Abstract
Objective
To systematically assess the prevalence of bacterial infection in adults with acute rhinosinusitis (ARS)
Data Sources
PubMed and CINAHL databases
Review Methods
Electronic databases were systematically searched for relevant studies published up to June 2012.
Results
29 articles, evaluating a total of 9,595 patients with a clinical diagnosis of ARS, were included in the study. 14 (48%) studies required radiographic confirmation of sinusitis, 1 (3%) required evidence of purulence, 10 (35%) required both for inclusion in the study population, and 4 (14%) required neither. The random effects model estimate of prevalence of bacterial growth on all cultures was 53.7% (CI 48.4%–59.0%), ranging from 52.5% (CI 46.7%–58.3%) in studies requiring radiographic confirmation of sinusitis to 61.1% (CI 54.0%–68.1%) in studies requiring neither radiographic evidence nor purulence on exam. Studies which obtained cultures from antral swab had a prevalence of bacterial growth of 61.0% (CI 54.7%–67.2%), while those utilizing endoscopic meatal sampling had a prevalence of 32.9% (CI 19.0%–46.8%).
Conclusion
Few studies evaluate the recovery of bacteria via culture in adults with a diagnosis of ABRS or ARS based on clinical criteria alone. With radiographic and/or endoscopic confirmation, antral puncture and endoscopically guided cultures produce positive bacterial cultures in approximately half of patients. Opportunities exist to improve diagnostic accuracy for bacterial infection in ARS.
Keywords: systematic review, meta-analysis, acute rhinosinusitis, bacterial infection, antral puncture, endoscopic middle meatus culture
Introduction
Acute rhinosinusitis (ARS) is among the most common conditions encountered by primary care providers, and ARS is one of the most common reasons for antibiotic prescriptions, with antibiotics prescribed in 82–88% of patient visits for ARS.1–4 A growing body of evidence suggests that antibiotics do not confer a distinct benefit in the majority of ARS cases,5–8 and guidelines do not recommend antibiotics for most cases of ARS.9–15 This is largely because only a small proportion of viral sinus infections is believed to progress to acute bacterial rhinosinusitis.9,10,16 In scientific literature, however, the reported prevalence of bacterial infection in ARS ranges widely, from 0.5% to 86%, depending on the population studied and the diagnostic methods used to confirm bacterial sinusitis. 13,16–24
ARS, as defined by the American Academy of Otolaryngology--Head and Neck Surgery Foundation clinical practice guideline, is defined by up to 4 weeks of purulent nasal drainage accompanied by nasal obstruction and/or facial pain/pressure/fullness.9 In ARS, an inflammatory reaction to a viral upper respiratory infection characterizes most cases. Viral, post-viral, and bacterial ARS show considerable overlap in inflammatory mechanisms and clinical presentation.7 The pathophysiology involves interplay between a predisposing condition (e.g. allergic rhinitis, septal deformity, concha bullosa, primary ciliary dyskinesia, immune deficiency, and environmental factors), infection, and consequent inflammatory response in the sinonasal mucosa. Viruses attach to host cells via intermolecular interaction between nucleocapsids (naked viruses) or viral membranes (enveloped viruses) and the host cell receptor.7 The inflammatory response involves edema, fluid extravasation, and mucus production. The inflammatory cascade involves T-helper type 1 cytokine polarization associated with tumor necrosis factor-β and interferon-γ. Proinflammatory cytokines such as interleukin (IL)-1β, IL-6, and IL-8 are potent chemoattractive agents for neutrophils.25 Mucosal inflammation may lead to obstruction of normal sinus outflow tracts. This obstruction impedes normal ventilation and drainage, leading to a lower partial pressure of oxygen, decreased ciliary clearance, and stasis of secretions. A secondary bacterial infection may develop.
The prevalence of bacterial infection in patients with clinically diagnosed ARS is not well defined given the difficulty distinguishing viral from bacterial infection. The clinical features of viral and bacterial ARS are similar. There are no clinical findings, including a change in the color or character of nasal discharge17, that predict whether ARS is of bacterial origin. Common imaging modalities are neither sufficiently sensitive nor specific. Several imaging, clinical, and laboratory tests have been used to increase the likelihood of a correct diagnosis of bacterial ARS.26–28
Culture of intrasinusal secretions from sinus puncture is considered the most widely accepted and gold standard method to define ABRS,26,29–31 but is not routinely feasible due to patient perceived of real discomfort of this invasive procedure.32 A recent meta-analysis revealed that endoscopically directed middle meatal cultures (EMMC) is a highly sensitive and accurate culture method for acute ABRS and may be more sensitive than maxillary sinus taps given the presence of pathogenic bacteria not found on antral lavage. The authors stated that EMMC is a viable, and possibly preferred, culture method for determining antimicrobial efficacy and bacterial resistance patterns.24
With the detrimental effects of inappropriate antibiotic prescribing in mind, the primary objective of this study was to review the literature to assess the prevalence of bacterial infection in adults with clinically diagnosed ARS who undergo culture from antral puncture or endoscopically directed middle meatus culture. A secondary objective was to compare the prevalence of bacterial infection in adults with clinically diagnosed ARS by method of culture: antral puncture vs. EMMC. We hypothesized bacterial recovery would be same between antral puncture and EMMC. Information regarding prevalence of bacterial infection in ARS and culture methods could direct efforts to improve the quality and quantity of antibiotic prescribing.
Materials and methods
This review was conducted based on the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.33 We searched PubMed and Ovid MEDLINE and CINAHL from date of database inception to June 12, 2012. For MEDLINE, we used search terms “acute sinusitis”[All Fields] OR “acute rhinosinusitis”[All Fields] OR “acute bacterial sinusitis”[All Fields] OR “acute bacterial rhinosinusitis”[All Fields] OR “viral rhinosinusitis”[All Fields] OR “viral sinusitis”[All Fields] AND (“humans”[MeSH Terms] AND English[lang]). For CINAHL, we searched boolean/phrases “acute sinusitis” or “acute rhinosinusitis” or “acute bacterial sinusitis” or “acute bacterial rhinosinusitis” or “viral sinusitis” or “viral rhinosinusitis.” We subsequently reviewed reference lists of review articles and other relevant publications for additional studies to include. A single patient was considered the unit of analysis in the study. Eligibility criteria were participants aged ≥13years with ARS by clinical, radiographic, or endoscopic diagnosis; English language; original research; experimental, quasi-experimental, or observational study designs; intervention with antral puncture or maxillary aspiration prior to antibiotic treatment; measurable outcome with bacterial culture; N<10. We excluded non- investigative studies (technical notes, letters, comments, reviews) and case reports. We also excluded studies when more than 1 sinus was cultured per patient, and patient level data were not explicit. In studies where both endoscopic middle meatus culture and sinus puncture were performed, with explicit bacteriologic results for each intervention, we included only the data for the puncture results in this review.
Radiographic confirmation was determined by a positive sinus X-ray or CT scan. Purulence was identified on physical exam, nasal endoscopy, or antral puncture. Bacterial cultures were obtained from antral puncture or endoscopic middle meatus secretion sampling. Bacterial culture results were considered positive in a qualitative fashion, without a minimal colony count.24
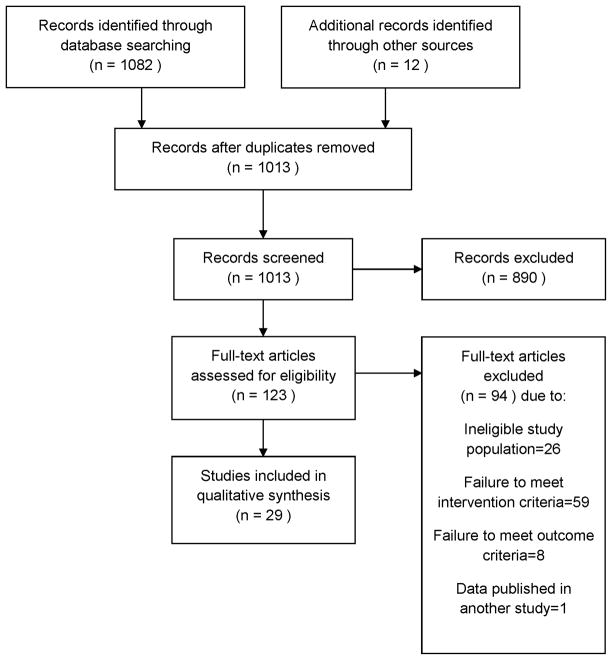
Two authors (SS and EF) independently screened all titles and abstracts of the retrieved publications for selection. Subsequently, the full texts of eligible studies were screened for a more detailed selection (Fig. 1). Disagreement between the authors was resolved by discussion. Inter-rater reliability was assessed by computing Kappa.
Figure 1.
Systematic Review Flowchart
Two authors (SS and EF) independently assessed the radiographic and purulence diagnostic criteria, intervention and site of diagnostic culture, number of included patients, and outcome (number of positive cultures out of all cultures taken). A single author (SS) gathered information for each study on design, study population, clinical basis of ARS diagnosis and original study design.
Two authors (SS and EF) independently assessed the level of evidence to provide an overall estimate of the strength of study design when the study design produced data directly pertaining to our research question.34 The risk of bias was assessed at the study level and outcome level by examining each study for specific markers of validity. These markers included randomization, concealment of subject allocation, and blinding of subjects or investigators. Disagreement was resolved by discussion. A random effects meta-analysis was performed for all of the included studies, for subsets based on whether diagnostic criteria included radiographic evidence and/or purulence on exam, and for a subset excluding 6 studies where patients were clinically diagnosed with ARS up to a duration of 3 months. The random effects model utilizes the effect sizes of studies to calculate a weighted average. Heterogeneity is described by i2, which describes the percentage of variation in study outcomes between studies not due to chance.
Results
Our search identified 1,013 unique articles, which were then screened for relevance (Figure 1). After screening titles and abstracts, 890 articles were excluded because they did not provide original data for the prevalence of positive bacterial cultures in adults with ARS diagnoses. Initial inter-rater reliability was substantial (Kappa=0.80), and there was 100% agreement on articles for full review following discussion of disagreements between authors. The remaining 123 articles were retrieved in full text for formal review. After independent review by two authors, 29 articles with a total of 9,595 patients met the inclusion and exclusion criteria and were eligible for full analysis. Inter-rater reliability was excellent (Kappa=1.0).
Descriptive characteristics and data from risk assessment of bias and level of evidence for individual studies are presented in Supplemental Table 1. 28 out of 29 studies were prospective inquiries. Assessment of randomization and concealment of subject allocation and blinding of subjects or investigators did not apply to the studies, because our inclusion criteria specified that all participants have a clinical diagnosis of ARS and undergo intervention of EMMC or antral puncture. There were no studies involving control aspirates. No studies reported lost bacteriologic data.
Of the 29 studies included for analysis, assessment on the level of evidence was not applicable in 22 studies, in which our outcome of interest was extrapolated from the original study situation.35 These 22 studies were drug studies, where the main outcome measure involved effects of antibiotics. While randomization, blinding, controlling, and/or handling of cases lost to follow up were pertinent to the interventions and/or outcomes of those studies, these quality measures were not applied to the interventions or outcomes assessed in our study. For example, in such studies all eligible participants with clinical diagnosis of ARS underwent antral puncture to confirm presence of bacterial pathogens prior to randomization to an antibiotic or placebo group. The outcome evaluations were not blinded (bacteriologic results were readily available in these studies). The level of evidence in the remaining 7 studies was level 4, reflecting the use of uncontrolled case series for data reporting.
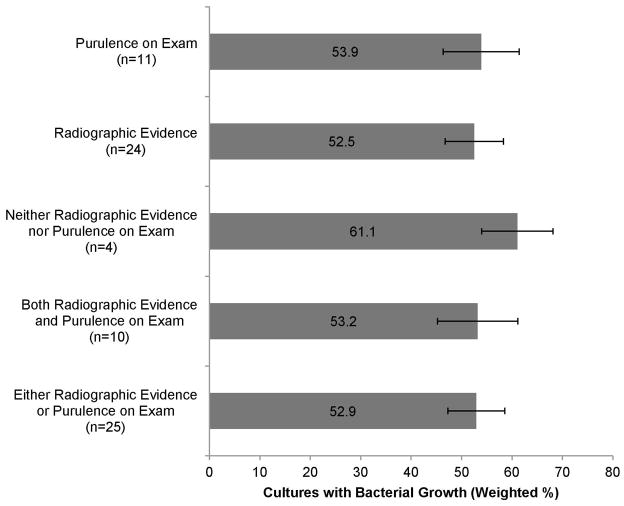
The primary findings of each prevalence study included for analysis are presented in Supplemental Table 2. Of the included studies, 14 (48%) required radiographic confirmation of sinusitis, 1 (3%) required evidence of purulence, 10 (35%) required both for inclusion in the study population, and 4(14%) required neither. Culture was obtained by antral puncture in 19 (66%) studies, endoscopic meatal sampling in 3 (10%), or either method in 7 (24%). The random effects model estimate of prevalence of bacterial growth ranged from 52.5% (CI 46.7%–58.3%) among studies requiring radiographic confirmation of sinusitis to 61.1% (CI 54.0%–68.1%) in studies requiring neither radiographic evidence nor purulence on exam (Figure 2).
Figure 2.
Bacterial growth based on objective diagnostic criteria (n = number of studies)
Studies which obtained cultures from antral swab had a random effects model estimate of prevalence of bacterial growth of 61.0% (CI 54.7%–67.2%), while those utilizing endoscopic meatal sampling had an estimated prevalence of 32.9% (CI 19.0%–46.8%). Studies which allowed for either method had an estimated prevalence of 43.4% (CI 35.7%–51.1%) (Figure 3). In the subset analysis excluding 6 studies where patients were clinically diagnosed with ARS up to a duration of 3 months, random effects model estimate of prevalence of bacterial growth of 51.2%.
Figure 3.
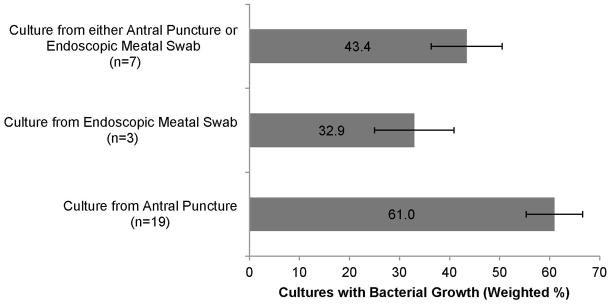
Bacteria growth based on method of culture (n = number of studies)
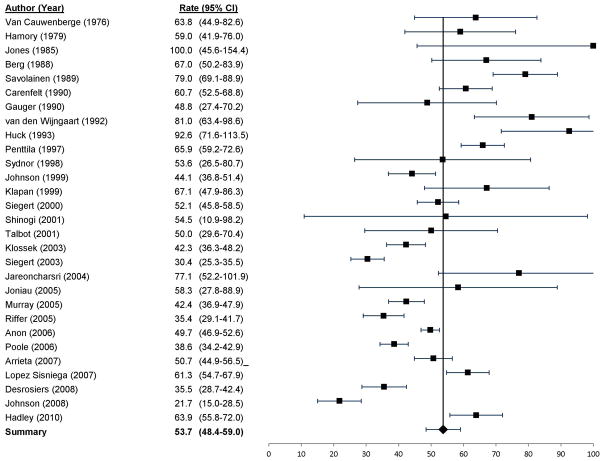
The random effects model estimate of prevalence of bacterial growth on culture was 53.7% (CI 48.4%–59.0%) among all studies, with i2 = 0.2% (Figure 4)
Figure 4.
Prevalence of bacterial infection in acute rhinosinusitis
Discussion
This study demonstrates four principal findings. First, there is a paucity of studies investigating bacterial prevalence in typical ARS adult patients without radiographic or purulent criterion. Second, bacterial pathogens are recovered by sinus puncture or EMMC in approximately half of patients with suspected bacterial ARS. Third, radiographic evidence and purulence was not associated with higher bacterial prevalence. Fourth, culture from EMMC had a lower rate of positive bacterial culture compared with antral puncture.
Initially, we set out to study recovery of bacterial pathogens in patients diagnosed with ARS in real world clinical settings. However, we found only 4 studies that met our inclusion criteria that did not also require radiographic and/or endoscopic evidence of ARS. All individual studies had strict ARS diagnostic inclusion criteria. Our meta-analysis shows that bacterial pathogens are recovered by sinus puncture or EMMC in 53% of patients with suspected bacterial ARS based on studies requiring patients to meet clinical criteria, with most studies also requiring radiographic and/or endoscopic confirmation.
To place these results in the context of existing literature, several highly regarded original articles,36 review articles,37,38 and clinical guidelines7,10,11 estimate that acute bacterial sinusitis complicates 0.5% to 2% of common colds and influenza-like illnesses in adults. Other review articles,16 and clinical guidelines13,17,18 suggest that acute bacterial sinusitis complicates 0.5% to 2% of acute viral sinus infections in adults. The citations in these publications can be traced back to 2 original studies as follows. Some publications11,17,36–38 cite a study by Berg et al. published in 1986.39 In that study of 100 patients with a common cold “or other acute ENT infection,” antral aspirations were performed in patients with ultrasound positive maxillary and frontal sinus secretions, and 2 aspirations revealed purulent fluid. Patients were excluded if they had “any complaints suggesting a sinusitis.”
Other publications36–38 cite a study by Dingle published in 1964.40 In that study, 53 (0.5%) of 11,134 patients with colds had sinusitis (implied acute community-acquired bacterial sinusitis) diagnosed by clinical criteria between 1947 and 1957. Still other publications 11,13,16,18 cite review articles with references that can be traced to the original research of Berg et al. and/or Dingle, and other publications 7,10 do not cite a reference. Studies examining the cost-effectiveness of managing acute sinusitis19,20 tend to use higher estimates for the prevalence of acute bacterial sinusitis in the range of 38%–50%, with wide sensitivity analyses.
Pharmacologic studies of patients meeting positive clinical plus radiographic criteria demonstrate positive bacterial cultures a higher proportion of cases, as demonstrated in our study. Despite this, randomized controlled studies demonstrate high cure or improvement rates in ARS in both placebo groups (80%) and antibiotic groups (90%),6 supporting the case that a substantial proportion of ARS cases are likely to be either viral or not from highly pathogenic bacterial strains.
Our analyses show that radiographic evidence and purulence did not more accurately predict which patients would have positive bacterial cultures by antral puncture or EMCC, demonstrating that mucosal inflammation or air fluid levels and purulence may be present in the absence of pathogenic bacteria. Of note, the four studies that did not require either radiographic evidence or purulence had the highest bacterial prevalence on culture (61.1%). These studies were from 1976,41 1979,42 and 1990,43,44 and are four out of the seven oldest studies in our meta-analyses, but no other common characteristics seem to make the patients in these studies more likely to have bacterial ARS compared to patients in later studies.
Our data show that EMMC had a lower rate of positive bacterial culture compared with antral puncture. Historically, it was theorized that specimens obtained via EMCC would be contaminated more easily than antral puncture.29 However, several studies suggest that carefully obtained samples of secretions from the middle meatus correlate with specimens obtained by antral puncture in both ARS24,45 and chronic rhinosinusitis (CRS).46 A meta-analysis revealed that endoscopically directed middle meatal cultures is a highly sensitive and accurate culture method for acute ABRS and may be more sensitive than maxillary sinus taps given the presence of pathogenic bacteria not found on antral lavage. The authors stated that EMMC is a viable, and possibly preferred, culture method for determining antimicrobial efficacy and bacterial resistance patterns.24 The slightly greater bacterial yield observed in antral puncture in this study may reflect oral or dental flora organisms; the speciation of bacteria isolated by cultures is beyond the scope of this paper.
The subset analysis that excluded 6 studies of patients with ARS symptoms of unspecified duration or lasting up to a duration of 3 months yielded a random effects model estimate of prevalence of bacterial growth that was only slightly lower (51.1 vs. 53.7%) than the overall analysis of all patients with clinically diagnosed ARS. This suggests that the excluded study patients may be similar to those with ARS classically defined by <4 weeks of symptoms. The authors hypothesize that many of the excluded study patients may, in fact, have had duration of symptoms <4 weeks. However, this cannot be determined within the scope of this study.
Though sinus puncture has been considered the gold standard method for detecting bacterial ARS,7,26,29–31 the validity of sinus puncture may be questioned based on innovative microbiology techniques. Antral cultures of healthy sinuses show conflicting results, with a predominance of sterility in some studies,47,48 and bacterial growth in others.49 Microbiome studies using culture-independent techniques have demonstrated that healthy sinuses are not sterile at baseline.50–52 Furthermore, conventional laboratory culture has been criticized for introducing “enrichment bias,” which selects for abundant, rapidly growing aerobic organisms with favorable growth characteristics in nutrient culture media.50,53
It is possible that bacterial infection within tissue may be present even if antral secretions are sterile. However, previous studies demonstrate that bacterial invasion of the mucosa is a rare phenomenon in sinusitis.54,55 The common pathogens of ARS (pneumococci, Haernophilus influenzae and various anaerobes from the mouth and throat flora) lack proteolytic enzymes.54 In a rabbit model of ARS, Marks characterized ARS by luminal exudates of neutrophils and eosinophils, mucosal infiltration with lymphocytes and plasma cells, and epithelial degeneration, and lymphoid follicles that appear to hypertrophy and liberate leukocytes into the sinus lumen. In severe infections, submucosal vacuole formation with overlying granulation tissue was observed.56 Histologic sections of CRS sinus mucosa demonstrate abundant bacteria on the surface of mucosa and in empty goblet cells, but not typically in deeper sections of mucosa.54 Using transmission electron microscopy, Ebenfelt found that bacterial invasion and phagosomes in sinus mucosa are rare in CRS, and concluded that the infectious process in CRS is situated mainly in sinus secretions where phagocytized bacteria and functionally active neutrophils have been found.55
The major limitation when considering the validity of the conclusion of this systematic review is the potential for selection bias. The included studies use a wide variety of diagnostic and inclusion criteria, with most studies requiring positive CT findings, which is not representative of real-world general outpatient medicine clinical scenarios. However, one would anticipate that selection bias would skew results to favor higher bacteria recovery in studies requiring CT and/or endoscopic evidence. The reverse was found in our study, where studies that did not require objective confirmation had higher bacterial prevalence, and so we conclude that selection bias did not significantly skew the results. A single patient was considered the unit of analysis in this study for two reasons: first, to most realistically represent a clinical scenario and improve external validity for a clinical population; and second, to reduce sampling bias (in which patients with more than one sinus cultured, or had two methods of culture at one time, would be underrepresented in the overall study population). This approach may introduce selection bias, by eliminating studies that use both EMMC and sinus puncture. However, we felt our method would be the most systematic approach and thereby, lead to the least biased results.
To summarize, the prevalence of bacterial infection in patients with clinically diagnosed ARS, without objective evidence, remains poorly defined. In the clinical setting, it is difficult to definitively distinguish viral from bacterial infection without invasive sinus-puncture studies, even when radiographic and/or endoscopic evidence is available. Although definitive clinical criteria that differentiate between ABRS and viral URTI are lacking, evaluation of the duration and severity of symptoms has historically provided the rational basis for diagnosing ABRS in primary care settings.57 However, the results of this systematic review demonstrate that even when strict clinical and even radiologic criteria are applied, only 53% of cultures are positive for pathogenic bacteria.
European guidelines published this year diagnose ABRS by the presence of 3 or more of the following: discolored discharge, severe local pain, fever, elevated CRP or ESR, and worsening of symptoms.7 Current U.S. guidelines9–15 provide fewer objective diagnostic criteria for ABRS. The lack of objective, reliable criteria to diagnose ABRS may partly explain why clinicians readily prescribe antibiotics for patients with ARS symptoms. Innovations in microbiology have greatly improved the sensitivity and accuracy of microbial detection and identification in CRS.50,51,58 There is opportunity for application of these tools to ARS.
Conclusion
This study sought to assess the prevalence of bacterial infection in adults with clinically diagnosed ARS who undergo culture from antral puncture or endoscopically directed middle meatus culture, and to compare the prevalence of bacterial infection in adults with clinically diagnosed ARS by antral puncture vs. EMMC. This systematic review provides evidence that the prevalence of bacterial infection in patients with clinically diagnosed ARS remains poorly defined, but is likely greater than the 0.5–2% figure previously widely quoted. We hypothesized bacterial recovery would be similar between antral puncture and EMMC; however, EMMC had a lower rate of positive bacterial culture compared with antral puncture in this systematic review. Despite the strength of this evidence, future studies are needed to better define the prevalence of bacterial infection in ARS. There is a need for additional high quality studies that compare antral puncture with EMMC, and for studies that investigate potential biomarkers and other culture-independent techniques to help distinguish viral and bacterial infections. Future studies should use standardized methods for the basis of diagnosis of ARS and severity of ARS symptoms. This will allow assessment of whether there is a particular subgroup of signs or symptoms that more reliably predict ABRS.
Supplementary Material
Acknowledgments
Research for this paper was done in large part while Dr. Stephanie Shintani Smith was a postdoctoral fellow at the Institute for Healthcare Studies, supported by an institutional award from the Agency for Healthcare Research and Quality, T-32 HS 000078 (PI: Jane L. Holl, MD MPH).
Funding: Supported by an institutional award from the Agency for Healthcare Research and Quality, T-32 HS 000078 (S.S.S., PI: Jane L. Holl, MD MPH), the National Institutes of Health/National Institute of Deafness and Communications Disorders 1K23DC012067 and the American College of Surgeons/Triological Society (B.K.T.), and the Department of Otolaryngology, Northwestern University Feinberg School of Medicine (S.S.S. and B.K.T.).
Footnotes
Presentations: Presented at the Triological Society meeting at COSM, April 10–14, 2013, in Orlando, FL, USA.
Disclosures: The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1.Gonzales R, Steiner JF, Lum A, Barrett PH., Jr Decreasing antibiotic use in ambulatory practice: impact of a multidimensional intervention on the treatment of uncomplicated acute bronchitis in adults. JAMA. 1999;281:1512–1519. doi: 10.1001/jama.281.16.1512. [DOI] [PubMed] [Google Scholar]
- 2.Sharp HJ, Denman D, Puumala S, Leopold DA. Treatment of acute and chronic rhinosinusitis in the United States, 1999–2002. Arch Otolaryngol Head Neck Surg. 2007;133:260–265. doi: 10.1001/archotol.133.3.260. [DOI] [PubMed] [Google Scholar]
- 3.Fairlie T, Shapiro DJ, Hersh AL, Hicks LA. National trends in visit rates and antibiotic prescribing for adults with acute sinusitis. Arch Intern Med. 2012;172:1513–1514. doi: 10.1001/archinternmed.2012.4089. [DOI] [PubMed] [Google Scholar]
- 4.Smith SS, Kern RC, Chandra RK, Tan BK, Evans CT. Variations in antibiotic prescribing of acute rhinosinusitis in United States ambulatory settings. Otolaryngol Head Neck Surg. 2013;148:852–859. doi: 10.1177/0194599813479768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garbutt JM, Banister C, Spitznagel E, Piccirillo JF. Amoxicillin for acute rhinosinusitis: a randomized controlled trial. JAMA. 2012;307:685–692. doi: 10.1001/jama.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahovuo-Saloranta A, Borisenko OV, Kovanen N, et al. Antibiotics for acute maxillary sinusitis. Cochrane Database Syst Rev. 2008:CD000243. doi: 10.1002/14651858.CD000243.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Fokkens WJ, Lund VJ, Mullol J, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinology. 2012;50:1–307. doi: 10.4193/Rhino12.000. [DOI] [PubMed] [Google Scholar]
- 8.Smith SR, Montgomery LG, Williams JW., Jr Treatment of mild to moderate sinusitis. Arch Intern Med. 2012;172:510–513. doi: 10.1001/archinternmed.2012.253. [DOI] [PubMed] [Google Scholar]
- 9.Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137:S1–31. doi: 10.1016/j.otohns.2007.06.726. [DOI] [PubMed] [Google Scholar]
- 10.Fokkens W, Lund V, Mullol J. European position paper on rhinosinusitis and nasal polyps 2007. Rhinol Suppl. 2007:1–136. [PubMed] [Google Scholar]
- 11.Slavin RG, Spector SL, Bernstein IL, et al. The diagnosis and management of sinusitis: a practice parameter update. J Allergy Clin Immunol. 2005;116:S13–47. doi: 10.1016/j.jaci.2005.09.048. [DOI] [PubMed] [Google Scholar]
- 12.American Academy of Allergy A, & Immunology. Choosing Wisely: Five Things Physicians and Patients Should Question. 2012. [Google Scholar]
- 13.Meltzer EO, Hamilos DL, Hadley JA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114:155–212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow AW, Benninger MS, Brook I, et al. IDSA Clinical Practice Guideline for Acute Bacterial Rhinosinusitis in Children and Adults. Clin Infect Dis. 2012;54:e72–e112. doi: 10.1093/cid/cir1043. [DOI] [PubMed] [Google Scholar]
- 15.Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinol Suppl. 2012;50:1–12. doi: 10.4193/Rhino12.000. [DOI] [PubMed] [Google Scholar]
- 16.Meltzer EO, Hamilos DL. Rhinosinusitis diagnosis and management for the clinician: a synopsis of recent consensus guidelines. Mayo Clin Proc. 2011;86:427–443. doi: 10.4065/mcp.2010.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anon JB, Jacobs MR, Poole MD, et al. Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Otolaryngol Head Neck Surg. 2004;130:1–45. doi: 10.1016/j.otohns.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenfeld RM. Clinical practice guideline on adult sinusitis. Otolaryngol Head Neck Surg. 2007;137:365–377. doi: 10.1016/j.otohns.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 19.Anzai Y, Jarvik JG, Sullivan SD, Hollingworth W. The cost-effectiveness of the management of acute sinusitis. Am J Rhinol. 2007;21:444–451. doi: 10.2500/ajr.2007.21.3009. [DOI] [PubMed] [Google Scholar]
- 20.Balk EM, Zucker DR, Engels EA, Wong JB, Williams JW, Jr, Lau J. Strategies for diagnosing and treating suspected acute bacterial sinusitis: a cost-effectiveness analysis. J Gen Intern Med. 2001;16:701–711. doi: 10.1111/j.1525-1497.2001.00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amin NM, Breadon G. An open-label, noncomparative study to evaluate the efficacy, safety, and tolerability of azithromycin in the treatment of patients with acute sinusitis. Clin Ther. 1995;17:701–707. doi: 10.1016/0149-2918(95)80046-8. [DOI] [PubMed] [Google Scholar]
- 22.Anon JB, Ferguson B, Twynholm M, Wynne B, Berkowitz E, Poole MD. Pharmacokinetically enhanced amoxicillin/clavulanate (2,000/125 mg) in acute bacterial rhinosinusitis caused by Streptococcus pneumoniae, including penicillin-resistant strains. Ear Nose Throat J. 2006;85:500, 502. 504 passim. [PubMed] [Google Scholar]
- 23.Arrieta JR, Galgano AS, Sakano E, et al. Moxifloxacin vs amoxicillin/clavulanate in the treatment of acute sinusitis. Am J Otolaryngol. 2007;28:78–82. doi: 10.1016/j.amjoto.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Benninger MS, Payne SC, Ferguson BJ, Hadley JA, Ahmad N. Endoscopically directed middle meatal cultures versus maxillary sinus taps in acute bacterial maxillary rhinosinusitis: a meta-analysis. Otolaryngol Head Neck Surg. 2006;134:3–9. doi: 10.1016/j.otohns.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Eloy P, Poirrier AL, De Dorlodot C, Van Zele T, Watelet JB, Bertrand B. Actual concepts in rhinosinusitis: a review of clinical presentations, inflammatory pathways, cytokine profiles, remodeling, and management. Curr Allergy Asthma Rep. 2011;11:146–162. doi: 10.1007/s11882-011-0180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berger G, Berger RL. The contribution of flexible endoscopy for diagnosis of acute bacterial rhinosinusitis. Eur Arch Otorhinolaryngol. 2011;268:235–240. doi: 10.1007/s00405-010-1329-5. [DOI] [PubMed] [Google Scholar]
- 27.Williams JW, Jr, Simel DL, Roberts L, Samsa GP. Clinical evaluation for sinusitis. Making the diagnosis by history and physical examination. Ann Intern Med. 1992;117:705–710. doi: 10.7326/0003-4819-117-9-705. [DOI] [PubMed] [Google Scholar]
- 28.Lindbaek M, Hjortdahl P, Johnsen UL. Use of symptoms, signs, and blood tests to diagnose acute sinus infections in primary care: comparison with computed tomography. Fam Med. 1996;28:183–188. [PubMed] [Google Scholar]
- 29.Tantilipikorn P, Fritz M, Tanabodee J, Lanza DC, Kennedy DW. A comparison of endoscopic culture techniques for chronic rhinosinusitis. Am J Rhinol. 2002;16:255–260. [PubMed] [Google Scholar]
- 30.Jardim Vieira FM, Nunes da Silva R, Stefanini R, et al. Safety of sphenoid aspiration for diagnosis and treatment of intensive care unit rhinosinusitis. Am J Rhinol Allergy. 2010;24:389–391. doi: 10.2500/ajra.2010.24.3512. [DOI] [PubMed] [Google Scholar]
- 31.Berg O, Bergstedt H, Carenfelt C, Lind MG, Perols O. Discrimination of purulent from nonpurulent maxillary sinusitis. Clinical and radiographic diagnosis. Ann Otol Rhinol Laryngol. 1981;90:272–275. doi: 10.1177/000348948109000316. [DOI] [PubMed] [Google Scholar]
- 32.Benninger MS, Appelbaum PC, Denneny JC, Osguthorpe DJ, Stankiewicz JA. Maxillary sinus puncture and culture in the diagnosis of acute rhinosinusitis: the case for pursuing alternative culture methods. Otolaryngol Head Neck Surg. 2002;127:7–12. doi: 10.1067/mhn.2002.124847. [DOI] [PubMed] [Google Scholar]
- 33.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips B, Ball C, Sackett D, et al. Levels of evidence and grades of recommendation. Oxford Centre for Evidence-Based Medicine; 2009. [Google Scholar]
- 35.Oxford Centre for Evidence Based Medicine Levels of Evidence Working Group. [Accessed August 20 2013];Levels of Evidence 1. 2013 Jul 1; Available at: http://www.cebm.net/index.aspx?o=1025.
- 36.Piccirillo JF, Mager DE, Frisse ME, Brophy RH, Goggin A. Impact of first-line vs second-line antibiotics for the treatment of acute uncomplicated sinusitis. JAMA. 2001;286:1849–1856. doi: 10.1001/jama.286.15.1849. [DOI] [PubMed] [Google Scholar]
- 37.Gwaltney JM, Jr, Wiesinger BA, Patrie JT. Acute community-acquired bacterial sinusitis: the value of antimicrobial treatment and the natural history. Clin Infect Dis. 2004;38:227–233. doi: 10.1086/380641. [DOI] [PubMed] [Google Scholar]
- 38.Gwaltney JM., Jr Acute community-acquired sinusitis. Clin Infect Dis. 1996;23:1209–1223. doi: 10.1093/clinids/23.6.1209. quiz 1224-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berg O, Carenfelt C, Rystedt G, Anggard A. Occurrence of asymptomatic sinusitis in common cold and other acute ENT-infections. Rhinology. 1986;24:223–225. [PubMed] [Google Scholar]
- 40.Dingle JH. Illness in the home; a study of 25,000 illnesses in a group of Cleveland families. Cleveland: Press of Western Reserve Univ; 1964. [Google Scholar]
- 41.Van Cauwenberge P, Verschraegen G, Van Renterghem L. Bacteriological findings in sinusitis (1963–1975) Scand J Infect Dis Suppl. 1976:72–77. [PubMed] [Google Scholar]
- 42.Hamory BH, Sande MA, Sydnor A, Jr, Seale DL, Gwaltney JM., Jr Etiology and antimicrobial therapy of acute maxillary sinusitis. J Infect Dis. 1979;139:197–202. doi: 10.1093/infdis/139.2.197. [DOI] [PubMed] [Google Scholar]
- 43.Carenfelt C, Melen I, Odkvist L, et al. Treatment of sinus empyema in adults. A coordinated Nordic multicenter trial of cefixime vs. cefaclor. Acta Otolaryngol. 1990;110:128–135. doi: 10.3109/00016489009122527. [DOI] [PubMed] [Google Scholar]
- 44.Gauger U, Inoka P, Germano G, Kissling M. Cefetamet in the treatment of acute sinusitis in adult patients. J Int Med Res. 1990;18:228–234. doi: 10.1177/030006059001800308. [DOI] [PubMed] [Google Scholar]
- 45.Vogan JC, Bolger WE, Keyes AS. Endoscopically guided sinonasal cultures: a direct comparison with maxillary sinus aspirate cultures. Otolaryngol Head Neck Surg. 2000;122:370–373. doi: 10.1016/S0194-5998(00)70051-9. [DOI] [PubMed] [Google Scholar]
- 46.Gold SM, Tami TA. Role of middle meatus aspiration culture in the diagnosis of chronic sinusitis. Laryngoscope. 1997;107:1586–1589. doi: 10.1097/00005537-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Sobin J, Engquist S, Nord CE. Bacteriology of the maxillary sinus in healthy volunteers. Scand J Infect Dis. 1992;24:633–635. doi: 10.3109/00365549209054650. [DOI] [PubMed] [Google Scholar]
- 48.Abou-Hamad W, Matar N, Elias M, et al. Bacterial flora in normal adult maxillary sinuses. Am J Rhinol Allergy. 2009;23:261–263. doi: 10.2500/ajra.2009.23.3317. [DOI] [PubMed] [Google Scholar]
- 49.Brook I. Aerobic and anaerobic bacterial flora of normal maxillary sinuses. Laryngoscope. 1981;91:372–376. [PubMed] [Google Scholar]
- 50.Boase S, Foreman A, Cleland E, et al. The microbiome of chronic rhinosinusitis: culture, molecular diagnostics and biofilm detection. BMC infectious diseases. 2013;13:210. doi: 10.1186/1471-2334-13-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feazel LM, Robertson CE, Ramakrishnan VR, Frank DN. Microbiome complexity and Staphylococcus aureus in chronic rhinosinusitis. Laryngoscope. 2012;122:467–472. doi: 10.1002/lary.22398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abreu NA, Nagalingam NA, Song Y, et al. Sinus microbiome diversity depletion and Corynebacterium tuberculostearicum enrichment mediates rhinosinusitis. Science translational medicine. 2012;4:151ra124. doi: 10.1126/scitranslmed.3003783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dunbar J, White S, Forney L. Genetic Diversity through the Looking Glass: Effect of Enrichment Bias. Applied and environmental microbiology. 1997;63:1326–1331. doi: 10.1128/aem.63.4.1326-1331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Engquist S, Lundberg C. Bacteria and inflammatory cells in maxillary sinusitis. Archives of oto-rhino-laryngology. 1984;239:173–180. doi: 10.1007/BF00463558. [DOI] [PubMed] [Google Scholar]
- 55.Ebenfelt A. Bacterial location in chronic sinusitis. Am J Rhinol. 2005;19:458–461. [PubMed] [Google Scholar]
- 56.Marks SC. Acute sinusitis in the rabbit model: histologic analysis. Laryngoscope. 1998;108:320–325. doi: 10.1097/00005537-199803000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Garau J, Dagan R. Accurate diagnosis and appropriate treatment of acute bacterial rhinosinusitis: minimizing bacterial resistance. Clin Ther. 2003;25:1936–1951. doi: 10.1016/s0149-2918(03)80197-2. [DOI] [PubMed] [Google Scholar]
- 58.Feazel LM, Frank DN, Ramakrishnan VR. Update on bacterial detection methods in chronic rhinosinusitis: implications for clinicians and research scientists. Int Forum Allergy Rhinol. 2011;1:451–459. doi: 10.1002/alr.20071. [DOI] [PubMed] [Google Scholar]
- 59.Anon JB, Berkowitz E, Breton J, Twynholm M. Efficacy/safety of amoxicillin/clavulanate in adults with bacterial rhinosinusitis. Am J Otolaryngol. 2006;27:248–254. doi: 10.1016/j.amjoto.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 60.Berg O, Carenfelt C, Kronvall G. Bacteriology of maxillary sinusitis in relation to character of inflammation and prior treatment. Scand J Infect Dis. 1988;20:511–516. doi: 10.3109/00365548809032499. [DOI] [PubMed] [Google Scholar]
- 61.Desrosiers M, Ferguson B, Klossek JM, Drugeon H, Mosges R. Clinical efficacy and time to symptom resolution of 5-day telithromycin versus 10-day amoxicillin-clavulanate in the treatment of acute bacterial sinusitis. Curr Med Res Opin. 2008;24:1691–1702. doi: 10.1185/03007990802133914. [DOI] [PubMed] [Google Scholar]
- 62.Hadley JA, Mosges R, Desrosiers M, Haverstock D, van Veenhuyzen D, Herman-Gnjidic Z. Moxifloxacin five-day therapy versus placebo in acute bacterial rhinosinusitis. Laryngoscope. 2010;120:1057–1062. doi: 10.1002/lary.20878. [DOI] [PubMed] [Google Scholar]
- 63.Huck W, Reed BD, Nielsen RW, et al. Cefaclor vs amoxicillin in the treatment of acute, recurrent, and chronic sinusitis. Arch Fam Med. 1993;2:497–503. doi: 10.1001/archfami.2.5.497. [DOI] [PubMed] [Google Scholar]
- 64.Jareoncharsri P, Bunnag C, Fooanant S, et al. An open label, randomized comparative study of levofloxacin and amoxicillin/clavulanic acid in the treatment of purulent sinusitis in adult Thai patients. Rhinology. 2004;42:23–29. [PubMed] [Google Scholar]
- 65.Johnson PA, Rodriguez HP, Wazen JJ, et al. Ciprofloxacin versus cefuroxime axetil in the treatment of acute bacterial sinusitis. Sinusitis Infection Study Group. J Otolaryngol. 1999;28:3–12. [PubMed] [Google Scholar]
- 66.Johnson P, Adelglass J, Rankin B, et al. Acute bacterial maxillary sinusitis: time to symptom resolution and return to normal activities with moxifloxacin. Int J Clin Pract. 2008;62:1366–1372. doi: 10.1111/j.1742-1241.2007.01518..x. [DOI] [PubMed] [Google Scholar]
- 67.Jones S, Yu VL, Johnson JT, Wagner RL, Kim HK. Pharmacokinetic and therapeutic trial of sultamicillin in acute sinusitis. Antimicrob Agents Chemother. 1985;28:832–833. doi: 10.1128/aac.28.6.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Joniau S, Vlaminck S, Van Landuyt H, Kuhweide R, Dick C. Microbiology of sinus puncture versus middle meatal aspiration in acute bacterial maxillary sinusitis. Am J Rhinol. 2005;19:135–140. [PubMed] [Google Scholar]
- 69.Klapan I, Culig J, Oreskovic K, Matrapazovski M, Radosevic S. Azithromycin versus amoxicillin/clavulanate in the treatment of acute sinusitis. Am J Otolaryngol. 1999;20:7–11. doi: 10.1016/s0196-0709(99)90044-3. [DOI] [PubMed] [Google Scholar]
- 70.Klossek JM, Siegert R, Nikolaidis P, Arvis P, Leberre MA. Comparison of the efficacy and safety of moxifloxacin and trovafloxacin for the treatment of acute, bacterial maxillary sinusitis in adults. J Laryngol Otol. 2003;117:43–51. doi: 10.1258/002221503321046630. [DOI] [PubMed] [Google Scholar]
- 71.Lopez Sisniega J, Profant M, Kostrica R, Waskin H. Oral garenoxacin in the treatment of acute bacterial maxillary sinusitis: a Phase II, multicenter, noncomparative, open-label study in adult patients undergoing sinus aspiration. Clin Ther. 2007;29:1632–1644. doi: 10.1016/j.clinthera.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 72.Murray JJ, Emparanza P, Lesinskas E, Tawadrous M, Breen JD. Efficacy and safety of a novel, single-dose azithromycin microsphere formulation versus 10 days of levofloxacin for the treatment of acute bacterial sinusitis in adults. Otolaryngol Head Neck Surg. 2005;133:194–200. doi: 10.1016/j.otohns.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 73.Penttila M, Savolainen S, Kiukaanniemi H, Forsblom B, Jousimies-Somer H. Bacterial findings in acute maxillary sinusitis--European study. Acta Otolaryngol Suppl. 1997;529:165–168. [PubMed] [Google Scholar]
- 74.Poole M, Anon J, Paglia M, Xiang J, Khashab M, Kahn J. A trial of high-dose, short-course levofloxacin for the treatment of acute bacterial sinusitis. Otolaryngol Head Neck Surg. 2006;134:10–17. doi: 10.1016/j.otohns.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 75.Riffer E, Spiller J, Palmer R, Shortridge V, Busman TA, Valdes J. Once daily clarithromycin extended-release vs twice-daily amoxicillin/clavulanate in patients with acute bacterial sinusitis: a randomized, investigator-blinded study. Curr Med Res Opin. 2005;21:61–70. doi: 10.1185/030079904x18009. [DOI] [PubMed] [Google Scholar]
- 76.Savolainen S, Jousimies-Somer H, Kleemola M, Ylikoski J. Serological evidence of viral or Mycoplasma pneumoniae infection in acute maxillary sinusitis. Eur J Clin Microbiol Infect Dis. 1989;8:131–135. doi: 10.1007/BF01963896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shinogi J, Harada T, Nonoyama T, Kishioka C, Sakakura Y, Majima Y. Quantitative analysis of mucin and lectin in maxillary sinus fluids in patients with acute and chronic sinusitis. Laryngoscope. 2001;111:240–245. doi: 10.1097/00005537-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 78.Siegert R, Berg O, Gehanno P, et al. Comparison of the efficacy and safety of faropenem daloxate and cefuroxime axetil for the treatment of acute bacterial maxillary sinusitis in adults. Eur Arch Otorhinolaryngol. 2003;260:186–194. doi: 10.1007/s00405-002-0532-4. [DOI] [PubMed] [Google Scholar]
- 79.Siegert R, Gehanno P, Nikolaidis P, et al. A comparison of the safety and efficacy of moxifloxacin (BAY 12-8039) and cefuroxime axetil in the treatment of acute bacterial sinusitis in adults. The Sinusitis Study Group. Respir Med. 2000;94:337–344. doi: 10.1053/rmed.1999.0769. [DOI] [PubMed] [Google Scholar]
- 80.Sydnor TA, Kopp EJ, Anthony KE, LoCoco JM, Kim SS, Fowler CL. Open-label assessment of levofloxacin for the treatment of acute bacterial sinusitis in adults. Ann Allergy Asthma Immunol. 1998;80:357–362. doi: 10.1016/S1081-1206(10)62983-3. [DOI] [PubMed] [Google Scholar]
- 81.Talbot GH, Kennedy DW, Scheld WM, Granito K. Rigid nasal endoscopy versus sinus puncture and aspiration for microbiologic documentation of acute bacterial maxillary sinusitis. Clin Infect Dis. 2001;33:1668–1675. doi: 10.1086/323813. [DOI] [PubMed] [Google Scholar]
- 82.van den Wijngaart W, Verbrugh H, Theopold HM, et al. A noncomparative study of cefprozil at two dose levels in the treatment of acute uncomplicated bacterial sinusitis. Clin Ther. 1992;14:306–313. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.