Abstract
We evaluated the safety and efficacy of closed-loop with meal announcement (CLMA) during reduction and omission of meal insulin boluses in adolescents with type 1 diabetes (T1D).
Twelve adolescents with T1D [M 6; age 15.9 (1.8) yrs; HbA1c 77 (27) mmol/mol] were studied in a randomised crossover study comparing CLMA versus conventional pump therapy over two 24h stays at clinical research facility. Identical meals were given on both occasions. Evening meal bolus was calculated for half of CHO content and no bolus was delivered for lunch.
Plasma glucose was in target 3.9–10mmol/l for 74(55,86)% of time during CLMA and 62 (49,75)% during conventional therapy, p=0.26. Time above 10 mmol/l [23 (13, 39) vs 27 (10, 50)%, p=0.88] or below 3.9 mmol/l [1 (0, 4) vs 5 (1, 10)%, p=0.24] and mean glucose [8.0 (7.6, 9.3) vs 7.7 (6.6, 10.1)mmol/l, p=0.79] were also similar.
In conclusion, these results assist home testing of closed-loop with meal announcement in adolescents with poorly controlled T1D miscalculating or missing meal insulin boluses.
INTRODUCTION
Closed-loop insulin delivery is an emerging therapeutic approach to improve glucose control whilst reducing the risk of hypoglycaemia in type 1 diabetes (T1D)(1). Clinical studies in adolescents with T1D demonstrated that closed-loop with algorithm-directed basal insulin delivery combined with standard insulin boluses at meal times reduces mean glucose and increases the time spent normoglycaemic(2). During adolescence, HbA1c is often higher than recommended(3) and reduced adherence to treatment guidelines is known to occur at a time when the need for independence might lead to the lack of engagement in diabetes care(4). The omission of or delayed insulin boluses with meals or snacks is commonly reported in up to 50% of adolescents on insulin pump therapy (4–6). In this scenario, the safety of closed-loop therapy might be compromised by algorithm-driven insulin overcorrection. The purpose of this study was to evaluate the safety and efficacy of closed-loop therapy during reduction or omission of meal boluses in adolescents with T1D and suboptimal glycaemic control.
METHODS
Subjects
We approached young people aged 12 to 18 years with T1D on insulin pump therapy and suboptimal glycaemic control (A1c 64–108mmol/mol), treated at four paediatric diabetes clinics (Cambridge, University College Hospital London, Basildon, and Norwich). Following ethical approval, participants/guardians signed consent/assent. Adolescents with insulin resistance (total daily dose >2IU/kg/day) and clinically significant nephropathy or retinopathy were excluded.
Study Protocol
In this open label, crossover study, participants received in random order closed-loop insulin delivery or conventional pump therapy during two 24 hour study periods at a clinical research facility, one to six weeks apart.
On each occasion, participants attended the Wellcome Trust Clinical Research Facility at Addenbrooke’s Hospital, Cambridge, from 17:30 on Day 1 until 18:00 on the following day. Continuous glucose monitoring (Enlite®, Medtronic Minimed, CA) was established 24–48 hours before each study visit by inserting a single sensor. During the study period, participants’ insulin pump was replaced by a study pump (Animas®2020, Animas, PA) connected to the existing infusion site and infusing insulin Aspart (Novo Nordisk, Bagsvaerd, Denmark). On each study visit, young people consumed an evening meal (70gCHO) at 19:00, breakfast (50gCHO) at 08:00 and lunch (55gCHO) at 12:30. Meals were self-selected, standardised and identical on the two study visits. Meal insulin boluses were calculated using subjects’ standard pump bolus calculator and pre-meal finger-stick glucose. On both study visits, the evening meal bolus was calculated for 35gCHO and the bolus for lunch was not delivered.
During closed-loop visits, an algorithm based on model-predictive control (MPC)(7) was used to adjust basal insulin delivery based on glucose sensor readings at 15 minutes intervals from 19:00 on Day 1 for 23 hour. At each closed-loop cycle, a sensor glucose level was entered into a laptop running the control algorithm. The algorithm generated advice on basal infusion rate set on the study insulin pump by a research nurse. The algorithm was initialised using subject’s weight, total daily insulin dose and basal insulin infusion. Information on the carbohydrate content of the meal and insulin bolus for dinner (35g) and breakfast, but not for lunch, was provided to the algorithm. Blood samples were taken to measure plasma glucose and insulin levels every 30–60 minutes. Glucose was measured in real-time by YSI2300 STAT Plus analyser (YSI, Farnborough, UK) and plasma insulin by immunochemiluminometric assay (Invitron, Monmouth, UK).
Statistical analysis
The primary outcome was time spent with plasma glucose in the target range (3.9–10mmol/l) between 19:00 on Day 1 and 18:00 on Day 2. A repeated measures regression model was fit to compare the two treatments adjusting for period effect and plasma glucose level at the start of closed-loop. Secondary analyses were additionally evaluated post-dinner (19:00 on Day 1 to 02:00 on Day 2) and post-lunch (12:30 to 18:00 on Day 2). Results are presented as median (interquartile range) or mean (SD) unless stated otherwise.
RESULTS
Twelve adolescents were studied [M 6; age 15.9(1.8)yrs; duration of diabetes 7.8(3.2)yrs (range 3.5–12.9yrs); A1C 9.2(1.2)% 77(27)mmol/mol; total daily dose 0.9(0.8,1.0)IU/kg/d]. One subject was excluded from the primary analysis due to early termination of conventional therapy visit (see below).
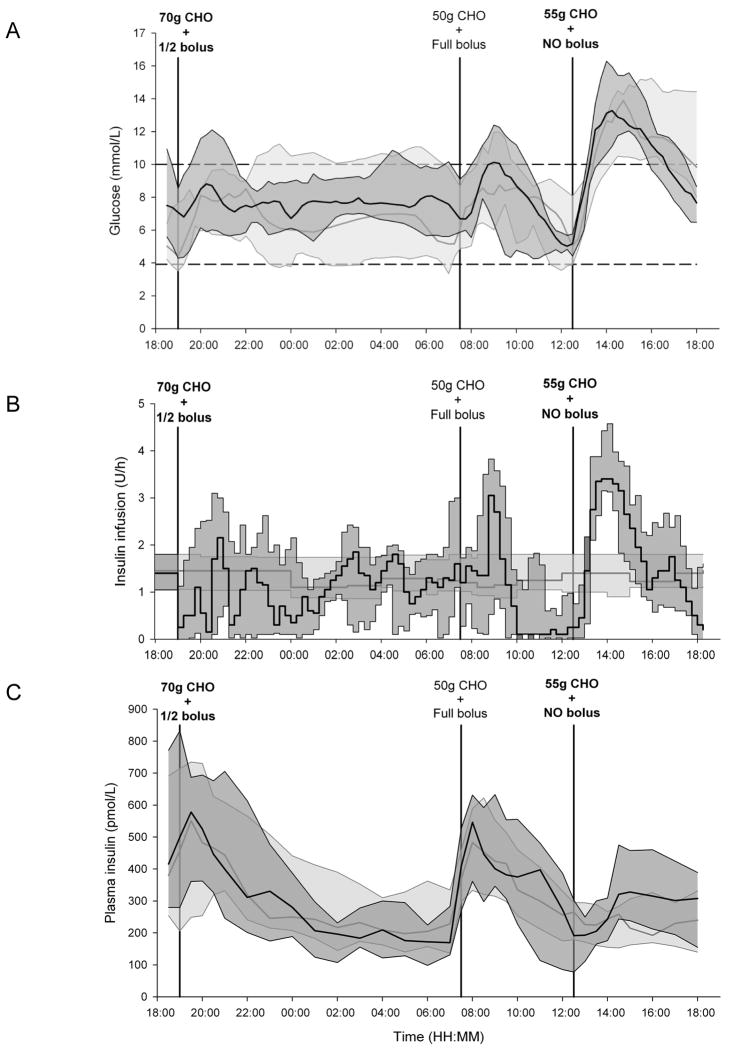
Plasma glucose, insulin delivery and plasma insulin are shown in Figure 1. The time spent with plasma glucose within the target range of 3.9–10mmol/l was 74(55,86)% during closed-loop versus 62(49,75)% during conventional insulin pump therapy (p=0.26). No difference was found in the time spent in the hyperglycaemic range above 10 mmol/l [23 (13, 39) % vs 27 (10, 50) %, p=0.88] or in the hypoglycaemic range below 3.9 mmol/l [1 (0, 4) % vs 5 (1, 10) %, p=0.24]. Insulin infusion rates were similar [1.3 (0.9, 1.9) vs 1.2 (1.0, 1.8) U/hr, p=0.91] resulting in comparable overall insulin delivery [40 (35, 57) vs 39 (36, 55) IU, p=0.69) and mean plasma insulin [279 (186, 396) vs 262 (210, 385) pmol/l, p=0.94)].
Figure 1.
Plasma glucose levels (panel A), insulin infusion rates (panel B) and plasma insulin levels (panel C) are shown for conventional insulin pump therapy (light grey shading) and closed-loop insulin delivery (dark grey shading) [median (interquartile range)]. Meals are indicated as amount of carbohydrates (CHO) consumed. Accompanying insulin boluses (half bolus, full bolus or no bolus) are also shown.
Closed-loop tended to increase the time spent in target from midnight until 07:30 [100 (71, 100) vs 41 (27, 91) %, p=0.08] and post-dinner [86 (63, 100) vs 78 (45, 97) %, p=0.07] whereas no difference was observed post-lunch [40 (29, 54) vs 35 (16, 55) %, p=0.56]. Insulin infusion rates were not different post-dinner [1.0 (0.8, 1.4) vs 1.3 (1.0, 1.8) U/h, p=0.12] whereas higher basal insulin delivery was observed during closed-loop post-lunch [2.0 (1.5, 2.5) vs 1.2 (1.1, 1.8) U/h, p<0.001]. This was reflected by plasma insulin levels [post-dinner: 348 (246, 507) vs 319 (252, 520) pmol/l, p=0.49; post-lunch: 272 (174, 390) vs 216 (158, 301) pmol/l, p=0.001].
Outcomes based on sensor glucose demonstrated increased time spent in target 3.9–10mmol/l during closed-loop [79 (62, 84) vs 51 (41, 73) %, p=0.01] with tendency towards reduced time spent hypoglycaemic [1 (0, 9) vs 15 (1, 21) %, p=0.08].
The median relative absolute difference of Enlite sensor was 13.2%. The Clarke error grid analysis showed that 95.5% of the values were in zone A+B, 0% in zone C, 4.5% in zone D and 0% in zone E.
Ten episodes of hypoglycaemia requiring treatment were observed in four subjects during standard insulin pump therapy (six overnight and four within three hours post-breakfast; of these, one episode required intravenous glucose and led to the termination of the visit). One hypoglycaemia occurred 2hr after breakfast during closed-loop.
CONCLUSIONS
Closed-loop therapy attained safe glucose control during reduction or omission of meal insulin boluses in adolescents with poorly controlled T1D. Although no statistically significant difference was detected between closed-loop and conventional insulin pump therapy, glucose levels were maintained between 3.9 and 10mmol/l for 74% of the time during closed-loop, as compared to 62% during conventional pump therapy. No episode of ketosis was documented.
As observed in previous studies, closed-loop was particularly beneficial overnight when a median time in target of 100% was achieved. Ten episodes of hypoglycaemia were observed overnight and post-breakfast during conventional pump therapy whereas only one hypoglycaemia occurred two hours post breakfast during closed-loop. This is explained by higher basal insulin delivery applied during conventional therapy to compensate for higher glucose levels/missing boluses in subjects with elevated A1C(5). The lack of post-dinner glucose rise levels during conventional pump therapy is explained by these compensatory basal pump settings.
At lunch, 55gCHO were consumed without insulin bolus. Closed-loop algorithm stepped up insulin delivery in response to increased glucose levels (Figure 1) but this did not prevent postprandial hyperglycaemia. Delays in insulin absorption related to the subcutaneous route of administration reduce efficacy in postprandial conditions during fully closed-loop(8;9) but postprandial insulin over-delivery may lead to late postprandial hypoglycaemia(8;10). We observed no such postprandial hypoglycaemia up to 5.5 hours after the lunch and glucose levels at 18:00 were safely reduced during closed-loop [7.6(7.0,8.4) vs 9.5(7.8,14)mmol/l]. Although the risk of hypoglycaemia was not evaluated beyond 5.5 hours post-lunch, it is unlikely that in real-life a meal or snack would not be eaten beyond this interval. Results based on sensor glucose showed a significant improvement in the median time spent in target during closed-loop (79 vs 51%, p=0.01). Due to sensor under-reading of postprandial glucose(11), these outcomes were not translated into improved plasma glucose control highlighting challenges in assessing outcomes based on sensor glucose alone.
In conclusion, we confirm safety and efficacy of closed-loop therapy in scenarios mimicking non-compliant behaviours in a group of adolescents with poorly controlled T1D. These outcomes support progression to testing closed-loop in the home setting.
Acknowledgments
Funding/support: This work was funded by the US National Institute of Diabetes and Digestive and Kidney Diseases (1R01DK085621). Support by the Juvenile Diabetes Research Foundation, NIHR Cambridge Biomedical Research Centre, Medical Research Council Centre for Obesity and Related metabolic Diseases is acknowledged.
Footnotes
Trial Registration
clinicaltrials.gov Identifier: NCT01629277
Conflict of interest disclosures: RH reports having received speaker honoraria from Minimed Medtronic, Lifescan, Eli Lilly, BBraun, and Novo Nordisk, serving on advisory panel for Animas, Minimed Medtronic, and Eli Lilly, receiving license fees from BBraun and Beckton Dickinson; and having served as a consultant to Beckton Dickinson, BBraun, Sanofi, and Profil. MEW has received license fees from Becton Dickinson and has served as a consultant to Beckton Dickinson. CK has served as a consultant to Medtronic International Trading Sàrl and Diabetes Technology Management. DE, GM, JMA, KK, LL, MN, HT, KC and PC declare no competing financial interests exist. RH, DBD and MEW report patent applications.
Author contributions: DE, GM and RH had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. RH coordinated the study. RH, DBD, DE, JMA and MEW co-designed the studies. DE, GM and JMA were responsible for screening and enrolment of participants and arranged informed consent from the participants. DE, GM, JMA, KK, LL, HT and KC provided patient care, collected the clinical and laboratory data, and contributed to biochemical analysis. MEW carried out randomization. RH, DE, GM, MN, PC and CK carried out or supported the data analysis, including the statistical analyses. RH designed and implemented the glucose controller. RH, DBD, DE and GM contributed to the interpretation of the results and the writing and critical review of the report.
Additional contributions: We are grateful to study volunteers for their participation and to staff at the Wellcome Trust Clinical Research Facility for their help in conducting studies. Prof Peter Hindmarsh (University College, London), Dr Birgit Van Meijgaarden (Basildon and Thurrock Hospitals) and Dr Nandu Thalange (Norfolk and Norwich University Hospital) helped identifying potential recruits. This research was conducted with support from the Investigator-Initiated Study Program of Animas Corporation. Animas supplied study pumps. Josephine Hayes (Institute of Metabolic Science, University of Cambridge) provided administrative support. Angie Watts and Karen Whitehaed (Department of Paediatrics, University of Cambridge) provided laboratory support. The Diabetes Research Network Laboratory Wales (Dr Steve Luzio) measured plasma insulin.
References
- 1.Elleri D, Dunger DB, Hovorka R. Closed-loop insulin delivery for treatment of type 1 diabetes. BMC Med. 2011;120:1–9. doi: 10.1186/1741-7015-9-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elleri D, Allen JM, Kumareswaran K, Leelarathna L, Nodale M, Caldwell K, Cheng P, Kollman C, Haidar A, Murphy HR, Wilinska ME, Acerini CL, Dunger DB, Hovorka R. Closed-loop basal insulin delivery over 36 hours in adolescents with type 1 diabetes: randomized clinical trial. Diabetes Care. 2013;36:838–844. doi: 10.2337/dc12-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diabetes Control and Complication Trial Study Group (DCCT) Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial.. Diabetes Control and Complications Trial Research Group. J Pediatr. 1994;125:177–188. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 4.Burdick J, Chase HP, Slover RH, Knievel K, Scrimgeour L, Maniatis AK, Klingensmith GJ. Missed insulin meal boluses and elevated hemoglobin A1c levels in children receiving insulin pump therapy. Pediatrics. 2004;113:e221–e224. doi: 10.1542/peds.113.3.e221. [DOI] [PubMed] [Google Scholar]
- 5.Olinder AL, Kernell A, Smide B. Missed bolus doses: devastating for metabolic control in CSII-treated adolescents with type 1 diabetes. Pediatr Diabetes. 2009;10:142–148. doi: 10.1111/j.1399-5448.2008.00462.x. [DOI] [PubMed] [Google Scholar]
- 6.Vanderwel BW, Messer LH, Horton LA, McNair B, Cobry EC, McFann KK, Chase HP. Missed insulin boluses for snacks in youth with type 1 diabetes. Diabetes Care. 2010;33:507–508. doi: 10.2337/dc09-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hovorka R, Allen JM, Elleri D, Chassin LJ, Harris J, Xing D, Kollman C, Hovorka T, Larsen AM, Nodale M, De PA, Wilinska ME, Acerini CL, Dunger DB. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet. 2010;375:743–751. doi: 10.1016/S0140-6736(09)61998-X. [DOI] [PubMed] [Google Scholar]
- 8.Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes. 2006;55:3344–3350. doi: 10.2337/db06-0419. [DOI] [PubMed] [Google Scholar]
- 9.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus sermautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31:934–939. doi: 10.2337/dc07-1967. [DOI] [PubMed] [Google Scholar]
- 10.Bruttomesso D, Farret A, Costa S, Marescotti MC, Vettore M, Avogaro A, Tiengo A, Dalla MC, Place J, Facchinetti A, Guerra S, Magni L, De NG, Cobelli C, Renard E, Maran A. Closed-loop artificial pancreas using subcutaneous glucose sensing and insulin delivery and a model predictive control algorithm: preliminary studies in Padova and Montpellier. J Diabetes Sci Technol. 2009;3:1014–1021. doi: 10.1177/193229680900300504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calhoun P, Lum J, Beck RW, Kollman C. Performance comparison of the medtronic sof-sensor and enlite glucose sensors in inpatient studies of individuals with type 1 diabetes. Diabetes Technol Ther. 2013;15:758–761. doi: 10.1089/dia.2013.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]