health care system, which increasingly relies on specialization, but it comes with its own set of challenges. Improved communication among HCQ/CQ-prescribing clinicians, patients, and eye-care providers could reduce the nonscreening of high-risk patients. Telemedicine may offer additional opportunities to enhance screening efforts. Retinal photography, now increasingly available, offers improved access to subspecialty care.41 OCT images and nonmydriatic fundus photographs acquired by nonophthalmologists can be sent to eye providers to determine which patients require referral for further evaluation.
There are several strengths of this study. First, because all of the patients in our study had health insurance, they should have all, at least theoretically, had access to eye-care services. Patients in the sample resided in communities throughout the country and received care from various types of providers in myriad practice settings. Unlike in cross-sectional studies, the patients were followed for ≥5 years. Finally, claims data enabled accurate estimation of duration and amount of HCQ/CQ exposure, without reliance on patient self-report which is of uncertain reliability.42
Our study has several limitations. The database lacked information on height and weight (to calculate mg/kg of HCQ/CQ exposure), best-corrected visual acuity, and the presence of symptoms of metamorphopsias, which could impact eye-care utilization. Likewise, diagnostic tests such as Amsler grid or color-vision testing lack CPT codes and therefore were not studied. We also cannot deduce from the claims data whether each visit to and test conducted at eye-care-providers' offices were done specifically to screen for HCQ/CQ toxicity, and we lacked information on HCQ/CQ use before patients' enrollment in the plan. Thus, only cumulative exposure in the 5-year study period, not overall cumulative drug exposure, could be calculated. Also, SD-OCT-imaging tests were recommended starting only in 2011, which may account for the substantial nonuse of this diagnostic test for much of the study period. Finally, our findings may be nongeneralizable to un- or underinsured patients, who likely have lower visit and testing rates.
We find that many HCQ/CQ users, including patients at high risk for retinal toxicity and long-term users over multiple years, are not undergoing regular visits to eye-care providers and diagnostic testing to check for maculopathy, as recommended by the AAO. Future studies will hopefully identify reasons for nonadherence to the recommendations and ways of improving adherence.
Supplementary Material
Acknowledgments
Grant support: National Eye Institute K23 Mentored Clinician Scientist Award (1K23EY019511) (JDS); Blue Cross Blue Shield of Michigan Foundation (JDS), Research to Prevent Blindness Physician Scientist Award (JDS).
The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Presented in part at Association for Research in Vision and Ophthalmology Meeting, Seattle, WA, May 8, 2013.
Financial Disclosure(s): The authors have no proprietary or commercial interest in any materials discussed in this article.
Joshua D. Stein, MD, MS, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Ruiz-Irastoza G, Ramos-Casais M, Brito-Zeron P, Khamashata MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. 2010;69(1):20–8. doi: 10.1136/ard.2008.101766. [DOI] [PubMed] [Google Scholar]
- 2.O'Dell JR, Haire CE, Erikson N, et al. Treatment of rheumatoid arthritis with methotrexate alone, sulfasalazine and hydroxychloroquine, or a combination of all three medications. N Engl J Med. 1996;334(20):1287–91. doi: 10.1056/NEJM199605163342002. [DOI] [PubMed] [Google Scholar]
- 3.Dubois EL. Antimalarials in the management of discoid and systemic lupus erythematosus. Semin Arthritis Rheum. 1978;8(1):33–51. doi: 10.1016/0049-0172(78)90033-1. [DOI] [PubMed] [Google Scholar]
- 4.Wallace DJ. The history of antimalarials. Lupus. 1996;5(Suppl 1):S2–S3. [PubMed] [Google Scholar]
- 5.Suarez-Almazor ME, Belseck E, Shea B, Homik J, Wells G, Tugwell P. Antimalarials for treating rheumatoid arthritis. Cochrane Database Syst Rev. 2000;(4):CD000959. doi: 10.1002/14651858.CD000959. [DOI] [PubMed] [Google Scholar]
- 6.Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 7.Daniel JW. Antimalarial drugs in the treatment of rheumatic disease. In: Basow DS, editor. UpToDate. Waltham, MA: 2013. UpToDate. [Google Scholar]
- 8.Singh JA, Furst DE, Bharat A, Curtis JR, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64(5):625–39. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis MJ, Dawes PT, Fowler PD, et al. Should disease-modifying agents be used in mild rheumatoid arthritis? Br J Rheumatol. 1991;30(6):451–4. doi: 10.1093/rheumatology/30.6.451. [DOI] [PubMed] [Google Scholar]
- 10.Wozniacka A, McCauliffe DP. Optimal use of antimalarials in treating cutaneous lupus erythematosus. Am J Clin Dermatol. 2005;6(1):1–11. doi: 10.2165/00128071-200506010-00001. [DOI] [PubMed] [Google Scholar]
- 11.Michaelides M, Stover NB, Farancis PJ, Weleber RG. Retinal toxicity associated with hydroxychloroquine and chloroquine: risk factors, screening, and progression despite cessation of therapy. Arch Ophthlamology. 2011;129(1):30–9. doi: 10.1001/archophthalmol.2010.321. [DOI] [PubMed] [Google Scholar]
- 12.Tehrani R, Ostrowski RA, Hariman R, Jay WM. Ocular toxicity of hydroxychloroquine. Semin Ophthalmol. 2008;23(3):201–9. doi: 10.1080/08820530802049962. [DOI] [PubMed] [Google Scholar]
- 13.Mavrikakis I, Sfikakis PP, Mavrikakis E, et al. The incidence of irreversible retinal toxicity in patients treated with hydroxychloroquine: a reappraisal. Ophthalmology. 2003;110(7):1321–6. doi: 10.1016/S0161-6420(03)00409-3. [DOI] [PubMed] [Google Scholar]
- 14.Marmor MF, Carr RE, Easterbrook M, Farjo AA, Mieler WF American Academy of Ophthalmology. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002;109(7):1377–82. doi: 10.1016/s0161-6420(02)01168-5. [DOI] [PubMed] [Google Scholar]
- 15.Marmor MF, Kellner U, Lai TY, Lyons JS, Mieler WF American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118(2):415–22. doi: 10.1016/j.ophtha.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 16.Mavrikakis M, Papazoglou S, Sfikakis PP, Vaiopoulos G, Rougas K. Retinal toxicity in long term hydroxychloroquine treatment. Ann Rheum Dis. 1996;55(3):187–9. doi: 10.1136/ard.55.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy GD, Munz SJ, Paschal J, Cohen HB, Price KJ, Peterson T. Incidence of hydroxychloroquine retinopathy in a large multicenter outpatient practice. Arthritis Rheum. 1997;40(8):1482–6. doi: 10.1002/art.1780400817. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe F, Marmor MF. Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2010;62(6):775–84. doi: 10.1002/acr.20133. [DOI] [PubMed] [Google Scholar]
- 19.Lyons JS, Severns ML. Detection of early hydroxychloroquine retinal toxicity enhanced by ring ratio analysis of multifocal electroretinography. Am J Ophthalmol. 2007;143(5):801–9. doi: 10.1016/j.ajo.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 20.ICD-9-CM for Physicians. Vol 1 and 2. Salt Lake City, UT: Contexo Media; 2006. International Classification of Diseases 9th Revision Clinical Modification. [Google Scholar]
- 21.CPT 2006: Current Procedural Terminology Professional Edition. Chicago, IL: American Medical Association; 2006. [Google Scholar]
- 22.Stein JD, Kim DS, Niziol LM, et al. Differences in rates of glaucoma among Asian Americans and other racial groups, and among various Asian ethnic groups. Ophthalmology. 2011;118(6):1031–7. doi: 10.1016/j.ophtha.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein JD, Talwar N, Laverne AM, Nan B, Lichter PR. Racial disparities in the use of ancillary testing to evaluate individuals with open-angle glaucoma. Arch Ophthalmol. 2012;130(12):1579–88. doi: 10.1001/archophthalmol.2012.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein JD, Niziol LM, Musch DC, et al. Longitudinal trends in resource use in an incident cohort of open-angle glaucoma patients: resource use in open-angle glaucoma. Am J Ophthalmol. 2012;154(3):452–459. doi: 10.1016/j.ajo.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel SJ, Lundy DC. Ocular manifestations of autoimmune disease. Am Fam Physician. 2002;66(6):991–8. [PubMed] [Google Scholar]
- 26.Mohsenin A, Huang JJ. Ocular manifestations of systemic inflammatory diseases. Conn Med. 2012;76(9):533–44. [PubMed] [Google Scholar]
- 27.Fraenkel L, Felson DT. Rheumatologists' attitudes toward routine screening for hydroxychloroquine retinopathy. J Rheumatol. 2001;28(6):1218–21. [PubMed] [Google Scholar]
- 28.Dillon CF, Gu Q, Hoffman HJ, Ko CW. Vision, hearing, balance, and sensory impairment in Americans aged 70 years and over: United States, 1999-2006. NCHS Data Brief. 2010;(31):1–8. [PubMed] [Google Scholar]
- 29.Evans JR. Risk factors of age-related macular degeneration. Prog Retin Eye Res. 2001;20(2):227–253. doi: 10.1016/s1350-9462(00)00023-9. [DOI] [PubMed] [Google Scholar]
- 30.Vajaranant TS, Nayak S, Wilensky JT, Joslin CE. Gender and glaucoma: what we know and what we need to know. Curr Opin Ophthalmol. 2010;21(2):91–9. doi: 10.1097/ICU.0b013e3283360b7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Congdon N, Vingerling JR, Klein BE, et al. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2005;122(4):487–94. doi: 10.1001/archopht.122.4.487. [DOI] [PubMed] [Google Scholar]
- 32.Bailey RN, Indian RW, Zhang X, Geiss LS, Duenas MR, Saaddine JB. Visual impairment and eye care among older adults - five States, 2005. MMWR Morb Mortal Wkly Rep. 2006;55(49):1321–5. [PubMed] [Google Scholar]
- 33.Lee DJ, Lam B, Arora S, et al. Reported eye care utilization and health insurance status among US adults. Arch Ophthalmol. 2009;127(3):303–10. doi: 10.1001/archophthalmol.2008.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orr P, Barron Y, Schein O, Rubin G, West SK. Eye care utilization by older Americans: the SEE project. Ophthalmology. 1999;106(5):904–9. doi: 10.1016/s0161-6420(99)00508-4. [DOI] [PubMed] [Google Scholar]
- 35.Ross JS, Bradley EH, Busch SH. Use of health care services by lower-income and higher-income uninsured adults. JAMA. 2006;295(17):2027–36. doi: 10.1001/jama.295.17.2027. [DOI] [PubMed] [Google Scholar]
- 36.Chou CF, Barker LE, Crews JE. Disparities in eye care utilization among the United States adults with visual impairment: findings from the behavioral risk factor surveillance system 2006-2009. Am J Ophthalmol. 2012;154(6 Suppl):S45–52. doi: 10.1016/j.ajo.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 37.Spencer C, Frick K, Gower EW, Kempen JH, Wolff JL. Disparities in access to medical care for individuals with vision impairment. Ophthalmic Epidemiol. 2009;16(5):281–8. [PubMed] [Google Scholar]
- 38.National Eye Institute. National Institutes of Health. Identification of variables that influence access to eye care: final report. [Accessed September 10, 2013]; Updated July 28, 2005. http://www.nei.nih.gov/nehep/research/FinalReport9_15_05.pdf.
- 39.Congdon N, O'Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–85. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 40.Ryskulova A, Turczyn K, Makuc DM, Cotch MF, Klein RJ, Janiszewski R. Self-reported age-related eye diseases and visual impairment in the United States: results of the 2002 National Health Interview Survey. Am J Public Health. 2008;98(3):454–61. doi: 10.2105/AJPH.2006.098202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in nonophthalmic settings and its potential for neurology. Neurologist. 2012;18(6):350–5. doi: 10.1097/NRL.0b013e318272f7d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patty L, Wu C, Torres M, Azen S, Varma R. Validity of self-reported eye disease and treatment in a population-based study: the Los Angeles Latino Eye Study. Ophthalmology. 2012;119(9):1725–30. doi: 10.1016/j.ophtha.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


