Abstract
Background
Approximately one-third of those with pediatric-onset MS experience cognitive impairment. Less is known concerning their change in cognitive functioning over time.
Objective
Changes in cognitive function over time were measured in the largest pediatric cohort to date through the U.S. Network of Pediatric MS Centers.
Methods
67 individuals with pediatric MS (n=62) or clinically isolated syndrome (CIS, n=5), ranging from 8 to 17 years of age (mean age ± SD = 14.37 ± 2.02) completed initial and followup neuropsychological testing after an average of 1.64 ± 0.63 years. The nine tests administered measure general intellect, attention and working memory, verbal memory, visuomotor integration, language, and executive functioning.
Results
Rate of impairment (having one-third or more scores in the impaired range) was 37% at baseline and 33% at follow-up. Tests commonly impaired were measures of visuomotor integration, speeded processing, and attention. Most tested did not decline over two years. There was no clear pattern of change on specific measures.
Conclusion
Findings suggest that over short timeframes, stable or even improved performances on measures of cognitive ability can occur. Rather than leading to decline, pediatric MS may instead prevent expected age-related gains.
Keywords: pediatric, multiple sclerosis, cognition, longitudinal study, cognitive impairment
INTRODUCTION
Multiple sclerosis (MS) is an immune-mediated disorder of the central nervous system marked by demyelinating events and accumulated neurological disability. Pediatric MS refers to the onset of the disorder before the age of 18 years and represents approximately three to five percent of all MS cases.1,2 Across studies, cognitive impairment has been estimated to occur in approximately one-third of pediatric MS cases compared to an estimated one-half or more in adult patients. Rates of cognitive impairment in pediatric MS patients are generally consistent despite variations in measures and definition of impairment, and comparison groups (i.e., to published normative values or matched groups of healthy control participants; 35%, n=373; 31%, n=634,5; 35%, n= 1876, 29%, n=287). Areas of dysfunction most often involved include visuomotor integration, attention and learning, and in some studies, language.4,8
Fewer studies have examined cognitive function across time and initial reports indicated that pediatric patients are at great risk for decline. For example, compared to published normative data, studies have found that some patients decline from previously normal functioning or, with prior dysfunction, experience further decline over time.9 In a sample of 56 pediatric MS participants followed at two years and compared to two sets of matched healthy control participants at each time point, Amato and colleagues8 classified 75% of the sample with cognitive deterioration based on a Cognitive Change Index, determined as a drop in two or more points in a total of scores weighted according to standard deviations from the normative mean. However, in a sample of 28 pediatric MS participants compared to a matched healthy control group both followed for one year, Till and colleagues7 found a lower rate of decline (25% in the MS vs. 4% in the control sample) based on reliable change indices or RCIs. However, MS participants showed an absence of expected age-related gains.
The U.S. Pediatric MS Network is a group of nine centers across the country, funded by the National MS Society and dedicated to clinical care and research for patients with pediatric MS. This is the first Network report on longitudinal changes in cognitive functioning from a cohort who have received serial neuropsychological evaluations.
METHOD
Participants underwent baseline neuropsychological testing between the ages of 8 and 18 years and met criteria for a diagnosis of pediatric MS or clinically isolated syndrome (CIS).2 Participants were excluded if they had a history of head injury, were diagnosed with another neurologic or a primary psychiatric disorder, or if they had another significant medical illness that could influence cognitive function. Written consent was obtained from a parent or guardian for all participants, all of whom provided their assent for the study. Participants were not provided compensation for their time. The study was approved by each participating institution’s review board.
Using a common neuropsychological protocol, all patients were evaluated consecutively at their baseline visit at each site then routinely followed after their baseline visits with the goal for repeat cognitive evaluation 18 months later. A cognitive evaluation was only completed if the participant was free of relapse and had no steroid dose for the prior 30 days. Parents were asked to complete a demographics questionnaire in which they provided the race and ethnicity of their child. Race and ethnicity were assessed to evaluate the racial and ethnic distribution in the pediatric population in MS.
A licensed clinical neuropsychologist at each site either administered or supervised a trained psychometrician to administer the battery of cognitive tests. The test administration took approximately two hours to complete (often as part of a longer neuropsychological battery) and breaks were provided as necessary. Where possible, test forms were alternated at each administration to minimize practice effects.
A subset of tests from the common battery, narrowed to the tests that were most consistently administered across the sites, was included in the current analyses (Table 1). These tests yielded 12 performance scores that were then compared between baseline and follow-up evaluations, as follows: Wechsler Abbreviated Scale of Intelligence – First and Second Editions (WASI, WASI-II)10,11 2-subtest Full Scale IQ score; California Verbal Learning Test – Child Version (CVLT-C, up to 16 years of age at time of testing)12 or Second Edition (CVLT-II, if 17 or more years of age at time of testing)13 scores for total trial learning and long-term delayed free recall; Expressive One-Word Picture Vocabulary Test (EOWPVT)14 total score; Pseudoword Decoding subtest total score of the Wechsler Individual Achievement Test – Second Edition (WIAT-II);15 Beery-Buktenica Developmental Test of Visual-Motor Integration (Beery-VMI)16 total score; Digit Span subtest from the Wechsler Intelligence Scale for Children – Fourth Edition (WISC-IV, up to 16 years of age at time of testing)17 or Wechsler Adult Intelligence Scale – Fourth Edition (WAIS-IV, if 17 years or more at time of testing),18 digit forward and digit backward total scores; the Trail Making Test (TMT) from the Delis-Kaplan Executive Functioning System (DKEFS)19 total scores for conditions of visual scanning, number sequencing, letter sequencing, number/letter switching, and motor speed.
Table 1.
Neuropsychological measures
| Domain of Function | Test | Dependent Measure(s) |
|---|---|---|
| General Intellect | Wechsler Abbreviated Scale of Intelligence (WASI)10,11 | Full scale IQ (2-subtest) |
| Attention & Working Memory | Age 8–16: Wechsler Intelligence Scale for Children – Fourth Edition (WISC-IV)17 Age 17 and over: Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV)18 |
Digit span forward Digit span backward |
| Verbal Memory | Age 8–16: California Verbal Learning Test – Child Version (CVLT-C)12 Age 17 and over: California Verbal Learning Test – Second Edition (CVLT-II)13 |
Total trials learning Total delayed free recall |
| Visuomotor Integration | Beery-Buktenica Developmental Visual-Motor Integration Test (Beery-VMI)16 | Total score |
| Language | Expressive One-Word Picture Vocabulary Test (EOWPVT)14 Wechsler Individual Achievement Test – Fourth Edition (WIAT), Pseudoword Decoding15 |
Total score Total score |
| Executive Functioning | Delis-Kaplan Executive Functioning System Trail Making Test (DKEFS TMT)19 | Visual scanning Number sequencing Letter sequencing Letter-number switching Motor speed |
RESULTS
There were a total of 67 participants, 62 diagnosed with pediatric MS and five with CIS. The majority of the cohort was enrolled at the Lourie Center for Pediatric MS at Stony Brook Medicine (n=40), followed by Partners Pediatric MS Center at the Massachusetts General Hospital for Children (n=16), the Center for Pediatric-Onset Demyelinating Disease at the Children’s Hospital of Alabama (n=4), University of California at San Francisco Regional Pediatric MS Center (n=4), and the Pediatric MS Center of the Jacobs Neurological Institute at SUNY Buffalo (n=3). Recruitment began in 2005; the final follow-up visit was completed in April 2013.
Demographics
Baseline characteristics are provided in Table 2. Race was not available for two participants. Seventy-two percent (n=48) of the cohort were white and thirty-one percent (n=21) of were of Hispanic ethnicity. Parent education (averaged parent total years of education) was an average of 13.85 ± 2.18 years, and ranged from 7.5 to 18 years. Time between baseline and follow-up testing was 1.64 ± 0.63 years with a range of 0.75 to 3.38 years.
Table 2.
Demographic and clinical features
| Characteristic | Participants (n=67) n (%) or mean ± SD |
|---|---|
| Diagnosis | MS: 62 (93) CIS: 5 (7) |
| Symptom duration at visit 1 (n=66), years | 1.56 ± 1.93 |
| Age of symptom onset (n=66), years | 12.89± 3.13 |
| Age at first testing, years | 14.4± 2.02 |
| Gender | Male: 25 (37) Female: 42 (63) |
| Race | White: 48 (72) African American: 13 (19) Asian: 2(3) Native American 1 (1) Mixed: 1 (1) Unknown: 2 (3) |
| Ethnicity | Non-Hispanic: 46 (69) Hispanic: 21 (31) |
| Parental education (n=52), years | 13.85±2.18 |
| Time between test dates, years | 1.64±0.63 |
| EDSS at baseline testing (n=54), median (range) | 1.25 (0 to 3.5) |
Abbreviations: MS- multiple sclerosis, CIS- clinically isolated syndrome, EDSS- expanded disability status scale.20
Clinical features
At the baseline neuropsychological visit, mean age at symptom onset was 12.94 ± 3.13 years with a range of 3.15 to 16.81 years. Mean symptom duration at first visit was 1.56 ± 1.93 years with a range of 0.09 to 9.27 years. Neurological disability as measured by the Expanded Disability Status Scale (EDSS)20 was a median score of 1.25 with a range of 0 to 3.5 at the baseline visit. At the return visit for follow-up neuropsychological testing, the median EDSS score was 1.00 with a range of 0 to 4.5.
Cognitive Functioning
Baseline and follow-up raw scores were initially transformed to z scores using published age-normative data, and averaged for each participant to provide an overall summary score to serve as an index of cognitive functioning. Across all analyses, cognitive impairment on a test was defined as a score falling one standard deviation or more below published normative means.
As a whole, the cohort functioned within the average range at both baseline and followup. At baseline, the mean summary score was z=−0.23 ± 0.74, with a range of −1.97 to 1.88. At follow-up, the mean summary score was z=−0.21 ± 0.59, with a range of −1.52 to .0.69. The group mean percentage of impaired test scores was 25.2% (median 23.1%, range 0 to 83%) at baseline and 24.9% (median 16.7%, range 0 to 69%) at follow-up. The overall percent of patients with cognitive impairment, defined as having one-third or more test scores in the impaired range, was 37.3% at baseline and 32.3% at follow-up.
While the mean of summary z-scores was within the average range, consistent with prior findings, the lowest performances for the group were for visuomotor integration (Beery-VMI), verbal recall (CVLT delayed recall), and attention (Digit Span). Table 3 shows the mean z-scores for each measure at baseline and follow-up.
Table 3.
Mean z-scores
| Baseline visit | Follow-up visit | Baseline vs. Follow-up |
|||
|---|---|---|---|---|---|
| Test | n | mean±SD | n | mean±SD | p |
| WASI Full scale IQ | 49 | 0.24±0.88 | 51 | 0.15±0.82 | 0.66 |
| Digit Span forward | 60 | −0.34±1.18 | 60 | −0.20±1.12 | 0.27 |
| Digit Span backward | 58 | −0.36±1.18 | 58 | −0.20±1.03 | 0.18 |
| CVLT Total Trial Learning | 67 | −0.01±0.98 | 67 | 0.09±1.12 | 0.39 |
| CVLT Delay Free Recall | 67 | −0.82±1.05 | 67 | 0.05±1.12 | 0.23 |
| Beery-VMI | 52 | −0.81±0.90 | 52 | −0.76±0.87 | 0.68 |
| EOWPVT | 61 | 0.20±1.05 | 61 | 0.12±0.99 | 0.25 |
| Pseudoword Decoding | 50 | −0.08±0.87 | 50 | −0.15±0.93 | 0.38 |
| TMT Visual scanning | 63 | −0.30±1.06 | 63 | −0.23±0.99 | 0.64 |
| TMT Number sequence | 64 | −0.61±1.31 | 64 | −0.61±1.26 | 0.97 |
| TMT Letter sequence | 63 | −0.16±3.50 | 63 | −0.38±1.12 | 0.63 |
| TMT Letter-number sequence | 64 | −0.16±3.47 | 64 | −0.45±0.97 | 0.52 |
| TMT Motor Speed | 63 | 0.13±0.84 | 63 | 0.34±0.80 | 0.03 |
| Composite Score | 67 | 0.13±0.84 | 67 | 0.13±0.84 | 0.72 |
Paired samples t-test comparisons, all p values NS at the corrected p<0.001 level
Demographic-adjusted impairment rates
Regression-based z-scores were generated for 5 of the 12 scores for a subset of the cohort with complete scores and demographic data (n=40). These regression formulas were based on normative data from the SUNY Buffalo site provided by locally-recruited healthy control participants (n=102), with normative data available for 6 of the 12 scores in this analysis: WASI Full Scale IQ, CVLT-C/II total trial learning and long delay free recall, EOWPVT, Beery-VMI, and Pseudoword Decoding. Following the method described by Smerbeck and colleagues,21,22 regression-based z-scores were calculated to adjust for demographic factors of age, gender, race, and years of parent education.
Measured by adjusted z-scores, the mean summary scores were slightly higher while remaining within the average range. At baseline, the mean ± SD z= −0.16 ± 0.83, with a range of 2.69 to 1.67. At follow-up, the mean ± SD z= −0.07 ± 0 .80, with a range of −2.20 to 1.39. Using these adjusted norms, the mean impaired test performance rate was generally consistent with that found with published normative data, 24.6% (vs. 25.2%) at baseline and 21.0% (vs. 24.9%) at follow-up. However, there was an overall higher percent of impairment, defined has having one-third or more test scores in the impaired range, of 40.0% (vs. 37.3%) at baseline and 37.5% (vs. 32.3%) at follow-up. These findings are consistent with the previous demonstration of improved sensitivity with the regression-based normative data for detecting impairment on certain tests.21,22 Table 4 shows the adjusted mean z scores at baseline and follow-up.
Table 4.
Mean adjusted z-scores
| Baseline | Follow-up | Baseline vs. Follow-up |
|||
|---|---|---|---|---|---|
| Test | n | mean±SD | n | mean±SD | p |
| WASI Full scale IQ | 28 | 0.23±1.21 | 28 | 0.14±1.10 | 0.60 |
| CVLT Total Trial Learning | 39 | −0.16±0.88 | 39 | 0.25±1.05 | 0.04 |
| CVLT Delay Free Recall | 37 | −0.25±1.14 | 37 | 0.17±1.20 | 0.02 |
| Beery-VMI | 37 | −0.67±1.24 | 37 | −0.59±1.18 | 0.69 |
| EOWPVT | 38 | 0.11±1.81 | 38 | 0.22±1.74 | 0.38 |
| Pseudoword Decoding | 39 | −0.28±1.37 | 39 | −0.59±1.32 | 0.05 |
| Composite Score | 40 | −0.16±0.83 | 40 | −0.07±0.80 | 0.18 |
Regression-based normative z-scores adjusting for demographic variable; Paired samples t-test comparisons, all p values NS at the corrected p<0.001 level all p’s nonsignificant at the corrected p<0.01 level
Change in Cognitive Functioning Over Time
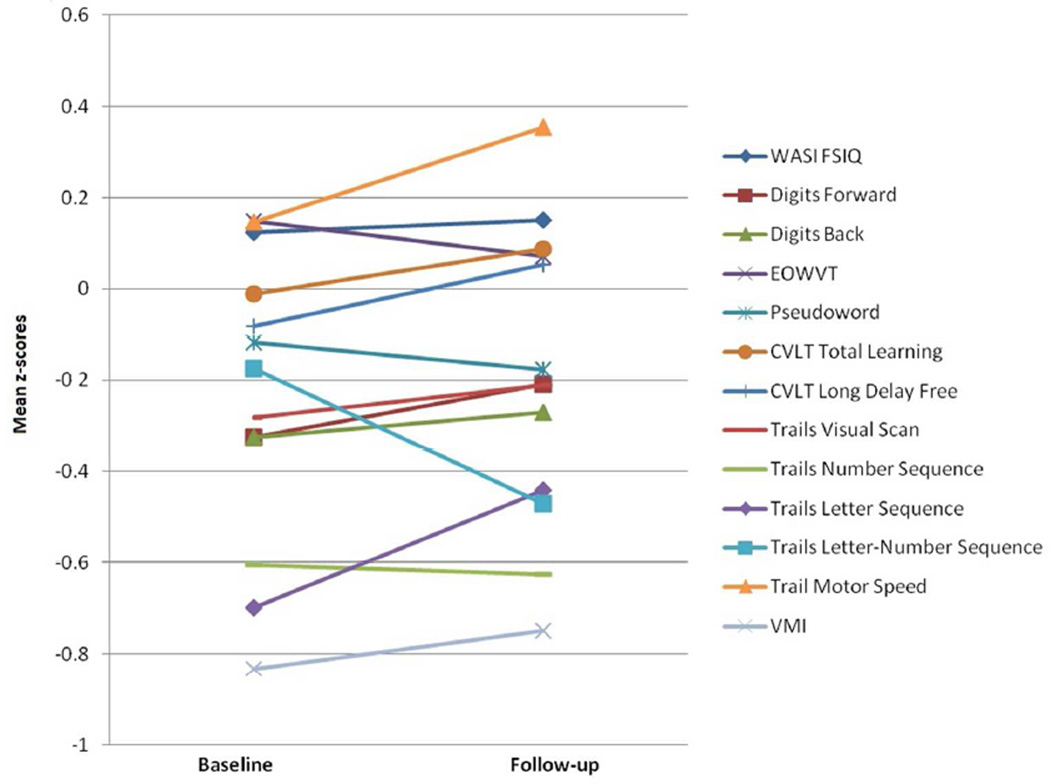
The mean change in summary score was z= 0.02 ± 0.45, with a range of −0.22 to 1.05, indicating variability in both directions with an average of no significant change. As shown in Figure 1, paired sample t-tests indicated no significant changes in mean z-scores on any individual test (Bonferroni corrected p < .001). None of the changes in the adjusted z-scores were significant (Bonferroni corrected p<.01), although scores for the CVLT-C/II (total trial learning and delay) and Pseudoword Decoding trended towards significant improvement.
Figure 1.
Performances on each test score at two time points (all p values > 0.05)
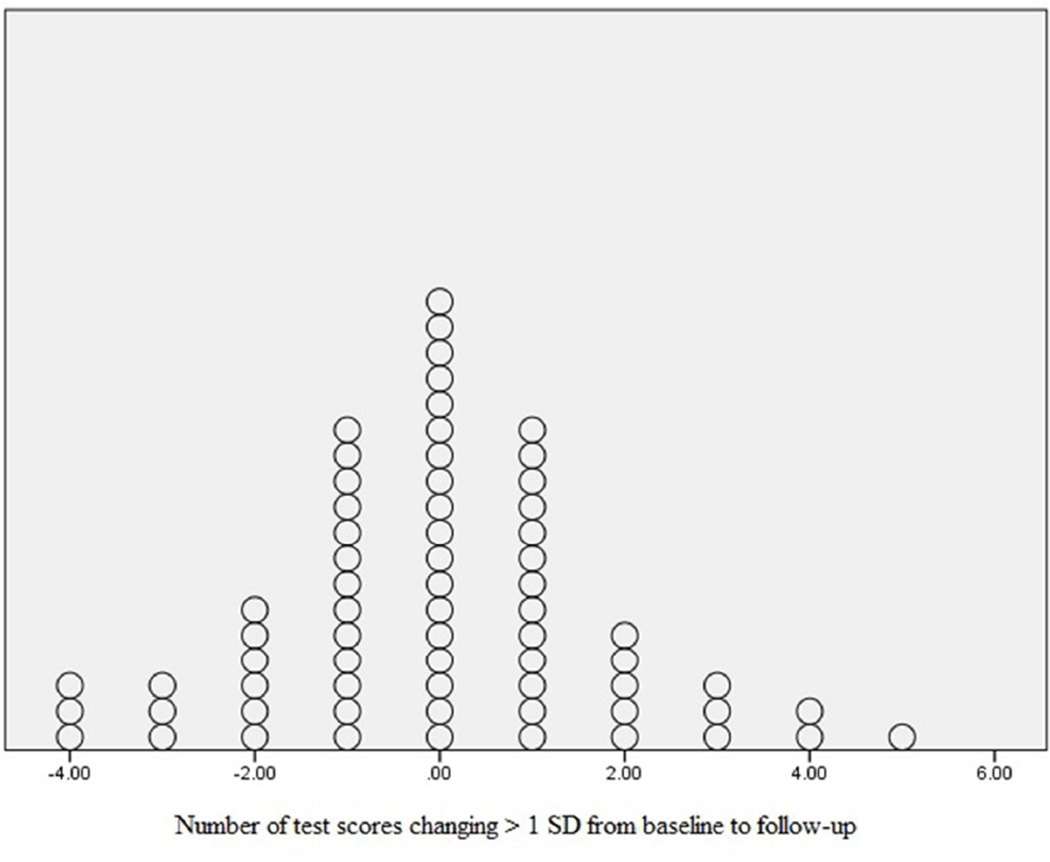
To determine individual change in impairment over time, the difference in the sum of impaired tests at baseline and follow-up was calculated. The group’s overall change was minimal with a mean of −0.15 ± 1.48 and ranged from −4.00 to 5.00. There was an approximately equal distribution of improvement and decline (Figure 2). Most participants showed no change while n=4 (6%) declined on two or more tasks and n=6 (9%) improved on 2 or more tasks. This analysis was repeated using a Cognitive Change Index (CCI),8 which assigns each score a weight indicating its categorical distance from the published normative mean (a weight of “1” is assigned to z-scores falling between 0 and −1, a weight of “2” is assigned to a score below −1 and above −2, etc.). This method provides the advantage of categorizing change only if the score moves to a different standard deviation range (e.g., from 0 to −1 to −1 to −2). The mean CCI at baseline was 11.37 ± 6.72 and 10.85 ± 6.03 at follow-up, with a mean change indicating slight improvement with −0.52 ± 4.12.
Figure 2.
Distribution of decline and improvement from baseline to follow-up
There was no pattern of decline or improvement on any test, or in any functional area. A logistic regression was performed to assess the influence of clinical and demographic characteristics predicting decline on one or more tasks at follow-up, and included age, gender, race, parent education, and EDSS. Decline at follow-up was not significantly predicted by any clinical or demographic features. The full model did not show any influence of these covariates (Χ2 4, N=44)= 2.99, p=0.56), explaining only 6 to 9% of the variance in those who declined versus remained stable or improved. As follows, none of the predictors approached significant contribution to prediction of classification (all p values> 0.22). In sum, decline at follow-up was not significantly predicted by any clinical or demographic features.
Reliable Change Indices (RCIs)
Reliable change indices (RCIs) were calculated for each raw score based on the Jacobson and Truax23 formula, RCI= (x2_x1)/SDiff, with x1 representing the baseline score, x2 the follow-up score, and SDiff the SE of the difference, calculated using test-retest reliability coefficients for each test. The RCIs may serve to mitigate any practice effects from repeat administration of the tests and provide a metric of more clinically meaningful change. Change was considered significant (in either direction) using the cut point of 1.96, which corresponds to the significance level of p<.05.23 In sum, six (9%) participants improved on one score and three (4.5%) significantly declined on one score. The area of decline was on the DKEFS TMT conditions of visual scanning condition (n=1) and number sequence (n=1), and on the CVLT total learning score (n=1). Improvements also included the DKEFS TMT condition of visual scanning (n=2), as well as letter sequencing (n=1) and the letter/number switching condition (n=2), and the CVLT delay score (n=1). There was no consistent pattern of improvement or decline, and no one participant consistently improved or declined on more than one score.
DISCUSSION
Taken together, the current findings indicate that cognitive impairment remains relatively stable in pediatric MS over a mean interval of 1.65 years. Frequency of cognitive impairment in this cohort was consistent with previous reports, involving the expected areas of visuomotor integration, learning and information processing. The finding of relative stability over time was consistent across a range of methodological approaches for measuring change over time including comparisons at the group and individual level, using both published and demographically-adjusted regression-based normative data, as well as with weighted scores (CCI) and RCIs.
Whereas cognitive stability is present over similar intervals in adult MS,24–27 these findings differ from the initial reports of significant decline in pediatric MS.8,9 In the largest previous cohort, Amato and colleagues reported a 75% rate of decline across an average of two years.8 It may be that the duration of this study, an average of 1.6 years, is not adequate to determine cognitive impairment. However, the current cohort also differed from the Amato et al. sample in the nature and severity of baseline deficits. For example, there was more intellectual impairment in their cohort (28%) while only one participant in our cohort had an IQ of less than 85.
In addition, the battery used by the Italian group both included more measures of language function than in the current study and found more language problems (in 20 to 40% of their cohort). While including fewer language measures, the current findings did not indicate language impairment or decline. Instead, using the demographically-adjusted regression-based normative data, there was a trend towards significant improvement in two of the language-based measures (CVLT and Pseudoword Decoding). The Amato et al.8 Italian cohort also had a younger age of symptom onset (11.7 years versus 12.94 years in this cohort) and reported that earlier onset was linked to greater risk of cognitive decline, similar to an earlier report by Banwell and Anderson28. We did not find that age of symptom onset was linked to either cognitive impairment or risk of decline. However, this cohort included relatively few of these youngest onset patients (n=8 or 11.9% with onset under the age of 10 years). Other clinical features may also explain the discrepant findings; unfortunately, additional clinical data were not available to include in analyses for our cohort.
Our findings of lower levels of decline are consistent with the findings reported by Till and colleagues in a Canadian sample.7 They concluded that there was most notably an absence of expected gains on tasks over time when compared to a matched control group that also received repeated assessments.
A major study limitation is the absence of a matched control group to compare cognitive changes over time. Analyses from the regression-based normative data suggest that this would be a preferred and more sensitive method for detecting impairment versus reliance on published normative data. A locally-recruited and matched control group would minimize the influence of regional and other demographic factors. Most importantly, a parallel group recruited and followed over time is the best approach to determine any meaningful change in cognitive functioning attributable to pediatric MS. The Canadian study 7 is the only study to date to include a longitudinal control group and suggests that MS occurring in the context of development may exact its greatest cost by inhibiting age-expected gains. However, even in the absence of a control comparison, our separate analytic approaches have clearly indicated that there was no pattern of decline in this sample.
Another limitation of the current study is that relatively few clinical descriptors of disease status were available to relate to the neuropsychological findings. For instance, with the limited data available on use of disease-modifying treatments (DMTs), it was not possible to accurately examine this potential clinical predictor. Of note, both the Italian and Canadian longitudinal studies reported similarly high rates of DMT use for their samples (88% and 81%, respectively) but differed in findings of cognitive decline. More importantly, the focus of this research was not directed towards the role of DMT on cognitive performance. To accurately answer the role of DMTs in prevention of cognitive decline, a prospective design with the collection of detailed DMT use should be used. Additional clinical information, including detailed measurement of disease activity through clinical relapses and brain magnetic resonance imaging would provide a more specific indicator of disease activity and severity. Nonetheless, with the general absence of decline in our sample, we would not have had adequate power to evaluate these clinical predictors of cognitive change.
The study was also limited by the measures of cognitive functioning included for analyses. While we administered measures that were representative of a general clinical battery, the inclusion of additional measures would have provided a richer assessment of functioning at the individual level. Specifically, the inclusion of additional tests of language functioning would have facilitated comparison to the Italian sample with its findings of language impairment. Finally, the interpretation of neuropsychological data with respect to real life impact is always limited in the absence of functional outcomes. Future studies should include measures of school performance and parent report.
Despite these limitations, we report on the largest longitudinally studied sample to date and the first longitudinal study of cognition with a multicenter US population. The finding of relatively stable cognitive functioning in this cohort is important to add to the ongoing issue of cognitive impairment in pediatric MS.
ACKNOWLEDGMENTS
Members of the U.S. Network of Pediatric MS Centers provided conceptual design feedback and revised the paper. Dana Serafin, BS collected study data at the Stony Brook Medicine site, created and managed the centralized study database, and assisted with analyses. Rebecca Cleary, BA collected study data at the Stony Brook Medicine site, assisted with study analyses, and revised the draft paper. Ralph Benedict, PhD provided the normative data for the healthy controls to calculate regression-based norms. Natalie Baruch, BS collected data at the Massachusetts General site. Laura Julian, PhD supervised and collected data at the UCSF site. Thomas Preston, PhD supervised and collected study data at the Stony Brook Medicine site.
The following are members of the U.S. Network for Pediatric MS: Greg Aaen (Loma Linda University Children’s Hospital); Anita Belman (Lourie Center for Pediatric MS, Stony Brook Medicine); Charles Casper (University of Utah); Tanuja Chitnis (Partners Pediatric MS Center at the Massachusetts General Hospital for Children); Osman Farooq (Pediatric MS Center of the Jacobs Neurological Institute, University at Buffalo); Mark Gorman (Pediatric MS and Related Disorders Program, Boston Children's Hospital); Lauren Krupp (Lourie Center for Pediatric MS, Stony Brook Medicine); Timothy E Lotze (The Blue Bird Circle Clinic for Multiple Sclerosis, Texas Children's Hospital); Jayne M Ness (Center for Pediatric-Onset Demyelinating Disease at Children's of Alabama); Marc Patterson (Pediatric MS Center, Mayo Clinic); Moses Rodriguez (Pediatric MS Center, Mayo Clinic); John Rose (University of Utah); Emmanuelle Waubant (University of California At San Francisco Regional Pediatric MS Center); Bianca Weinstock-Guttman (Pediatric MS Center of the Jacobs Neurological Institute, University at Buffalo).
This study was supported by The Lourie Foundation, Inc., National Multiple Sclerosis Society (10020073405), NIH R01 NS071463, and the Slomo and Cindy Silvian Foundation, all of whom provided support for data collection.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest.
REFERENCES
- 1.Chitnis T, Glanz B, Jaffin S, et al. Demographics of pediatric-onset multiple sclerosis in an MS center population from the Northeastern United States. Mult Scler. 2009;15(5):627–631. doi: 10.1177/1352458508101933. [DOI] [PubMed] [Google Scholar]
- 2.Krupp LB, Banwell B, Tenembaum, et al. International Pediatric MS Study Group. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology. 2007;68:S7–S12. doi: 10.1212/01.wnl.0000259422.44235.a8. demyelinating disorders: revisions to the 2007 definitions. Mult Scler 2013; 19(10): 1261–1267. [DOI] [PubMed] [Google Scholar]
- 3.MacAllister WS, Belman AL, Milazzo M, et al. Cognitive functioning in children and adolescents with multiple sclerosis. Neurology. 2005;64(8):1422–1425. doi: 10.1212/01.WNL.0000158474.24191.BC. [DOI] [PubMed] [Google Scholar]
- 4.Amato MP, Goretti B, Ghezzi A, et al. Cognitive and psychosocial features of childhood and juvenile MS. Neurology. 2008;70(20):1891–1897. doi: 10.1212/01.wnl.0000312276.23177.fa. [DOI] [PubMed] [Google Scholar]
- 5.Portaccio E, Goretti B, Lori S, et al. The brief neuropsychological battery for children: a screening tool for cognitive impairment in childhood and juvenile multiple sclerosis. Mult Scler. 2009;15(5):620–626. doi: 10.1177/1352458508101950. [DOI] [PubMed] [Google Scholar]
- 6.Julian L, Serafin D, Charvet L, et al. Cognitive impairment occurs in children and adolescents with multiple sclerosis: results from a United States network. J Child Neurol. 2013;28:102–107. doi: 10.1177/0883073812464816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Till C, Racine N, Araujo D, et al. Changes in cognitive performance over a 1-year period in children and adolescents with multiple sclerosis. Neuropsychology. 2013;27(2):210–219. doi: 10.1037/a0031665. [DOI] [PubMed] [Google Scholar]
- 8.Amato MP, Goretti B, Ghezzi A, et al. Cognitive and psychosocial features in childhood and juvenile MS: two-year follow-up. Neurology. 2010;75(13):1134–1140. doi: 10.1212/WNL.0b013e3181f4d821. [DOI] [PubMed] [Google Scholar]
- 9.MacAllister WS, Christodoulou C, Milazzo M, et al. Longitudinal neuropsychological assessment in pediatric multiple sclerosis. Dev Neuropsychol. 2007;32(2):625–644. doi: 10.1080/87565640701375872. [DOI] [PubMed] [Google Scholar]
- 10.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Pearson Assessments; 1999. [Google Scholar]
- 11.Wechsler D. Wechsler Abbreviated Scale of Intelligence - Second Edition (WASI-II) San Antonio, TX: Pearson Assessments; 2001. [Google Scholar]
- 12.Delis DC, Kramer JH, Kaplan E, et al. California Verbal Learning Test - Child Version (CVLT-C) San Antonio, TX: Pearson Assessments; 1994. [Google Scholar]
- 13.Delis DC, Kramer JH, Kaplan E, et al. California Verbal Learning Test - Second Version (CVLT-II) San Antonio, TX: Pearson Assessments; 2000. [Google Scholar]
- 14.Bronwell R. Expressive One-Word Picture Vocabulary Test (EOWPVT) San Antonio, TX: Pearson Assessments; 2000. [Google Scholar]
- 15.Wechsler D. Wechsler Individual Achievement Test - Second Edition (WIAT-II) San Antonio, TX: Pearson Assessments; 2001. [Google Scholar]
- 16.Beery KE, Buktenica NA, Beery NA. Beery-Buktenica Developmental Test of Visual-Motor Integration (Beery-VMI) San Antonio, TX: Pearson Assessments; 2010. [Google Scholar]
- 17.Wechsler D. Wechsler Intelligence Scale for Children - Fourth Edition (WISC-IV) San Antonio, TX: Pearson Assessments; 2004. [Google Scholar]
- 18.Wechsler D. Wechsler Adult Intelligence Scale - Fourth Edition (WAIS-IV) San Antonio, TX: Pearson Assessments; 2008. [Google Scholar]
- 19.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. San Antonio, TX: Pearson Assessments; 2001. [Google Scholar]
- 20.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 21.Smerbeck AM, Parrish J, Yeh EA, et al. Regression-based pediatric norms for the brief visuospatial memory test: revised and the symbol digit modalities test. Clin Neuropsychol. 2011;25(3):402–412. doi: 10.1080/13854046.2011.554445. [DOI] [PubMed] [Google Scholar]
- 22.Smerbeck AM, Parrish J, Yeh EA, et al. Regression-based norms improve the sensitivity of the National MS Society Consensus Neuropsychological Battery for Pediatric Multiple Sclerosis (NBPMS) Clin Neuropsychol. 2012;26(6):985–1002. doi: 10.1080/13854046.2012.704074. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson NS and Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59(1):12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 24.Fischer JS, Priore RL, Jacobs LD, et al. Neuropsychological effects of interferon beta-1a in relapsing multiple sclerosis. Multiple Sclerosis Collaborative Research Group. Ann Neurol. 2000;48(6):885–892. [PubMed] [Google Scholar]
- 25.Weinstein A, Schwid SR, Schiffer RB, et al. Neuropsychologic status in multiple sclerosis after treatment with glatiramer. Arch Neurol. 1999;56(3):319–324. doi: 10.1001/archneur.56.3.319. [DOI] [PubMed] [Google Scholar]
- 26.Qu ZX, Pliskin N, Jensen MW, et al. Etretinate augments interferon beta-1b effects on suppressor cells in multiple sclerosis. Arch Neurol. 2001;58(1):87–90. doi: 10.1001/archneur.58.1.87. [DOI] [PubMed] [Google Scholar]
- 27.Kappos L, Freedman MS, Polmant CH, et al. Long-term effect of early treatment with interferon beta-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol. 2009;8(11):987–997. doi: 10.1016/S1474-4422(09)70237-6. [DOI] [PubMed] [Google Scholar]
- 28.Banwell BL, Anderson PE. The cognitive burden of multiple sclerosis in children. Neurology. 2005;8(5):891–894. doi: 10.1212/01.WNL.0000152896.35341.51. 64. [DOI] [PubMed] [Google Scholar]