Abstract
Problem
Inflammation and infection play a major role in preterm birth. The purpose of this study was to: 1) determine the prevalence and clinical significance of sterile intra-amniotic inflammation; and 2) examine the relationship between amniotic fluid (AF) concentrations of high mobility group box-1 (HMGB1) and the interval from amniocentesis-to-delivery in patients with sterile intra-amniotic inflammation.
Method of Study
AF samples obtained from 135 women with preterm labor and intact membranes were analyzed using cultivation techniques as well as broad-range PCR and mass spectrometry (PCR/ESI-MS). Sterile intra-amniotic inflammation was defined when patients with negative AF cultures and without evidence of microbial footprints had intra-amniotic inflammation (AF interleukin-6 ≥ 2.6 ng/mL).
Results
1) The frequency of sterile intra-amniotic inflammation was significantly greater than that of microbial-associated intra-amniotic inflammation [26% (35/135) vs. 11% (15/135); (p=0.005)]; 2) patients with sterile intra-amniotic inflammation delivered at comparable gestational ages, had similar rates of acute placental inflammation and adverse neonatal outcomes as patients with microbial-associated intra-amniotic inflammation; and 3) patients with sterile intra-amniotic inflammation and high AF concentrations of HMGB1 (≥ 8.55 ng/mL) delivered earlier than those with low AF concentrations of HMGB1 (p=0.02).
Conclusions
1) sterile intra-amniotic inflammation is more frequent than microbial-associated intra-amniotic inflammation; and 2) we propose that danger signals participate in sterile intra-amniotic inflammation in the setting of preterm labor.
Keywords: alarmins, danger signal, HMGB1, polymerase chain reaction with electrospray ionization mass spectrometry, pregnancy, preterm delivery
Introduction
Preterm labor is the leading cause of perinatal morbidity and mortality worldwide.1-6 Two-thirds of preterm deliveries occur after the spontaneous onset of preterm labor, with either intact or ruptured membranes.7-11 Preterm labor is one of the great obstetrical syndromes,11-15 in which the presenting symptoms and signs largely represent activation of the common pathway of parturition [i.e. increased uterine contractility,16-26 cervical remodeling,27-39 and membrane/decidual activation40-55]. Activation of the common pathway of parturition can be the result of multiple pathological processes.11,14 Intra-amniotic inflammation due to microbial invasion of the amniotic cavity (MIAC) is an important cause of spontaneous preterm delivery,56-80 and the molecular mechanisms responsible for parturition in this scenario have been extensively studied.81-119
Intra-amniotic inflammation can be due to microorganisms (bacteria, parasites or viruses) or to other mechanisms of disease in which necrosis or cellular stress induces a release of mediators which activate the innate immune system.120-123 We have used the term “sterile intra-amniotic inflammation” to refer to an inflammatory process in which microorganisms cannot be detected.124-130 The precise stimuli for sterile intra-amniotic inflammation in patients with preterm labor and intact membranes have not been identified. One possibility is that Damage-associated molecular patterns (DAMPs) are responsible for this inflammatory process, as well as in a subset of patients with clinical chorioamnionitis at term.127, 129, 131 Several alarmins, including interleukin (IL)-1α,89, 132 S100 calcium binding protein B (S100B),125 high mobility group box-1 (HMGB1),129, 131 and heat shock proteins,127 are elevated in the amniotic fluid (AF) of women with intra-amniotic inflammation.126 HMGB1 is a prototypic alarmin,133-142 and elevated concentrations of this alarmin may reflect engagement of DAMPs-induced inflammation.141-151 HMGB1 plays a key role in mediating inflammation in response to microorganisms, as well as sterile inflammation due to cell injury. HMGB1 is secreted actively during microbial invasion or cellular stress and passively by damage of cellular integrity.152-154 In this study, we sought to: 1) determine the frequency and clinical significance of sterile intra-amniotic inflammation; and 2) examine the relationship between the AF concentrations of HMGB1 and the interval from amniocentesis-to-delivery in patients with sterile intra-amniotic inflammation.
Materials and Methods
Study population
A prospective cohort of women with singleton pregnancies who presented with spontaneous preterm labor and intact membranes was selected from the clinical database and Bank of Biological Samples maintained by Wayne State University, the Detroit Medical Center, and the Perinatology Research Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) if they met the following criteria: 1) had an amniocentesis (transabdominal) performed between 20 and 35 weeks of gestation prior to the rupture of the chorioamniotic membranes; 2) absence of chromosomal or structural fetal anomalies; and 3) the pregnancy outcome was known. This population represents a subset of that included in a study previously reported in this journal130 focusing on the use of molecular microbiologic techniques. Women with an insufficient volume of AF required for the determination of HMGB1 were excluded from this analysis [5%, (7/142)]. Each patient provided written informed consent and the use of biological specimens and clinical data for research purposes was approved by the Institutional Review Boards of NICHD and Wayne State University.
Clinical definitions
Preterm labor was diagnosed by the presence of regular uterine contractions (at least 3 in 30 minutes) and documented cervical changes in patients with a gestational age between 20 and 36 6/7 weeks. Preterm delivery was defined as birth prior to the 37th week of gestation. MIAC was defined as either a positive culture for bacteria in AF or the detection of microbial footprints for either viruses or bacteria, using polymerase chain reaction (PCR) coupled with electrospray ionization mass spectrometry (ESI-MS) (Ibis® technology - Athogen, Carlsbad, CA). Intra-amniotic inflammation was diagnosed when AF IL-6 concentration was ≥ 2.6 ng/mL.72,155 Microbial-associated intra-amniotic inflammation was defined as the presence of MIAC with intra-amniotic inflammation. Sterile intra-amniotic inflammation was diagnosed when the AF IL-6 concentration was ≥ 2.6 ng/mL and there was no evidence of microbial footprints for viruses or bacteria (negative AF culture and no detection of microbial footprints using PCR/ESI-MS).
Composite neonatal morbidity was defined as the presence of: respiratory distress syndrome, bronchopulmonary dysplasia, grade III or IV intraventricular hemorrhage, periventricular leukomalacia, proven neonatal sepsis, necrotizing enterocolitis or perinatal mortality. The diagnostic criteria of these complications have been previously reported.156 Acute placental inflammation was diagnosed based on the presence of inflammatory cells in the chorionic plate, chorioamniotic membranes (histologic chorioamnionitis),157-159 and/or umbilical cord (funisitis).157,158
Amniocentesis and amniotic fluid processing
Patients with preterm labor and intact membranes who had transabdominal ultrasound-guided amniocentesis to evaluate possible intra-amniotic infection (within the standard of care at the Detroit Medical Center) were eligible for the study. AF was immediately transported in a capped sterile syringe to the clinical laboratory. Evaluation of white blood cell (WBC) count, glucose concentration and Gram stain of AF were also performed shortly after collection. AF not required for clinical assessment was centrifuged for 10 minutes at 4°C shortly after the amniocentesis, and the supernatant was aliquoted and stored at -70°C until analysis. The current management for preterm labor in our hospital is to administer corticosteroids (betamethasone or dexamethasone) between 24 and 34 weeks of gestation. Betamethasone is given intramuscularly in two doses (12 mg), 24-hours apart and dexamethasone is administered intramuscularly in 4 doses (6 mg), 12-hours apart.
Detection of microorganisms with cultivation and molecular methods
AF was analyzed using cultivation techniques (aerobic, anaerobic and genital mycoplasmas) as well as with PCR/ESI-MS (Ibis®). Briefly, DNA was extracted from 300 uL of AF using a method that combines bead-beating cell lysis with a magnetic-bead based extraction method.160,161 Extracted DNA was amplified by the previously described broad bacteria and candida (BAC) detection assay according to the manufacturer's instructions.162 PCR/ESI-MS can identify 3400 bacteria and 40 Candida spp, which are represented in the platform's signature database.163-165 For viral detection, the nucleic acids were extracted from 300 uL of AF using a method that combines chemical lysis with a magnetic-bead based extraction method. The extracted RNA/DNA was amplified on the broad viral assay according to the manufacturer's instructions. In the eight wells, there were 14 primer pairs used to detect the following viruses: Herpes simplex virus 1 (HHV-1), Herpes simplex virus 2 (HHV-2), Varicella-zoster virus (HHV-3), Epstein-Barr virus (HHV-4), Cytomegalovirus (HHV-5), Kaposi's sarcoma-associated herpes virus (HHV-8), human adenoviruses, human enteroviruses, BK polyomavirus, JC polyomavirus and Parvovirus B19.165
After PCR amplification, 30-μL aliquots of each PCR product were desalted and analyzed via ESI-MS as previously described.163,166 The presence of microorganisms was determined by signal processing and triangulation analysis of all base composition signatures obtained from each sample and compared to a database. The sensitivity [level of detection (LOD)] of the assay for the detection of bacteria in blood is on average 100 colony-forming units (CFU) per mL [95% confidence interval (CI), 6 – 600 CFU/mL].164 A comparison of detection limits between blood and AF show comparable detection limits (100 CFU/mL). The sensitivity for the broad viral in plasma ranges from 400 to 6600 copies/mL.167 Detection limits in AF ranged from ~800 to 1600 copies/mL (depending upon the specific microorganism).
IL-6 and HMGB1 concentrations in amniotic fluid
AF concentrations of IL-6 and HMGB1 were determined by sensitive and specific enzyme immunoassays obtained from R&D Systems (Minneapolis, MN) and IBL International (Toronto, Canada), respectively. An initial assay validation was performed in our laboratory prior to conducting this study. Briefly, the immunoassays utilized a quantitative sandwich enzyme immunoassay technique and the concentrations were determined by interpolation from the standard curves. The inter- and intra-assay coefficients of variation for IL-6 were 8.7% and 4.6%, and for HMGB1 3.1% and 4.4%, respectively. The detection limits of the assay for IL-6 and HMGB1 were 0.09 pg/mL and 0.2 ng/mL, respectively.
Statistical analysis
The Kolmogorov-Smirnov test and visual plot inspection were used to assess the normality of continuous data distributions. A Kruskal-Wallis test and a two-tailed Mann–Whitney U test were used to compare differences of arithmetic variables among and between the groups. Comparisons of proportions were performed using Chi-square or Fisher's exact tests. Differences in the frequency of sterile intra-amniotic inflammation and microbial-associated inflammation were examined using McNemar's test. A receiver operating characteristic (ROC) curve for the identification of patients who delivered within seven days was used to select a cutoff for AF concentrations of HMGB1. Kaplan-Meier survival curves were plotted and the log rank test was used to determine whether there were differences in time-to-delivery (censoring observations for patients delivered for maternal or fetal indications). Logistic and Cox proportional hazard regression models were fit to examine magnitudes of association. Spearman's correlation was used to assess the relationship between two continuous variables. A two tailed p-value of <0.05 was considered statistically significant. The statistical package used was SPSS v.15.0 (SPSS, Chicago, IL).
Results
Prevalence of sterile and microbial-associated intra-amniotic inflammation
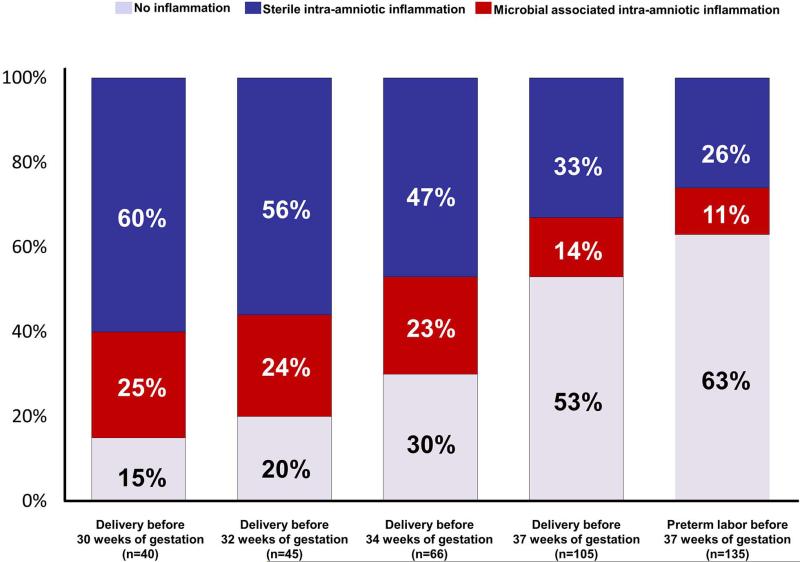
Clinical characteristics are displayed in Table I. The frequency of sterile intra-amniotic inflammation was significantly greater than that of microbial-associated intra-amniotic inflammation [26% (35/135) vs. 11% (15/135) (p=0.005)] (Figure 1). When analysis was restricted to patients who delivered before 37, 34, 32 and 30 weeks of gestation, the frequency of sterile intra-amniotic inflammation was higher than that of microbial-associated intra-amniotic inflammation in all gestational age subgroups (p=0.01, 0.03, 0.02 and 0.02, respectively) (see Figure 1). The earlier the gestational age at delivery, the higher the prevalence of both microbial-associated and sterile intra-amniotic inflammation.
Table I.
Clinical and demographic characteristics of the study population.
| No intra-amniotic inflammation (n=85) | Microbial-associated intra-amniotic inflammation (n=15) | Sterile intra-amniotic inflammation (n=35) | p value | |
|---|---|---|---|---|
| Maternal age (years) | 23 (20 – 26) | 24 (20 – 30) | 23 (20 – 26.2) | 0.8 |
| BMI (kg/m2) | 23 (20 – 29) | 27 (23 – 29) | 23 (20 – 32) | 0.1 |
| Nulliparity | 32% (27) | 73% (11) | 40% (14) | 0.009 |
| Race | 0.1 | |||
| African-American | 89% (76) | 73% (11) | 91% (30) | |
| Caucasian | 6% (5) | 20% (3) | 6% (2) | |
| Hispanic | 0 | 7% (1) | 3% (1) | |
| Others | 5% (4) | 0 | 0 | |
| Tobacco use during pregnancy | 20% (17) | 13% (2) | 21% (7) | 0.8 |
| Cervical dilatation at admission (cms) | 3 (2 – 4) | 4 (2 – 4) | 3 (2 – 4) | 0.1 |
| Gestational age at amniocentesis (weeks) | 32 (29 – 33) | 26 (23 – 32) | 25 (23 – 32) | <0.001* |
| AF white blood cells count (cells/mm3) | 1 (0 – 5) | 295 (2 – 960) | 3 (1 – 17) | <0.001* |
| AF glucose (mg/dL) | 29 (24 – 34) | 11 (10 – 20) | 22 (18 – 28) | <0.001* |
| AF interleukin-6 (ng/mL) | 0.8 (0.5 – 1.1) | 96 (17 – 266) | 12 (5 – 21) | <0.001* |
| Gestational age at delivery (weeks) | 36 (34 – 38) | 26 (24 – 33) | 27 (24 – 32) | <0.001* |
| Composite neonatal morbidity | 11% (9) | 67% (10) | 68% (24) | <0.001 |
| Acute placental inflammation¥ | 22.5% (18/80) | 79% (11/14) | 61% (19/31) | <0.001* |
| Acute histologic chorioamnionitis | 21% (17/80) | 79% (11/14) | 58% (18/31) | |
| Funisitis | 13% (10/80) | 57% (8/14) | 29% (9/31) |
Data presented as median (interquartile) and percentage and (n); AF: amniotic fluid; BMI: body mass index.
Acute placental inflammation was calculated over a total of 125 specimens.
Kruskal Wallis test.
Figure 1.
Frequency of sterile and microbial-associated intra-amniotic inflammation in patients with preterm labor and intact membranes as a function of the gestational age at delivery. The frequency of sterile intra-amniotic inflammation was significantly greater than that of microbial-associated intra-amniotic inflammation [26% (35/135) vs. 11% (15/135) (p= 0.005)].
Clinical characteristics of sterile intra-amniotic inflammation
The median gestational age [interquartile range, (IQR)] at amniocentesis was significantly lower in patients with sterile and microbial associated intra-amniotic inflammation than in women without intra-amniotic inflammation [25 (IQR: 23 – 32) and 26 (IQR: 23 – 32) weeks vs. 32 (29 – 33) weeks, respectively (each p<0.001); Table I]. There were no significant differences in other clinical characteristics, such as maternal age, race, tobacco use or cervical dilatation at admission among these three clinical groups (Table I).
The median (IQR) AF concentrations of IL-6 and WBC count in patients with sterile intra-amniotic inflammation were lower than those of patients with microbial-associated intra-amniotic inflammation [AF IL-6: 12 (5 - 21) vs. 96 (17 - 266) ng/mL; p<0.001; and WBC count: 3 (1 - 17) vs. 295 (2 - 960) cells/mm3; p=0.02]. There was no significant difference in the median (IQR) gestational age at delivery between patients with microbial-associated intra-amniotic inflammation and those with sterile intra-amniotic inflammation [26 (24 – 33) vs. 27 (24 – 32) weeks; p=0.6]. The prevalence of acute placental inflammation (acute histologic chorioamnionitis and/or funisitis) was similar in patients with intra-amniotic inflammation with or without detectable microorganisms [79% (11/14) vs. 61% (19/31); p=0.3].
Neonatal morbid events (assessed by composite neonatal morbidity) were significantly more common in patients with microbial-associated intra-amniotic inflammation and sterile intra-amniotic inflammation than in those without intra-amniotic inflammation [67% (10/15) and 68% (24/35) vs. 11% (9/85); each p<0.001; see Table I]. Importantly, there was no significant difference in the prevalence of neonatal morbid events between neonates born to mothers with sterile intra-amniotic inflammation and those born to mothers with microbial-associated intra-amniotic inflammation (p=1.0).
Amniotic fluid concentrations of HMGB1 in women with sterile intra-amniotic inflammation and the interval to delivery
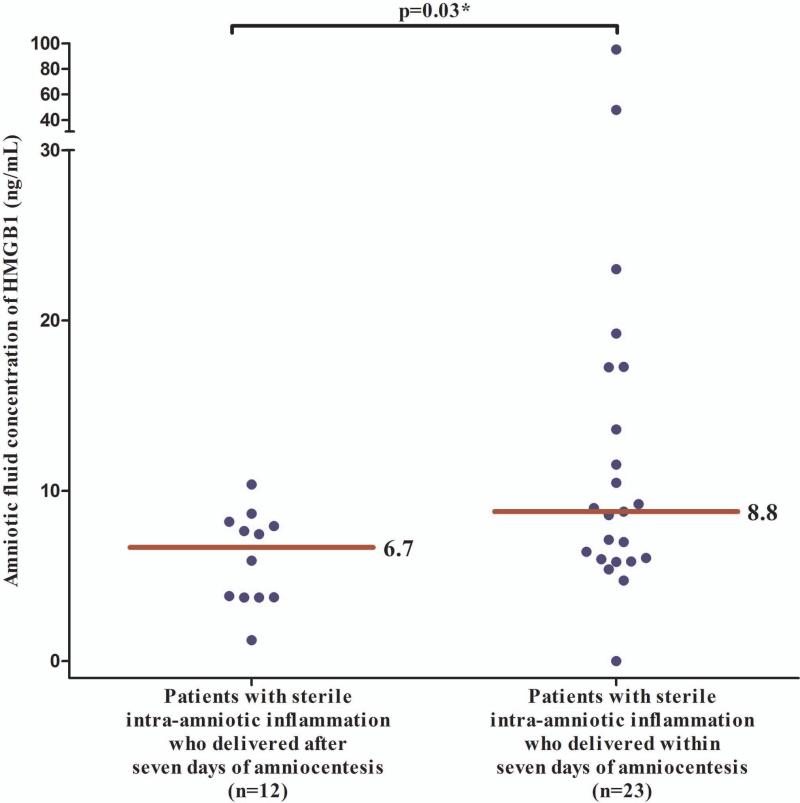
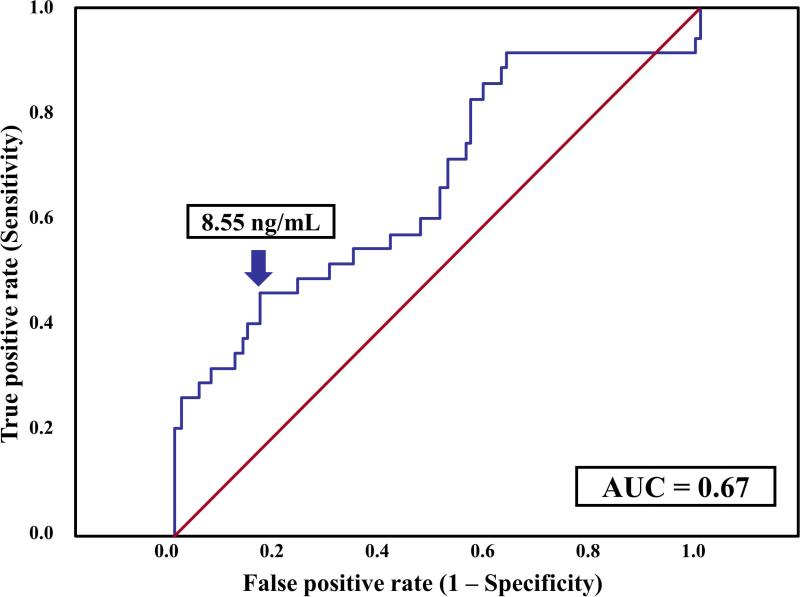
The median (IQR) AF concentration of HMGB1 was significantly higher in patients with sterile intra-amniotic inflammation (n=35) than in those without intra-amniotic inflammation (n=85) [7.6 (5.8 – 10.4) vs. 6 (4 – 7.7) ng/mL; p=0.007]. Among patients with sterile intra-amniotic inflammation, those who delivered within seven days after amniocentesis (n=23) had a significantly higher median (IQR) AF concentration of HMGB1 than patients who delivered after seven days (n=12) [8.8 (5.9 - 17.3) vs. 6.7 (3.7 - 8.1) ng/mL; p=0.03) (Figure 2)]. A cutoff of 8.55 ng/mL was selected to define an elevated AF HMGB1 concentration upon examining a ROC curve for the identification of patients who delivered within seven days of amniocentesis, excluding women with microbial-associated intra-amniotic inflammation (Figure 3). Patients with AF concentrations of HMGB1 at or above this cutoff were 5-fold more likely (than those with concentrations below 8.55 ng/mL) to deliver within seven days of amniocentesis [adjusted odds ratio (OR): 5.1; 95% CI 1.6 – 15.5], adjusting for gestational age at amniocentesis and cervical dilatation with logistic regression.
Figure 2.
AF concentrations of HMGB1 in patients with sterile intra-amniotic inflammation according to whether delivery occurred within or after seven days of amniocentesis. Patients who delivered within seven days after amniocentesis had a significantly higher median (IQR) AF concentration of HMGB1 than patients who delivered after seven days [8.8 (5.9 - 17.3) vs. 6.7 (3.7 - 8.1); p=0.03].
Figure 3.
Receiver operator characteristic curve analysis of AF concentrations of HMGB1 for the identification of patients without microbial-associated intra-amniotic inflammation who subsequently delivered within seven days after amniocentesis. AF HMGB1 concentration of 8.55 ng/mL had an area under the ROC curve of 0.67 (95% CI 0.55 – 0.78; p=0.005), a sensitivity of 46% (16/35), a specificity of 84% (71/85), a positive predictive value of 53% (16/30), and a negative predictive value of 80% (71/90) for the identification of patients who subsequently delivered within 7 days.
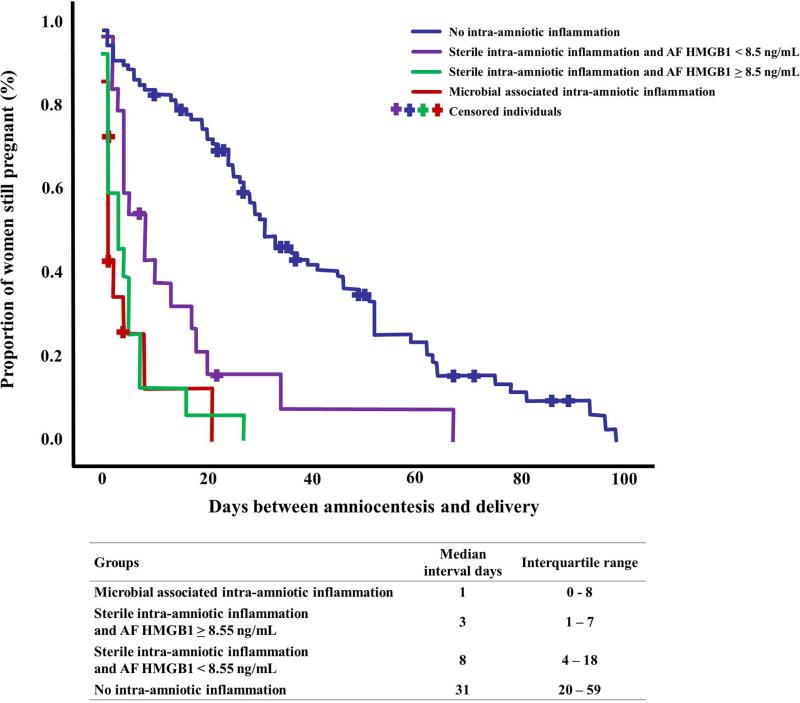
The amniocentesis-to-delivery interval of women with sterile intra-amniotic inflammation who had AF HMGB1 concentrations > 8.55 ng/mL was significantly shorter than that of women with sterile intra-amniotic inflammation who had AF HMGB1 concentrations below 8.55 ng/mL and women without intra-amniotic inflammation [median 3, IQR: 1 – 7 days vs. median 8, IQR: 4 – 18 days, and median 31, IQR: 20 – 59 days (p=0.02 and p<0.0001, respectively, Figure 4)]. Importantly, there was no significant difference in the amniocentesis-to-delivery interval between patients with sterile intra-amniotic inflammation and AF HMGB1 > 8.55 ng/mL compared to patients with microbial-associated intra-amniotic inflammation [median 3, IQR: 1 – 7 days vs. median 1, IQR: 0- 8 days; p=0.6, Figure 4)].
Figure 4.
Survival analysis of amniocentesis-to-delivery interval (days) according to the presence of microbial-associated intra-amniotic inflammation or sterile intra-amniotic inflammation with the presence of higher concentration of alarmins. Patients in whom labor was induced were censored and are represented by crosses. The amniocentesis-to-delivery interval among women with sterile intra-amniotic inflammation who had elevated AF HMGB1 concentrations (≥8.55 ng/mL) was significantly shorter than that of: 1) women with sterile intra-amniotic inflammation who had AF HMGB1 concentrations below 8.55 ng/mL; and 2) women without intra-amniotic inflammation [median 3, IQR: 1 – 7 days vs. median 8, IQR: 4 – 18 days and median 31, IQR: 20 – 59 days (p=0.02 and p<0.0001), respectively]. There was no significant difference in the amniocentesis-to-delivery interval between patients with sterile intra-amniotic inflammation and AF HMGB1 concentrations ≥ 8.55 ng/mL and those with microbial-associated intra-amniotic inflammation.
Multivariable survival analysis (Cox proportional hazard modeling) was used to explore the amniocentesis-to-delivery interval among different clinical groups taking into account microbial invasion, intra-amniotic inflammation, and AF concentration of HMGB1 while adjusting for cervical dilatation at admission and gestational age at amniocentesis. Patients with either microbial-associated or sterile intra-amniotic inflammation with AF HMGB1 ≥ 8.55 ng/mL had a shorter amniocentesis-to-delivery interval than those without intra-amniotic inflammation [hazard ratio of 14.6 (95% CI, 6.2 - 34), and 17.7 (95% CI, 7.3 - 42), respectively]. Moreover, patients with sterile intra-amniotic inflammation and AF HMGB1 < 8.55 ng/mL also had a shorter amniocentesis-to-delivery interval than those without intra-amniotic inflammation [hazard ratio of 6.5 (95% CI%, 3.2 – 13.3)].
There was an association between AF HMGB1 concentrations and acute inflammatory lesions in the placenta (acute histologic chorioamnionitis and/or funisitis). The median (IQR) AF concentration of HMGB1 was significantly higher in patients with acute placental inflammation (n=48) than that of patients without these lesions (n=77) [8.5 (5.1 – 17.8) vs. 6.6 (4.7 – 8.3) ng/mL; p=0.009]. The prevalence of acute placental inflammation was significantly higher in patients with elevated AF HMGB1 (> 8.55 ng/mL) than in those with a low AF concentration of HMGB1 [58% (24/41) vs. 29% (24/84); p=0.001].
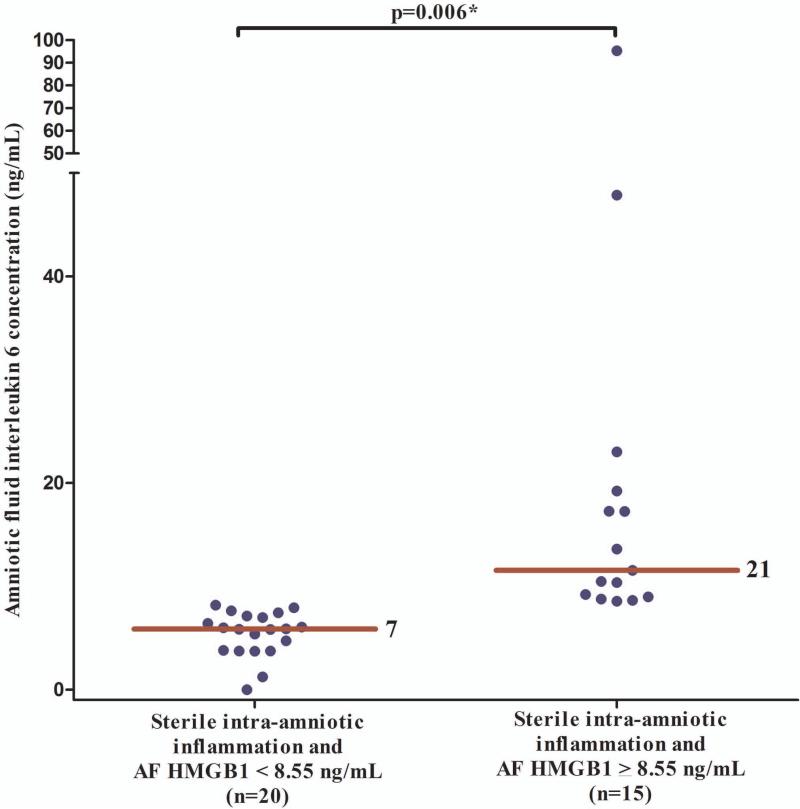
Amniotic fluid concentrations of IL-6 in patients with sterile intra-amniotic inflammation and elevated HMGB1 concentrations
Figure 5 displays AF IL-6 concentrations in patients with sterile intra-amniotic inflammation according to the AF concentration of HMGB1 [below 8.55 ng/mL; (n=20) and above 8.55 ng/mL; (n=15)]. The median (IQR) AF concentration of IL-6 was significantly higher in patients with AF HMGB1 ≥ 8.55 ng/mL compared to those with AF HMGB1 < 8.55 ng/mL [21 (10 – 27) vs. 7 (3.5 – 15.3) ng/mL; p=0.006; Figure 5]. AF concentrations of HMGB1 were moderately correlated with AF IL-6 concentrations (Spearman's Rho 0.6; p<0.001) and inversely correlated with the interval from amniocentesis to delivery (Spearman's Rho −0.4; p=0.03) in patients with sterile intra-amniotic inflammation.
Figure 5.
AF concentrations of IL-6 in patients with sterile intra-amniotic inflammation according to the AF concentration of HMGB1 (above or below 8.55 ng/mL). The median (IQR) AF concentration of IL-6 was significantly higher in patients with AF HMGB1 ≥ 8.55 ng/mL than in those with AF HMGB1 < 8.55 ng/mL [21 (10 – 27) vs. 7 (3.5 – 15.3) ng/mL; p=0.006].
Antibiotics were administered in 29% (39/135) of patients before amniocentesis. Most patients [60% (81/135)] did not receive corticosteroids before amniocentesis. We repeated the analysis adjusting for the administration of antibiotics (within 6 hours) and corticosteroids (within 7 days) before amniocentesis using analysis of covariance, and the results of amniotic fluid IL-6 and HMGB1 concentrations remained significant among groups.
Discussion
Principal findings of the study
1) Sterile intra-amniotic inflammation is more common than microbial-associated intra-amniotic inflammation in patients with preterm labor and intact membranes; 2) patients with sterile intra-amniotic inflammation had early preterm deliveries at a comparable gestational age to women with microbial-associated intra-amniotic inflammation; 3) the AF glucose concentration and WBC count in cases of sterile intra-amniotic inflammation were within normal ranges; 4) acute histologic chorioamnionitis and/or funisitis were identified in patients with sterile intra-amniotic inflammation, as well as in those with microbial-associated intra-amniotic inflammation; 5) the intensity of the inflammatory response (assessed by AF IL-6 concentrations) was stronger in patients with microbial-associated intra-amniotic inflammation than in patients with sterile intra-amniotic inflammation; and 6) patients with sterile intra-amniotic inflammation and high AF concentrations of HMGB1, delivered earlier than those with low AF concentrations of HMGB1. This is compelling evidence that sterile intra-amniotic inflammation is of major importance in preterm parturition.
Sterile vs. microbial-associated intra-amniotic inflammation
The key finding of this study is that in patients with preterm labor and intact membranes sterile intra-amniotic inflammation is more common than microbial-associated intra-amniotic inflammation. We have previously proposed that intra-amniotic inflammation without detectable microorganisms is a mechanism of disease in preterm labor71,72,74,76,125-129,131,168 based on observations of elevated AF concentrations of inflammatory mediators [IL-6,71,72,74,91,96,168-175 IL-8,106 matrix metalloproteinase 8 (MMP-8)76,128, monocyte chemotactic protein-1 (MCP-1),113, 176 and other inflammatory markers79,80,107,172,177] in the absence of detectable microorganisms. The designation of “sterile intra-amniotic inflammation” depends on the techniques employed to exclude the presence of microbes. In this study, we used a new sensitive method which uses electrospray ionization mass spectrometry (ESI-MS) for base composition analysis of polymerase chain reaction (PCR) products to rapidly create a signature that allows for the identification of a large number of microorganisms (bacteria and viruses) at the genus and species level within 8 hours. Therefore, the classification of intra-amniotic inflammation as “sterile” was determined with a high degree of confidence. It is possible that future technological advances will allow discovery of microorganisms which have escaped detection. The use of next generation sequencing may result in the detection of low quantities of microbial nucleic acids in biological fluids. Yet, determining whether this reflects the true presence of bacteria and viruses (rather than contamination) is a challenge.
Clinical and laboratory characteristics of sterile intra-amniotic inflammation
Although the clinical characteristics of patients with sterile intra-amniotic inflammation were similar to those with microbial-associated intra-amniotic inflammation (e.g. gestational age at presentation, short interval-to-delivery, and a high rate of adverse neonatal events), the laboratory findings were different. Specifically, the AF WBC count and AF glucose concentrations of patients with sterile intra-amniotic inflammation were within normal range (see Table I). Therefore, a work-up based on AF WBC count and AF glucose concentration would fail to identify the most common type of intra-amniotic inflammation in patients with preterm labor and intact membranes. Interestingly, the AF concentrations of IL-6 are significantly lower in patients with sterile intra-amniotic inflammation than in those with microbial-associated intra-amniotic inflammation. Further studies are required to characterize the behavior of the cytokinechemokine network in amniotic fluid and maternal blood in patients with sterile intra-amniotic inflammation.
Acute histologic chorioamnionitis can occur in the absence of microbial-associated intra-amniotic inflammation
Acute histologic chorioamnionitis and funisitis are generally considered to be due to amniotic fluid infection.91,95,170,178-187 Early studies using amniocentesis in patients with spontaneous preterm labor who delivered within 48 hours showed that 71% (27/38) of patients with acute histologic chorioamnionitis and 81% (29/36) with funisitis had positive AF cultures for bacteria.188 Hillier et al. reported that in 73% (16/22) of placentas with acute histologic chorioamnionitis, bacteria could be isolated from the chorioamniotic space.92 The findings reported herein indicate that 58% (n=18/31) of patients with sterile intra-amniotic inflammation have acute histologic chorioamnionitis, and that of all patients with acute histologic chorioamnionitis, 38% (n=18/48) had sterile intra-amniotic inflammation at the time of amniocentesis. We propose that DAMPs-induced inflammation occurs after a danger signal engages a pattern recognition receptor (PRR), such as toll like receptors (TLRs)-2, -4, and -9, leading to the release of HMGB1 and other alarmins that can induce inflammation by stimulating the production of inflammatory cytokines such as IL-6, IL-1β, IL-8 and TNF-α.144,145,189,190
Although our observations suggest that MIAC was not present at the time of amniocentesis in patients with sterile intra-amniotic inflammation who subsequently delivered and had acute histologic chorioamnionitis, it is possible that microorganisms may have invaded the amniotic cavity at some point before the procedure and induced intra-amniotic inflammation, which eradicated the microbes. This possibility is extremely difficult to exclude in humans. It is also possible that danger-associated intra-amniotic inflammation led to preterm labor, and that a secondary microbial invasion of the amniotic cavity occurred which was not detected at the time of amniocentesis. Studies in which placentas and membranes are examined for microorganisms using culture, sequence-based techniques and morphologic approaches are required to address this possibility. The biology of infection during pregnancy is extremely complex. Recent observations in animal models suggest that systemic and localized infections in the lower genital tract during pregnancy may increase the susceptibility to microbial products (i.e. endotoxin) or bacteria (two-hit hypothesis model).191-195 Whether this is the case in humans remains to be determined.
Danger signals in patients with sterile intra-amniotic inflammation and preterm labor
Inflammation is a mechanism of host defense in response to infection and non-infection related insults.196-200 The innate immune system initiates an inflammatory response when PRRs sense exogenous or endogenous signals considered potentially harmful to the host.146,196,199-213 During infection, these signals include molecular structures that are conserved among microbial species, known as pathogen-associated molecular patterns (PAMPs).203,207,214 Tissue damaged from non-infectious origins (e.g. trauma, ischemia-reperfusion injury, urate crystals, chemical injuries etc.) induce the release alarmins or “danger signals”,154,203,207,214 known as DAMPs. When recognized by PRRs, these multifunctional proteins are also capable of inducing an inflammatory response.139,144,145,189,207,212,215
In this study, AF concentrations of HMGB1, a prototypical “alarmin”, were significantly higher in patients with sterile intra-amniotic inflammation than in patients without intra-amniotic inflammation. In addition, we report that patients with sterile intra-amniotic inflammation and high AF concentrations of HMGB1 (≥ 8.55 ng/mL) had a significantly shorter amniocentesis-to-delivery interval than patients with AF HMGB1 < 8.55 ng/mL. These findings suggest that alarmins can play a role in preterm labor in the setting of sterile intra-amniotic inflammation.
HMGB1 is a chromatin component located in the nucleus of eukaryotic cells, whose functional role is to inform other cells that damage has occurred.133,135,149,189,216-218 This protein has chemotactic activity for monocytes,152,153,217, 219, 220 macrophages,221, 222 neutrophils,221-224 and dendritic cells.137,138,217,225,226 HMGB1 is released into the extracellular space either actively or passively. Active secretion occurs from stressed cells of the immune system (e.g. macrophages, monocytes and cells at the frontline of host defense).133,135,137,145,149 Passive release occurs during sterile cell injury and is almost immediate.147 During infection, HMGB1 is transferred from the nucleus of monocytes, macrophages and other immune cells to the cytosol, where it accumulates in intracellular vesicles prior to secretion which takes approximately 8 hours.147,153,227 In mice, serum concentrations of HMGB1 increase 8 to 32 hours after endotoxin exposure; thus, HMGB1 has been considered a “late” mediator of inflammation.227-230 Alarmins are potential therapeutic targets. Blocking HMGB1 with neutralizing antibodies reduces mortality in animal models of endotoxemia227,230 and sepsis,228,231 this has generated considerable interest in HMGB1 as a potential pharmacologic target in sepsis and sterile inflammation.
Evidence that “alarmins” can induce preterm labor
Systemic administration of IL-1α has been shown to induce preterm delivery in mice,232,233 an effect that was abrogated by pretreatment with the IL-1 receptor antagonist.233 Subsequently, Bry et al. reported that intra-amniotic injection of IL-1α in rabbits similarly induces preterm labor.234 Elevated AF concentrations of other alarmins have been reported in women with preterm labor with intact membranes or preterm prelabor rupture of membranes, including S100B,125 HMGB1,129,131 and heat shock proteins.126,127 Whether these multifunctional proteins can induce preterm labor and delivery remains to be determined. Further studies of the effect of HMGB1 on the chorioamniotic membranes and pregnancy are required.
Although we have reported that HMGB1 concentrations are elevated in a subset of patients with preterm labor,129 the mechanisms whereby HMGB1 may lead to the onset of labor are unknown. Additional research is required to answer these questions. Necrotic or stress cells can release alarmins including HMGB1. These alarmins would be responsible, at least in part, for inducing inflammation and the onset of labor. The effect of IL-1α in inducing parturition232-234 may be mediated through the activation of the components of the inflammasome.235-237
Conclusion
1) Sterile intra-amniotic inflammation is more frequent than microbial-associated inflammation in patients with preterm labor and intact membranes; 2) we propose that danger signals participate in sterile intra-amniotic inflammation in the setting of preterm labor with intact membranes; and 3) future research on the mechanisms responsible for sterile intra-amniotic inflammation, biomarkers, and therapeutic interventions are required to address the challenges offered by this clinical condition.
Acknowledgements
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH); and, in part, with Federal funds from NICHD, NIH under Contract No. HHSN275201300006C.
References
- 1.World Health Organization . WHO Library Cataloguing-in-Publication. World Health Organization; Geneva: 2012. Born too soon: the global action report in preterm birth. [Google Scholar]
- 2.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, Lawn JE. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 3.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, Mathers C, Black RE. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton BE, Hoyert DL, Martin JA, Strobino DM, Guyer B. Annual summary of vital statistics: 2010-2011. Pediatrics. 2013;131:548–558. doi: 10.1542/peds.2012-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.March of Dimes. [May 19, 2014];Premature Birth. Available at http://www.marchofdimes.com/peristats/.
- 6.Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med. 2010;362:529–535. doi: 10.1056/NEJMra0904308. [DOI] [PubMed] [Google Scholar]
- 7.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414–429. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 8.Berkowitz GS, Blackmore-Prince C, Lapinski RH, Savitz DA. Risk factors for preterm birth subtypes. Epidemiology. 1998;9:279–285. [PubMed] [Google Scholar]
- 9.Moutquin JM. Classification and heterogeneity of preterm birth. BJOG. 2003;110(Suppl 20):30–33. doi: 10.1016/s1470-0328(03)00021-1. [DOI] [PubMed] [Google Scholar]
- 10.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romero R, Lockwood CJ. Pathogenesis of Spontaneous Preterm Labor. In: Creasy RK, Resnik R, Iams J, editors. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. Sixth edn Elsevier; Philadelphia: 2009. pp. 521–543. [Google Scholar]
- 12.Romero R. The child is the father of the man. Prenat Neonat Med. 1996;1:8–11. [Google Scholar]
- 13.Romero R, Gomez R, Mazor M, Ghezzi F, Yoon BH. The preterm labor syndrome. In: Elder MG, Romero R, Lamont RF, editors. Preterm labor. Churchill Livingstone; New York: 1997. pp. 29–49. [Google Scholar]
- 14.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero R. Prenatal medicine: the child is the father of the man. 1996. J Matern Fetal Neonatal Med. 2009;22:636–639. doi: 10.1080/14767050902784171. [DOI] [PubMed] [Google Scholar]
- 16.Garfield RE, Hayashi RH. Appearance of gap junctions in the myometrium of women during labor. Am J Obstet Gynecol. 1981;140:254–260. doi: 10.1016/0002-9378(81)90270-2. [DOI] [PubMed] [Google Scholar]
- 17.Huszar G, Roberts JM. Biochemistry and pharmacology of the myometrium and labor: regulation at the cellular and molecular levels. Am J Obstet Gynecol. 1982;142:225–237. doi: 10.1016/s0002-9378(16)32341-9. [DOI] [PubMed] [Google Scholar]
- 18.Hsu HW, Figueroa JP, Honnebier MB, Wentworth R, Nathanielsz PW. Power spectrum analysis of myometrial electromyogram and intrauterine pressure changes in the pregnant rhesus monkey in late gestation. Am J Obstet Gynecol. 1989;161:467–473. doi: 10.1016/0002-9378(89)90543-7. [DOI] [PubMed] [Google Scholar]
- 19.Balducci J, Risek B, Gilula NB, Hand A, Egan JF, Vintzileos AM. Gap junction formation in human myometrium: a key to preterm labor? Am J Obstet Gynecol. 1993;168:1609–1615. doi: 10.1016/s0002-9378(11)90806-0. [DOI] [PubMed] [Google Scholar]
- 20.Chow L, Lye SJ. Expression of the gap junction protein connexin-43 is increased in the human myometrium toward term and with the onset of labor. Am J Obstet Gynecol. 1994;170:788–795. doi: 10.1016/s0002-9378(94)70284-5. [DOI] [PubMed] [Google Scholar]
- 21.Asboth G, Phaneuf S, Lopez Bernal AL. Prostaglandin E receptors in myometrial cells. Acta Physiol Hung. 1997;85:39–50. [PubMed] [Google Scholar]
- 22.Cook JL, Zaragoza DB, Sung DH, Olson DM. Expression of myometrial activation and stimulation genes in a mouse model of preterm labor: myometrial activation, stimulation, and preterm labor. Endocrinology. 2000;141:1718–1728. doi: 10.1210/endo.141.5.7474. [DOI] [PubMed] [Google Scholar]
- 23.Lye SJ, Mitchell J, Nashman N, Oldenhof A, Ou R, Shynlova O, Langille L. Role of mechanical signals in the onset of term and preterm labor. Front Horm Res. 2001;27:165–178. doi: 10.1159/000061025. [DOI] [PubMed] [Google Scholar]
- 24.Chan EC, Fraser S, Yin S, Yeo G, Kwek K, Fairclough RJ, Smith R. Human myometrial genes are differentially expressed in labor: a suppression subtractive hybridization study. J Clin Endocrinol Metab. 2002;87:2435–2441. doi: 10.1210/jcem.87.6.8439. [DOI] [PubMed] [Google Scholar]
- 25.Smith R. Parturition. N Engl J Med. 2007;356:271–283. doi: 10.1056/NEJMra061360. [DOI] [PubMed] [Google Scholar]
- 26.Pierce SL, England SK. SK3 channel expression during pregnancy is regulated through estrogen and Sp factor-mediated transcriptional control of the KCNN3 gene. American journal of physiology Endocrinology and metabolism. 2010;299:E640–646. doi: 10.1152/ajpendo.00063.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liggins C. Cervical ripening as an inflammatory reaction. In: Anderson A, editor. The Cervix in Pregnancy and Labour: Clinical and Biochemical Investigations D Ellwood. Churchill Livingstone; Edinburgh: 1981. pp. 1–9. [Google Scholar]
- 28.Leppert PC. Anatomy and physiology of cervical ripening. Clin Obstet Gynecol. 1995;38:267–279. doi: 10.1097/00003081-199506000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Ludmir J, Sehdev HM. Anatomy and physiology of the uterine cervix. Clin Obstet Gynecol. 2000;43:433–439. doi: 10.1097/00003081-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Kelly RW. Inflammatory mediators and cervical ripening. J Reprod Immunol. 2002;57:217–224. doi: 10.1016/s0165-0378(02)00007-4. [DOI] [PubMed] [Google Scholar]
- 31.Straach KJ, Shelton JM, Richardson JA, Hascall VC, Mahendroo MS. Regulation of hyaluronan expression during cervical ripening. Glycobiology. 2005;15:55–65. doi: 10.1093/glycob/cwh137. [DOI] [PubMed] [Google Scholar]
- 32.Hassan SS, Romero R, Haddad R, Hendler I, Khalek N, Tromp G, Diamond MP, Sorokin Y, Malone J., Jr. The transcriptome of the uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol. 2006;195:778–786. doi: 10.1016/j.ajog.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 33.Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med. 2007;25:69–79. doi: 10.1055/s-2006-956777. [DOI] [PubMed] [Google Scholar]
- 34.Hassan SS, Romero R, Tarca AL, Nhan-Chang CL, Vaisbuch E, Erez O, Mittal P, Kusanovic JP, Mazaki-Tovi S, Yeo L, Draghici S, Kim JS, Uldbjerg N, Kim CJ. The transcriptome of cervical ripening in human pregnancy before the onset of labor at term: identification of novel molecular functions involved in this process. J Matern Fetal Neonatal Med. 2009;22:1183–1193. doi: 10.3109/14767050903353216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lyttle B, Chai J, Gonzalez JM, Xu H, Sammel M, Elovitz MA. The negative regulators of the host immune response: an unexplored pathway in preterm birth. Am J Obstet Gynecol. 2009;201:284, e281–287. doi: 10.1016/j.ajog.2009.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez JM, Dong Z, Romero R, Girardi G. Cervical remodeling/ripening at term and preterm delivery: the same mechanism initiated by different mediators and different effector cells. PLoS One. 2011;6:e26877. doi: 10.1371/journal.pone.0026877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahendroo M. Cervical remodeling in term and preterm birth: insights from an animal model. Reproduction. 2012;143:429–438. doi: 10.1530/REP-11-0466. [DOI] [PubMed] [Google Scholar]
- 38.Akgul Y, Holt R, Mummert M, Word A, Mahendroo M. Dynamic changes in cervical glycosaminoglycan composition during normal pregnancy and preterm birth. Endocrinology. 2012;153:3493–3503. doi: 10.1210/en.2011-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez JM, Romero R, Girardi G. Comparison of the mechanisms responsible for cervical remodeling in preterm and term labor. J Reprod Immunol. 2013;97:112–119. doi: 10.1016/j.jri.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skinner SJ, Liggins GC. Glycosaminoglycans and collagen in human amnion from pregnancies with and without premature rupture of the membranes. J Dev Physiol. 1981;3:111–121. [PubMed] [Google Scholar]
- 41.Athayde N, Edwin SS, Romero R, Gomez R, Maymon E, Pacora P, Menon R. A role for matrix metalloproteinase-9 in spontaneous rupture of the fetal membranes. Am J Obstet Gynecol. 1998;179:1248–1253. doi: 10.1016/s0002-9378(98)70141-3. [DOI] [PubMed] [Google Scholar]
- 42.McLaren J, Malak TM, Bell SC. Structural characteristics of term human fetal membranes prior to labour: identification of an area of altered morphology overlying the cervix. Hum Reprod. 1999;14:237–241. doi: 10.1093/humrep/14.1.237. [DOI] [PubMed] [Google Scholar]
- 43.Keelan JA, Marvin KW, Sato TA, Coleman M, McCowan LM, Mitchell MD. Cytokine abundance in placental tissues: evidence of inflammatory activation in gestational membranes with term and preterm parturition. Am J Obstet Gynecol. 1999;181:1530–1536. doi: 10.1016/s0002-9378(99)70400-x. [DOI] [PubMed] [Google Scholar]
- 44.Maymon E, Romero R, Pacora P, Gomez R, Athayde N, Edwin S, Yoon BH. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;183:94–99. doi: 10.1067/mob.2000.105344. [DOI] [PubMed] [Google Scholar]
- 45.Maymon E, Romero R, Pacora P, Gervasi MT, Bianco K, Ghezzi F, Yoon BH. Evidence for the participation of interstitial collagenase (matrix metalloproteinase 1) in preterm premature rupture of membranes. Am J Obstet Gynecol. 2000;183:914–920. doi: 10.1067/mob.2000.108879. [DOI] [PubMed] [Google Scholar]
- 46.McLaren J, Taylor DJ, Bell SC. Increased concentration of pro-matrix metalloproteinase 9 in term fetal membranes overlying the cervix before labor: implications for membrane remodeling and rupture. Am J Obstet Gynecol. 2000;182:409–416. doi: 10.1016/s0002-9378(00)70232-8. [DOI] [PubMed] [Google Scholar]
- 47.Ulug U, Goldman S, Ben-Shlomo I, Shalev E. Matrix metalloproteinase (MMP)-2 and MMP-9 and their inhibitor, TIMP-1, in human term decidua and fetal membranes: the effect of prostaglandin F(2alpha) and indomethacin. Mol Hum Reprod. 2001;7:1187–1193. doi: 10.1093/molehr/7.12.1187. [DOI] [PubMed] [Google Scholar]
- 48.Maymon E, Romero R, Pacora P, Gomez R, Mazor M, Edwin S, Chaiworapongsa T, Kim JC, Yoon BH, Menon R, Fortunato S, Berry SM. A role for the 72 kDa gelatinase (MMP-2) and its inhibitor (TIMP-2) in human parturition, premature rupture of membranes and intraamniotic infection. J Perinat Med. 2001;29:308–316. doi: 10.1515/JPM.2001.044. [DOI] [PubMed] [Google Scholar]
- 49.Helmig BR, Romero R, Espinoza J, Chaiworapongsa T, Bujold E, Gomez R, Ohlsson K, Uldbjerg N. Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. J Matern Fetal Neonatal Med. 2002;12:237–246. doi: 10.1080/jmf.12.4.237.246. [DOI] [PubMed] [Google Scholar]
- 50.Fortunato SJ, Menon R. Screening of novel matrix metalloproteinases (MMPs) in human fetal membranes. J Assist Reprod Genet. 2002;19:483–486. doi: 10.1023/A:1020362519981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldman S, Weiss A, Eyali V, Shalev E. Differential activity of the gelatinases (matrix metalloproteinases 2 and 9) in the fetal membranes and decidua, associated with labour. Mol Hum Reprod. 2003;9:367–373. doi: 10.1093/molehr/gag040. [DOI] [PubMed] [Google Scholar]
- 52.Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M, Romero R. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol. 2006;195:394, e391–324. doi: 10.1016/j.ajog.2005.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Than NG, Romero R, Tarca AL, Draghici S, Erez O, Chaiworapongsa T, Kim YM, Kim SK, Vaisbuch E, Tromp G. Mitochondrial manganese superoxide dismutase mRNA expression in human chorioamniotic membranes and its association with labor, inflammation, and infection. J Matern Fetal Neonatal Med. 2009;22:1000–1013. doi: 10.3109/14767050903019676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bollapragada S, Youssef R, Jordan F, Greer I, Norman J, Nelson S. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am J Obstet Gynecol. 2009;200:104, e101–111. doi: 10.1016/j.ajog.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 55.Holzman C, Senagore PK, Wang J. Mononuclear leukocyte infiltrate in extraplacental membranes and preterm delivery. Am J Epidemiol. 2013;177:1053–1064. doi: 10.1093/aje/kws351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bobitt JR, Ledger WJ. Unrecognized amnionitis and prematurity: a preliminary report. J Reprod Med. 1977;19:8–12. [PubMed] [Google Scholar]
- 57.Bobitt JR, Ledger WJ. Amniotic fluid analysis. Its role in maternal neonatal infection. Obstet Gynecol. 1978;51:56–62. [PubMed] [Google Scholar]
- 58.Miller JM, Jr., Pupkin MJ, Hill GB. Bacterial colonization of amniotic fluid from intact fetal membranes. Am J Obstet Gynecol. 1980;136:796–804. doi: 10.1016/0002-9378(80)90458-5. [DOI] [PubMed] [Google Scholar]
- 59.Bobitt JR, Hayslip CC, Damato JD. Amniotic fluid infection as determined by transabdominal amniocentesis in patients with intact membranes in premature labor. Am J Obstet Gynecol. 1981;140:947–952. doi: 10.1016/0002-9378(81)90090-9. [DOI] [PubMed] [Google Scholar]
- 60.Wallace RL, Herrick CN. Amniocentesis in the evaluation of premature labor. Obstet Gynecol. 1981;57:483–486. [PubMed] [Google Scholar]
- 61.Wahbeh CJ, Hill GB, Eden RD, Gall SA. Intra-amniotic bacterial colonization in premature labor. Am J Obstet Gynecol. 1984;148:739–743. doi: 10.1016/0002-9378(84)90558-1. [DOI] [PubMed] [Google Scholar]
- 62.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, Hobbins JC. Infection in the pathogenesis of preterm labor. Semin Perinatol. 1988;12:262–279. [PubMed] [Google Scholar]
- 63.Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31:553–584. doi: 10.1097/00003081-198809000-00006. [DOI] [PubMed] [Google Scholar]
- 64.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, Hobbins JC. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–824. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 65.Romero R, Avila C, Brekus CA, Morotti R. The role of systemic and intrauterine infection in preterm parturition. Ann N Y Acad Sci. 1991;622:355–375. doi: 10.1111/j.1749-6632.1991.tb37880.x. [DOI] [PubMed] [Google Scholar]
- 66.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol. 1992;79:351–357. doi: 10.1097/00006250-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 67.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166:1515–1528. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 68.Romero R, Munoz H, Gomez R, Galasso M, Sherer DM, Cotton DB, Mitchell MD. Does infection cause premature labor and delivery? Seminars in Reproductive Endocrinology. 1994;12:227–239. [Google Scholar]
- 69.Gomez R, Romero R, Edwin SS, David C. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect Dis Clin North Am. 1997;11:135–176. doi: 10.1016/s0891-5520(05)70347-0. [DOI] [PubMed] [Google Scholar]
- 70.Romero R, Gomez R, Chaiworapongsa T, Conoscenti G, Kim JC, Kim YM. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol. 2001;15(Suppl 2):41–56. doi: 10.1046/j.1365-3016.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- 71.Yoon BH, Romero R, Moon JB, Oh SY, Han SY, Kim JC, Shim SS. The frequency and clinical significance of intra-amniotic inflammation in patients with a positive cervical fetal fibronectin. Am J Obstet Gynecol. 2001;185:1137–1142. doi: 10.1067/mob.2001.118162. [DOI] [PubMed] [Google Scholar]
- 72.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 73.Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Semin Neonatol. 2002;7:259–274. doi: 10.1016/s1084-2756(02)90121-1. [DOI] [PubMed] [Google Scholar]
- 74.Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI, Yoon BH. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004;191:1339–1345. doi: 10.1016/j.ajog.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 75.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65:S194–202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 76.Lee SE, Romero R, Jung H, Park CW, Park JS, Yoon BH. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2007;197:294, e291–296. doi: 10.1016/j.ajog.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 77.Lee SE, Romero R, Park CW, Jun JK, Yoon BH. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol. 2008;198:633, e631–638. doi: 10.1016/j.ajog.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 78.Lee SE, Romero R, Lee SM, Yoon BH. Amniotic fluid volume in intra-amniotic inflammation with and without culture-proven amniotic fluid infection in preterm premature rupture of membranes. J Perinat Med. 2010;38:39–44. doi: 10.1515/JPM.2009.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Madan I, Romero R, Kusanovic JP, Mittal P, Chaiworapongsa T, Dong Z, Mazaki-Tovi S, Vaisbuch E, Alpay Savasan Z, Yeo L, Kim CJ, Hassan SS. The frequency and clinical significance of intra-amniotic infection and/or inflammation in women with placenta previa and vaginal bleeding: an unexpected observation. J Perinat Med. 2010;38:275–279. doi: 10.1515/JPM.2010.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim SM, Romero R, Lee J, Mi Lee S, Park CW, Shin Park J, Yoon BH. The frequency and clinical significance of intra-amniotic inflammation in women with preterm uterine contractility but without cervical change: do the diagnostic criteria for preterm labor need to be changed? J Matern Fetal Neonatal Med. 2012;25:1212–1221. doi: 10.3109/14767058.2011.629256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Minkoff H. Prematurity: infection as an etiologic factor. Obstet Gynecol. 1983;62:137–144. [PubMed] [Google Scholar]
- 82.Romero R, Kadar N, Hobbins JC, Duff GW. Infection and labor: the detection of endotoxin in amniotic fluid. Am J Obstet Gynecol. 1987;157:815–819. doi: 10.1016/s0002-9378(87)80061-3. [DOI] [PubMed] [Google Scholar]
- 83.Romero R, Emamian M, Wan M, Quintero R, Hobbins JC, Mitchell MD. Prostaglandin concentrations in amniotic fluid of women with intra-amniotic infection and preterm labor. Am J Obstet Gynecol. 1987;157:1461–1467. doi: 10.1016/s0002-9378(87)80245-4. [DOI] [PubMed] [Google Scholar]
- 84.Cox SM, MacDonald PC, Casey ML. Assay of bacterial endotoxin (lipopolysaccharide) in human amniotic fluid: potential usefulness in diagnosis and management of preterm labor. Am J Obstet Gynecol. 1988;159:99–106. doi: 10.1016/0002-9378(88)90501-7. [DOI] [PubMed] [Google Scholar]
- 85.Romero R, Roslansky P, Oyarzun E, Wan M, Emamian M, Novitsky TJ, Gould MJ, Hobbins JC. Labor and infection. II. Bacterial endotoxin in amniotic fluid and its relationship to the onset of preterm labor. Am J Obstet Gynecol. 1988;158:1044–1049. doi: 10.1016/0002-9378(88)90216-5. [DOI] [PubMed] [Google Scholar]
- 86.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, Durum SK. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160:1117–1123. doi: 10.1016/0002-9378(89)90172-5. [DOI] [PubMed] [Google Scholar]
- 87.Romero R, Manogue KR, Mitchell MD, Wu YK, Oyarzun E, Hobbins JC, Cerami A. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol. 1989;161:336–341. doi: 10.1016/0002-9378(89)90515-2. [DOI] [PubMed] [Google Scholar]
- 88.McDuffie RS, Jr., Sherman MP, Gibbs RS. Amniotic fluid tumor necrosis factor-alpha and interleukin-1 in a rabbit model of bacterially induced preterm pregnancy loss. Am J Obstet Gynecol. 1992;167:1583–1588. doi: 10.1016/0002-9378(92)91745-v. [DOI] [PubMed] [Google Scholar]
- 89.Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, Dinarello CA. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992;27:117–123. doi: 10.1111/j.1600-0897.1992.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 90.Romero R, Sepulveda W, Kenney JS, Archer LE, Allison AC, Sehgal PB. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found Symp. 1992;167:205–220. doi: 10.1002/9780470514269.ch13. discussion 220-203. [DOI] [PubMed] [Google Scholar]
- 91.Greig PC, Ernest JM, Teot L, Erikson M, Talley R. Amniotic fluid interleukin-6 levels correlate with histologic chorioamnionitis and amniotic fluid cultures in patients in premature labor with intact membranes. Am J Obstet Gynecol. 1993;169:1035–1044. doi: 10.1016/0002-9378(93)90050-s. [DOI] [PubMed] [Google Scholar]
- 92.Hillier SL, Witkin SS, Krohn MA, Watts DH, Kiviat NB, Eschenbach DA. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol. 1993;81:941–948. [PubMed] [Google Scholar]
- 93.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994;171:1660–1667. doi: 10.1016/0002-9378(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 94.Fidel PL, Jr., Romero R, Wolf N, Cutright J, Ramirez M, Araneda H, Cotton DB. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol. 1994;170:1467–1475. doi: 10.1016/s0002-9378(94)70180-6. [DOI] [PubMed] [Google Scholar]
- 95.Gomez R, Ghezzi F, Romero R, Munoz H, Tolosa JE, Rojas I. Premature labor and intra amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin Perinatol. 1995;22:281–342. [PubMed] [Google Scholar]
- 96.Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, Syn HC. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995;172:960–970. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 97.Garry D, Figueroa R, Aguero-Rosenfeld M, Martinez E, Visintainer P, Tejani N. A comparison of rapid amniotic fluid markers in the prediction of microbial invasion of the uterine cavity and preterm delivery. Am J Obstet Gynecol. 1996;175:1336–1341. doi: 10.1016/s0002-9378(96)70051-0. [DOI] [PubMed] [Google Scholar]
- 98.Fortunato SJ, Menon RP, Swan KF, Menon R. Inflammatory cytokine (interleukins 1, 6 and 8 and tumor necrosis factor-alpha) release from cultured human fetal membranes in response to endotoxic lipopolysaccharide mirrors amniotic fluid concentrations. Am J Obstet Gynecol. 1996;174:1855–1861. doi: 10.1016/s0002-9378(96)70221-1. discussion 1861-1852. [DOI] [PubMed] [Google Scholar]
- 99.Hsu CD, Meaddough E, Aversa K, Hong SF, Lu LC, Jones DC, Copel JA. Elevated amniotic fluid levels of leukemia inhibitory factor, interleukin 6, and interleukin 8 in intra amniotic infection. Am J Obstet Gynecol. 1998;179:1267–1270. doi: 10.1016/s0002-9378(98)70144-9. [DOI] [PubMed] [Google Scholar]
- 100.Odibo AO, Rodis JF, Sanders MM, Borgida AF, Wilson M, Egan JF, Campbell WA. Relationship of amniotic fluid markers of intra-amniotic infection with histopathology in cases of preterm labor with intact membranes. J Perinatol. 1999;19:407–412. doi: 10.1038/sj.jp.7200210. [DOI] [PubMed] [Google Scholar]
- 101.Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol. 1992;166:1576–1587. doi: 10.1016/0002-9378(92)91636-o. [DOI] [PubMed] [Google Scholar]
- 102.Baumann P, Romero R, Berry S, Gomez R, McFarlin B, Araneda H, Cotton DB, Fidel P. Evidence of participation of the soluble tumor necrosis factor receptor I in the host response to intrauterine infection in preterm labor. Am J Reprod Immunol. 1993;30:184–193. doi: 10.1111/j.1600-0897.1993.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 103.Laham N, Brennecke SP, Bendtzen K, Rice GE. Tumour necrosis factor alpha during human pregnancy and labour: maternal plasma and amniotic fluid concentrations and release from intrauterine tissues. Eur J Endocrinol. 1994;131:607–614. doi: 10.1530/eje.0.1310607. [DOI] [PubMed] [Google Scholar]
- 104.Maymon E, Ghezzi F, Edwin SS, Mazor M, Yoon BH, Gomez R, Romero R. The tumor necrosis factor alpha and its soluble receptor profile in term and preterm parturition. Am J Obstet Gynecol. 1999;181:1142–1148. doi: 10.1016/s0002-9378(99)70097-9. [DOI] [PubMed] [Google Scholar]
- 105.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol. 1991;165:813–820. doi: 10.1016/0002-9378(91)90422-n. [DOI] [PubMed] [Google Scholar]
- 106.Cherouny PH, Pankuch GA, Romero R, Botti JJ, Kuhn DC, Demers LM, Appelbaum PC. Neutrophil attractant/activating peptide-1/interleukin-8: association with histologic chorioamnionitis, preterm delivery, and bioactive amniotic fluid leukoattractants. Am J Obstet Gynecol. 1993;169:1299–1303. doi: 10.1016/0002-9378(93)90297-v. [DOI] [PubMed] [Google Scholar]
- 107.Romero R, Gomez R, Galasso M, Munoz H, Acosta L, Yoon BH, Svinarich D, Cotton DB. Macrophage inflammatory protein-1 alpha in term and preterm parturition: effect of microbial invasion of the amniotic cavity. Am J Reprod Immunol. 1994;32:108–113. doi: 10.1111/j.1600-0897.1994.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 108.Gravett MG, Novy MJ. Endocrine-immune interactions in pregnant non-human primates with intrauterine infection. Infect Dis Obstet Gynecol. 1997;5:142–153. doi: 10.1155/S1064744997000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dudley DJ. Pre-term labor: an intra-uterine inflammatory response syndrome? J Reprod Immunol. 1997;36:93–109. doi: 10.1016/s0165-0378(97)00065-x. [DOI] [PubMed] [Google Scholar]
- 110.Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Araneda H, Yoon BH. A role for the novel cytokine RANTES in pregnancy and parturition. Am J Obstet Gynecol. 1999;181:989–994. doi: 10.1016/s0002-9378(99)70337-6. [DOI] [PubMed] [Google Scholar]
- 111.Pacora P, Romero R, Maymon E, Gervasi MT, Gomez R, Edwin SS, Yoon BH. Participation of the novel cytokine interleukin 18 in the host response to intra-amniotic infection. Am J Obstet Gynecol. 2000;183:1138–1143. doi: 10.1067/mob.2000.108881. [DOI] [PubMed] [Google Scholar]
- 112.Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol. 2003;163:2103–2111. doi: 10.1016/S0002-9440(10)63567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, Mazor M, Adashi EY. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med. 2005;17:365–373. doi: 10.1080/14767050500141329. [DOI] [PubMed] [Google Scholar]
- 114.Figueroa R, Garry D, Elimian A, Patel K, Sehgal PB, Tejani N. Evaluation of amniotic fluid cytokines in preterm labor and intact membranes. J Matern Fetal Neonatal Med. 2005;18:241–247. doi: 10.1080/13506120500223241. [DOI] [PubMed] [Google Scholar]
- 115.Chaiworapongsa T, Romero R, Espinoza J, Kim YM, Edwin S, Bujold E, Gomez R, Kuivaniemi H. Macrophage migration inhibitory factor in patients with preterm parturition and microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med. 2005;18:405–416. doi: 10.1080/14767050500361703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thaxton JE, Nevers TA, Sharma S. TLR-mediated preterm birth in response to pathogenic agents. Infect Dis Obstet Gynecol. 2010 doi: 10.1155/2010/378472. pii: 378472. doi: 10.1155/2010/378472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Choi SJ, Jung SH, Eom M, Han KH, Chung IB, Kim SK. Immunohistochemical distribution of toll-like receptor 4 in preterm human fetal membrane. J Obstet Gynaecol Res. 2012;38:108–112. doi: 10.1111/j.1447-0756.2011.01626.x. [DOI] [PubMed] [Google Scholar]
- 118.Kacerovsky M, Musilova I, Khatibi A, Skogstrand K, Hougaard DM, Tambor V, Tosner J, Jacobsson B. Intraamniotic inflammatory response to bacteria: analysis of multiple amniotic fluid proteins in women with preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2012;25:2014–2019. doi: 10.3109/14767058.2012.671873. [DOI] [PubMed] [Google Scholar]
- 119.Abrahams VM, Potter JA, Bhat G, Peltier MR, Saade G, Menon R. Bacterial modulation of human fetal membrane Toll-like receptor expression. Am J Reprod Immunol. 2013;69:33–40. doi: 10.1111/aji.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jacobsson B. Intra-amniotic infection and inflammation in preterm birth--is bacteria always the connection? Commentary on the article by Miralles . on page 570. Pediatr Res. 2005;57:473–474. doi: 10.1203/01.PDR.0000156475.50488.B0. [DOI] [PubMed] [Google Scholar]
- 121.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008;79:50–57. doi: 10.1016/j.jri.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 123.Vrachnis N, Vitoratos N, Iliodromiti Z, Sifakis S, Deligeoroglou E, Creatsas G. Intrauterine inflammation and preterm delivery. Ann N Y Acad Sci. 2010;1205:118–122. doi: 10.1111/j.1749-6632.2010.05684.x. [DOI] [PubMed] [Google Scholar]
- 124.Espinoza J, Romero R, Chaiworapongsa T, Kim JC, Yoshimatsu J, Edwin S, Rathnasabapathy C, Tolosa J, Donnenfeld A, Craparo F, Gomez R, Bujold E. Lipopolysaccharide-binding protein in microbial invasion of the amniotic cavity and human parturition. J Matern Fetal Neonatal Med. 2002;12:313–321. doi: 10.1080/jmf.12.5.313.321. [DOI] [PubMed] [Google Scholar]
- 125.Friel LA, Romero R, Edwin S, Nien JK, Gomez R, Chaiworapongsa T, Kusanovic JP, Tolosa JE, Hassan SS, Espinoza J. The calcium binding protein, S100B, is increased in the amniotic fluid of women with intra-amniotic infection/inflammation and preterm labor with intact or ruptured membranes. J Perinat Med. 2007;35:385–393. doi: 10.1515/JPM.2007.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Romero R, Espinoza J, Hassan S, Gotsch F, Kusanovic JP, Avila C, Erez O, Edwin S, Schmidt AM. Soluble receptor for advanced glycation end products (sRAGE) and endogenous secretory RAGE (esRAGE) in amniotic fluid: modulation by infection and inflammation. J Perinat Med. 2008;36:388–398. doi: 10.1515/JPM.2008.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chaiworapongsa T, Erez O, Kusanovic JP, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Than NG, Mittal P, Kim YM, Camacho N, Edwin S, Gomez R, Hassan SS, Romero R. Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. J Matern Fetal Neonatal Med. 2008;21:449–461. doi: 10.1080/14767050802054550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee SE, Park IS, Romero R, Yoon BH. Amniotic fluid prostaglandin F2 increases even in sterile amniotic fluid and is an independent predictor of impending delivery in preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2009;22:880–886. doi: 10.1080/14767050902994648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Romero R, Chaiworapongsa T, Alpay Savasan Z, Xu Y, Hussein Y, Dong Z, Kusanovic JP, Kim CJ, Hassan SS. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med. 2011;24:1444–1455. doi: 10.3109/14767058.2011.591460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chaiworapongsa T, Romero R, Chaemsaithong P, Miranda J, Dong Z, Hassan S. Rapid detection of microbial invasion of amniotic cavity with molecular microbiologic techniques and the intra-amniotic inflammatory response in preterm labor with intact membranes: diagnostic, prognostic and therapeutic implications. Am J Reprod Immunol. 2013;69:48. [Google Scholar]
- 131.Romero R, Chaiworapongsa T, Savasan ZA, Hussein Y, Dong Z, Kusanovic JP, Kim CJ, Hassan SS. Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. J Matern Fetal Neonatal Med. 2012;25:558–567. doi: 10.3109/14767058.2011.599083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Romero R, Durum S, Dinarello CA, Oyarzun E, Hobbins JC, Mitchell MD. Interleukin-1 stimulates prostaglandin biosynthesis by human amnion. Prostaglandins. 1989;37:13–22. doi: 10.1016/0090-6980(89)90028-2. [DOI] [PubMed] [Google Scholar]
- 133.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 134.Erlandsson Harris H, Andersson U. Mini-review: The nuclear protein HMGB1 as a proinflammatory mediator. Eur J Immunol. 2004;34:1503–1512. doi: 10.1002/eji.200424916. [DOI] [PubMed] [Google Scholar]
- 135.Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, Dumitriu IE, Muller S, Iannacone M, Traversari C, Bianchi ME, Manfredi AA. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004;5:825–830. doi: 10.1038/sj.embor.7400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen G, Ward MF, Sama AE, Wang H. Extracellular HMGB1 as a proinflammatory cytokine. J Interferon Cytokine Res. 2004;24:329–333. doi: 10.1089/107999004323142187. [DOI] [PubMed] [Google Scholar]
- 137.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 138.Dumitriu IE, Baruah P, Manfredi AA, Bianchi ME, Rovere-Querini P. HMGB1: guiding immunity from within. Trends Immunol. 2005;26:381–387. doi: 10.1016/j.it.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 139.Harris HE, Raucci A. Alarmin(g) news about danger: workshop on innate danger signals and HMGB1. EMBO Rep. 2006;7:774–778. doi: 10.1038/sj.embor.7400759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 141.Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med. 2008;14:476–484. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Castiglioni A, Canti V, Rovere-Querini P, Manfredi AA. High-mobility group box 1 (HMGB1) as a master regulator of innate immunity. Cell Tissue Res. 2011;343:189–199. doi: 10.1007/s00441-010-1033-1. [DOI] [PubMed] [Google Scholar]
- 143.Andersson U, Tracey KJ. HMGB1 in sepsis. Scand J Infect Dis. 2003;35:577–584. doi: 10.1080/00365540310016286. [DOI] [PubMed] [Google Scholar]
- 144.Oppenheim JJ, Tewary P, de la Rosa G, Yang D. Alarmins initiate host defense. Adv Exp Med Biol. 2007;601:185–194. doi: 10.1007/978-0-387-72005-0_19. [DOI] [PubMed] [Google Scholar]
- 145.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 146.Foell D, Wittkowski H, Roth J. Mechanisms of disease: a ‘DAMP’ view of inflammatory arthritis. Nat Clin Pract Rheumatol. 2007;3:382–390. doi: 10.1038/ncprheum0531. [DOI] [PubMed] [Google Scholar]
- 147.Gauley J, Pisetsky DS. The translocation of HMGB1 during cell activation and cell death. Autoimmunity. 2009;42:299–301. doi: 10.1080/08916930902831522. [DOI] [PubMed] [Google Scholar]
- 148.Hreggvidsdottir HS, Ostberg T, Wahamaa H, Schierbeck H, Aveberger AC, Klevenvall L, Palmblad K, Ottosson L, Andersson U, Harris HE. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J Leukoc Biol. 2009;86:655–662. doi: 10.1189/jlb.0908548. [DOI] [PubMed] [Google Scholar]
- 149.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol. 2012;8:195–202. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- 151.Diener KR, Al-Dasooqi N, Lousberg EL, Hayball JD. The multifunctional alarmin HMGB1 with roles in the pathophysiology of sepsis and cancer. Immunol Cell Biol. 2013;91:443–450. doi: 10.1038/icb.2013.25. [DOI] [PubMed] [Google Scholar]
- 152.Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim KW, Romero R, Park HS, Park CW, Shim SS, Jun JK, Yoon BH. A rapid matrix metalloproteinase-8 bedside test for the detection of intraamniotic inflammation in women with preterm premature rupture of membranes. Am J Obstet Gynecol. 2007;197:292, e291–295. doi: 10.1016/j.ajog.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 156.Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ, Jun JK. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol. 2000;183:1124–1129. doi: 10.1067/mob.2000.109035. [DOI] [PubMed] [Google Scholar]
- 157.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, Ghezzi F, Berry SM, Qureshi F, Jacques SM, Kim JC, Kadar N, Romero R. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 158.Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation--a workshop report. Placenta. 2005;26(Suppl A):S114–117. doi: 10.1016/j.placenta.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 159.Redline RW. Inflammatory responses in the placenta and umbilical cord. Semin Fetal Neonatal Med. 2006;11:296–301. doi: 10.1016/j.siny.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 160.Eshoo MW, Crowder CC, Rebman AW, Rounds MA, Matthews HE, Picuri JM, Soloski MJ, Ecker DJ, Schutzer SE, Aucott JN. Direct molecular detection and genotyping of Borrelia burgdorferi from whole blood of patients with early Lyme disease. PLoS One. 2012;7:e36825. doi: 10.1371/journal.pone.0036825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shin JH, Ranken R, Sefers SE, Lovari R, Quinn CD, Meng S, Carolan HE, Toleno D, Li H, Lee JN, Stratton CW, Massire C, Tang YW. Detection, identification, and distribution of fungi in bronchoalveolar lavage specimens by use of multilocus PCR coupled with electrospray ionization/mass spectrometry. J Clin Microbiol. 2013;51:136–141. doi: 10.1128/JCM.01907-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kaleta EJ, Clark AE, Johnson DR, Gamage DC, Wysocki VH, Cherkaoui A, Schrenzel J, Wolk DM. Use of PCR coupled with electrospray ionization mass spectrometry for rapid identification of bacterial and yeast bloodstream pathogens from blood culture bottles. J Clin Microbiol. 2011;49:345–353. doi: 10.1128/JCM.00936-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ecker DJ, Sampath R, Li H, Massire C, Matthews HE, Toleno D, Hall TA, Blyn LB, Eshoo MW, Ranken R, Hofstadler SA, Tang YW. New technology for rapid molecular diagnosis of bloodstream infections. Expert Rev Mol Diagn. 2010;10:399–415. doi: 10.1586/erm.10.24. [DOI] [PubMed] [Google Scholar]
- 164.Metzgar D, Frinder M, Lovari R, Toleno D, Massire C, Blyn LB, Ranken R, Carolan HE, Hall TA, Moore D, Hansen CJ, Sampath R, Ecker DJ. Broad-spectrum biosensor capable of detecting and identifying diverse bacterial and Candida species in blood. J Clin Microbiol. 2013;51:2670–2678. doi: 10.1128/JCM.00966-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, Bertin J, Coyle A, Grant EP, Akira S, Nunez G. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 166.Ecker JA, Massire C, Hall TA, Ranken R, Pennella TT, Agasino Ivy C, Blyn LB, Hofstadler SA, Endy TP, Scott PT, Lindler L, Hamilton T, Gaddy C, Snow K, Pe M, Fishbain J, Craft D, Deye G, Riddell S, Milstrey E, Petruccelli B, Brisse S, Harpin V, Schink A, Ecker DJ, Sampath R, Eshoo MW. Identification of Acinetobacter species and genotyping of Acinetobacter baumannii by multilocus PCR and mass spectrometry. J Clin Microbiol. 2006;44:2921–2932. doi: 10.1128/JCM.00619-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Legoff J, Feghoul L, Mercier-Delarue S, Dalle JH, Scieux C, Cherot J, Sicre de Fontbrune F, Baruchel A, Socie G, Simon F. Broad-Range PCR/Electrospray Ionization Mass Spectrometry for Detection and Typing of Adenovirus and Other Opportunistic Viruses in Stem Cell Transplant Patients. J Clin Microbiol. 2013;51:4186–4192. doi: 10.1128/JCM.01978-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Romero R, Yoon BH, Kenney JS, Gomez R, Allison AC, Sehgal PB. Amniotic fluid interleukin-6 determinations are of diagnostic and prognostic value in preterm labor. Am J Reprod Immunol. 1993;30:167–183. doi: 10.1111/j.1600-0897.1993.tb00618.x. [DOI] [PubMed] [Google Scholar]
- 169.Romero R, Yoon BH, Mazor M, Gomez R, Diamond MP, Kenney JS, Ramirez M, Fidel PL, Sorokin Y, Cotton D, Sehgal P. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 1993;169:805–816. doi: 10.1016/0002-9378(93)90009-8. [DOI] [PubMed] [Google Scholar]
- 170.Andrews WW, Hauth JC, Goldenberg RL, Gomez R, Romero R, Cassell GH. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol. 1995;173:606–612. doi: 10.1016/0002-9378(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 171.Greci LS, Gilson GJ, Nevils B, Izquierdo LA, Qualls CR, Curet LB. Is amniotic fluid analysis the key to preterm labor? A model using interleukin-6 for predicting rapid delivery. Am J Obstet Gynecol. 1998;179:172–178. doi: 10.1016/s0002-9378(98)70269-8. [DOI] [PubMed] [Google Scholar]
- 172.Yoon BH, Romero R, Park JS, Chang JW, Kim YA, Kim JC, Kim KS. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1998;179:1254–1260. doi: 10.1016/s0002-9378(98)70142-5. [DOI] [PubMed] [Google Scholar]
- 173.Maymon E, Romero R, Chaiworapongsa T, Berman S, Conoscenti G, Gomez R, Edwin S. Amniotic fluid matrix metalloproteinase-8 in preterm labor with intact membranes. Am J Obstet Gynecol. 2001;185:1149–1155. doi: 10.1067/mob.2001.118165. [DOI] [PubMed] [Google Scholar]
- 174.Cobo T, Palacio M, Martinez-Terron M, Navarro-Sastre A, Bosch J, Filella X, Gratacos E. Clinical and inflammatory markers in amniotic fluid as predictors of adverse outcomes in preterm premature rupture of membranes. Am J Obstet Gynecol. 2011;205:126, e121–128. doi: 10.1016/j.ajog.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 175.Cobo T, Kacerovsky M, Holst RM, Hougaard DM, Skogstrand K, Wennerholm UB, Hagberg H, Jacobsson B. Intra-amniotic inflammation predicts microbial invasion of the amniotic cavity but not spontaneous preterm delivery in preterm prelabor membrane rupture. Acta Obstet Gynecol Scand. 2012;91:930–935. doi: 10.1111/j.1600-0412.2012.01427.x. [DOI] [PubMed] [Google Scholar]
- 176.Jacobsson B, Holst RM, Wennerholm UB, Andersson B, Lilja H, Hagberg H. Monocyte chemotactic protein-1 in cervical and amniotic fluid: relationship to microbial invasion of the amniotic cavity, intra-amniotic inflammation, and preterm delivery. Am J Obstet Gynecol. 2003;189:1161–1167. doi: 10.1067/s0002-9378(03)00594-5. [DOI] [PubMed] [Google Scholar]
- 177.Soto E, Espinoza J, Nien JK, Kusanovic JP, Erez O, Richani K, Santolaya-Forgas J, Romero R. Human beta-defensin-2: a natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med. 2007;20:15–22. doi: 10.1080/14767050601036212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bennett P, Fisk N. Chorioamnionitis and pre-term delivery. Baillieres Clin Obstet Gynaecol. 1993;7:25–43. doi: 10.1016/s0950-3552(05)80146-4. [DOI] [PubMed] [Google Scholar]
- 179.Jacobsson B, Mattsby-Baltzer I, Hagberg H. Interleukin-6 and interleukin-8 in cervical and amniotic fluid: relationship to microbial invasion of the chorioamniotic membranes. BJOG. 2005;112:719–724. doi: 10.1111/j.1471-0528.2005.00536.x. [DOI] [PubMed] [Google Scholar]
- 180.Miralles R, Hodge R, McParland PC, Field DJ, Bell SC, Taylor DJ, Grant WD, Kotecha S. Relationship between antenatal inflammation and antenatal infection identified by detection of microbial genes by polymerase chain reaction. Pediatr Res. 2005;57:570–577. doi: 10.1203/01.PDR.0000155944.48195.97. [DOI] [PubMed] [Google Scholar]
- 181.Lee SE, Romero R, Kim CJ, Shim SS, Yoon BH. Funisitis in term pregnancy is associated with microbial invasion of the amniotic cavity and intra-amniotic inflammation. J Matern Fetal Neonatal Med. 2006;19:693–697. doi: 10.1080/14767050600927353. [DOI] [PubMed] [Google Scholar]
- 182.Hecht JL, Onderdonk A, Delaney M, Allred EN, Kliman HJ, Zambrano E, Pflueger SM, Livasy CA, Bhan I, Leviton A. Characterization of chorioamnionitis in 2nd-trimester C-section placentas and correlation with microorganism recovery from subamniotic tissues. Pediatr Dev Pathol. 2008;11:15–22. doi: 10.2350/07-06-0285.1. [DOI] [PubMed] [Google Scholar]
- 183.DiGiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, Kim CJ, Erez O, Edwin S, Relman DA. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3:e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kim MJ, Romero R, Gervasi MT, Kim JS, Yoo W, Lee DC, Mittal P, Erez O, Kusanovic JP, Hassan SS, Kim CJ. Widespread microbial invasion of the chorioamniotic membranes is a consequence and not a cause of intra-amniotic infection. Lab Invest. 2009;89:924–936. doi: 10.1038/labinvest.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kacerovsky M, Pliskova L, Bolehovska R, Musilova I, Hornychova H, Tambor V, Jacobsson B. The microbial load with genital mycoplasmas correlates with the degree of histologic chorioamnionitis in preterm PROM. Am J Obstet Gynecol. 2011;205:213, e211–217. doi: 10.1016/j.ajog.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 186.Chen ML, Allred EN, Hecht JL, Onderdonk A, VanderVeen D, Wallace DK, Leviton A, Dammann O. Placenta microbiology and histology and the risk for severe retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2011;52:7052–7058. doi: 10.1167/iovs.11-7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Redline RW. Inflammatory response in acute chorioamnionitis. Semin Fetal Neonatal Med. 2012;17:20–25. doi: 10.1016/j.siny.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 188.Romero R, Salafia CM, Athanassiadis AP, Hanaoka S, Mazor M, Sepulveda W, Bracken MB. The relationship between acute inflammatory lesions of the preterm placenta and amniotic fluid microbiology. Am J Obstet Gynecol. 1992;166:1382–1388. doi: 10.1016/0002-9378(92)91609-e. [DOI] [PubMed] [Google Scholar]
- 189.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 190.Huang H, Evankovich J, Yan W, Nace G, Zhang L, Ross M, Liao X, Billiar T, Xu J, Esmon CT, Tsung A. Endogenous histones function as alarmins in sterile inflammatory liver injury through Toll-like receptor 9 in mice. Hepatology. 2011;54:999–1008. doi: 10.1002/hep.24501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cardenas I, Means RE, Aldo P, Koga K, Lang SM, Booth CJ, Manzur A, Oyarzun E, Romero R, Mor G. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol. 2010;185:1248–1257. doi: 10.4049/jimmunol.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. American journal of reproductive immunology. 2010;63:425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ilievski V, Hirsch E. Synergy between viral and bacterial toll-like receptors leads to amplification of inflammatory responses and preterm labor in the mouse. Biol Reprod. 2010;83:767–773. doi: 10.1095/biolreprod.110.085464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cardenas I, Mor G, Aldo P, Lang SM, Stabach P, Sharp A, Romero R, Mazaki-Tovi S, Gervasi M, Means RE. Placental viral infection sensitizes to endotoxin-induced pre-term labor: a double hit hypothesis. American journal of reproductive immunology. 2011;65:110–117. doi: 10.1111/j.1600-0897.2010.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Racicot K, Cardenas I, Wunsche V, Aldo P, Guller S, Means RE, Romero R, Mor G. Viral infection of the pregnant cervix predisposes to ascending bacterial infection. J Immunol. 2013;191:934–941. doi: 10.4049/jimmunol.1300661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Janeway CA, Jr., Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 197.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 198.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 200.Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 201.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 202.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 203.Medzhitov R, Janeway CA., Jr. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 204.Medzhitov R, Janeway CA., Jr. Self-defense: the fruit fly style. Proc Natl Acad Sci U S A. 1998;95:429–430. doi: 10.1073/pnas.95.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Medzhitov R, Janeway CA., Jr. How does the immune system distinguish self from nonself? Semin Immunol. 2000;12:185–188. doi: 10.1006/smim.2000.0230. discussion 257-344. [DOI] [PubMed] [Google Scholar]
- 206.Medzhitov R, Janeway C., Jr. Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173:89–97. doi: 10.1034/j.1600-065x.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- 207.Medzhitov R, Janeway CA., Jr. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 208.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 209.Kopp E, Medzhitov R. Recognition of microbial infection by Toll-like receptors. Curr Opin Immunol. 2003;15:396–401. doi: 10.1016/s0952-7915(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 210.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect. 2004;6:1382–1387. doi: 10.1016/j.micinf.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 211.Hargreaves DC, Medzhitov R. Innate sensors of microbial infection. J Clin Immunol. 2005;25:503–510. doi: 10.1007/s10875-005-8065-4. [DOI] [PubMed] [Google Scholar]
- 212.Bianchi ME, Manfredi AA. Immunology. Dangers in and out. Science. 2009;323:1683–1684. doi: 10.1126/science.1172794. [DOI] [PubMed] [Google Scholar]
- 213.Nunez G. Intracellular sensors of microbes and danger. Immunol Rev. 2011;243:5–8. doi: 10.1111/j.1600-065X.2011.01058.x. [DOI] [PubMed] [Google Scholar]
- 214.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 215.Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010 doi: 10.1155/2010/672395. pii: 672395. doi: 10.1155/2010/672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yang D, Chen Q, Yang H, Tracey KJ, Bustin M, Oppenheim JJ. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol. 2007;81:59–66. doi: 10.1189/jlb.0306180. [DOI] [PubMed] [Google Scholar]
- 218.Dumitriu IE, Bianchi ME, Bacci M, Manfredi AA, Rovere-Querini P. The secretion of HMGB1 is required for the migration of maturing dendritic cells. J Leukoc Biol. 2007;81:84–91. doi: 10.1189/jlb.0306171. [DOI] [PubMed] [Google Scholar]
- 219.Rouhiainen A, Kuja-Panula J, Wilkman E, Pakkanen J, Stenfors J, Tuominen RK, Lepantalo M, Carpen O, Parkkinen J, Rauvala H. Regulation of monocyte migration by amphoterin (HMGB1). Blood. 2004;104:1174–1182. doi: 10.1182/blood-2003-10-3536. [DOI] [PubMed] [Google Scholar]
- 220.DeMarco RA, Fink MP, Lotze MT. Monocytes promote natural killer cell interferon gamma production in response to the endogenous danger signal HMGB1. Mol Immunol. 2005;42:433–444. doi: 10.1016/j.molimm.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 221.Li J, Kokkola R, Tabibzadeh S, Yang R, Ochani M, Qiang X, Harris HE, Czura CJ, Wang H, Ulloa L, Warren HS, Moldawer LL, Fink MP, Andersson U, Tracey KJ, Yang H. Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol Med. 2003;9:37–45. [PMC free article] [PubMed] [Google Scholar]
- 222.Park JS, Arcaroli J, Yum HK, Yang H, Wang H, Yang KY, Choe KH, Strassheim D, Pitts TM, Tracey KJ, Abraham E. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am J Physiol Cell Physiol. 2003;284:C870–879. doi: 10.1152/ajpcell.00322.2002. [DOI] [PubMed] [Google Scholar]
- 223.Silva E, Arcaroli J, He Q, Svetkauskaite D, Coldren C, Nick JA, Poch K, Park JS, Banerjee A, Abraham E. HMGB1 and LPS induce distinct patterns of gene expression and activation in neutrophils from patients with sepsis-induced acute lung injury. Intensive Care Med. 2007;33:1829–1839. doi: 10.1007/s00134-007-0748-2. [DOI] [PubMed] [Google Scholar]
- 224.Fan J, Li Y, Levy RM, Fan JJ, Hackam DJ, Vodovotz Y, Yang H, Tracey KJ, Billiar TR. 2007;178:6573–6580. doi: 10.4049/jimmunol.178.10.6573. [DOI] [PubMed] [Google Scholar]
- 225.Messmer D, Yang H, Telusma G, Knoll F, Li J, Messmer B, Tracey KJ, Chiorazzi N. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol. 2004;173:307–313. doi: 10.4049/jimmunol.173.1.307. [DOI] [PubMed] [Google Scholar]
- 226.Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP, Arnold B, Bianchi ME, Manfredi AA, Rovere-Querini P. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol. 2005;174:7506–7515. doi: 10.4049/jimmunol.174.12.7506. [DOI] [PubMed] [Google Scholar]
- 227.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 228.Wang H, Yang H, Czura CJ, Sama AE, Tracey KJ. HMGB1 as a late mediator of lethal systemic inflammation. Am J Respir Crit Care Med. 2001;164:1768–1773. doi: 10.1164/ajrccm.164.10.2106117. [DOI] [PubMed] [Google Scholar]
- 229.Czura CJ, Tracey KJ. Targeting high mobility group box 1 as a late-acting mediator of inflammation. Crit Care Med. 2003;31:S46–50. doi: 10.1097/00003246-200301001-00007. [DOI] [PubMed] [Google Scholar]
- 230.Tang D, Kang R, Xiao W, Zhang H, Lotze MT, Wang H, Xiao X. Quercetin prevents LPS-induced high-mobility group box 1 release and proinflammatory function. Am J Respir Cell Mol Biol. 2009;41:651–660. doi: 10.1165/rcmb.2008-0119OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wang H, Ward MF, Sama AE. Novel HMGB1-inhibiting therapeutic agents for experimental sepsis. Shock. 2009;32:348–357. doi: 10.1097/SHK.0b013e3181a551bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol. 1991;165:969–971. doi: 10.1016/0002-9378(91)90450-6. [DOI] [PubMed] [Google Scholar]
- 233.Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol. 1992;167:1041–1045. doi: 10.1016/s0002-9378(12)80035-4. [DOI] [PubMed] [Google Scholar]
- 234.Bry K, Hallman M. Transforming growth factor-beta 2 prevents preterm delivery induced by interleukin-1 alpha and tumor necrosis factor-alpha in the rabbit. Am J Obstet Gynecol. 1993;168:1318–1322. doi: 10.1016/0002-9378(93)90388-y. [DOI] [PubMed] [Google Scholar]
- 235.Gotsch F, Romero R, Chaiworapongsa T, Erez O, Vaisbuch E, Espinoza J, Kusanovic JP, Mittal P, Mazaki-Tovi S, Kim CJ, Kim JS, Edwin S, Nhan-Chang CL, Hamill N, Friel L, Than NG, Mazor M, Yoon BH, Hassan SS. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med. 2008;21:605–616. doi: 10.1080/14767050802212109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Gross O, Yazdi AS, Thomas CJ, Masin M, Heinz LX, Guarda G, Quadroni M, Drexler SK, Tschopp J. Inflammasome activators induce interleukin-1alpha secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity. 2012;36:388–400. doi: 10.1016/j.immuni.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 237.Yazdi AS, Drexler SK. Regulation of interleukin 1alpha secretion by inflammasomes. Ann Rheum Dis. 2013;72(Suppl 2):ii96–99. doi: 10.1136/annrheumdis-2012-202252. [DOI] [PubMed] [Google Scholar]