Abstract
Mouse lemurs are suggested to represent promising novel non-human primate models for aging research. However, standardized and cross-taxa cognitive testing methods are still lacking. Touchscreen-based testing procedures have proven high stimulus control and reliability in humans and rodents. The aim of this study was to adapt these procedures to mouse lemurs, thereby exploring the effect of age. We measured appetitive learning and cognitive flexibility of two age groups by applying pairwise visual discrimination (PD) and reversal learning (PDR) tasks. On average, mouse lemurs needed 24 days of training before starting with the PD task. Individual performances in PD and PDR tasks correlate significantly, suggesting that individual learning performance is unrelated to the respective task. Compared to the young, aged mouse lemurs showed impairments in both PD and PDR tasks. They needed significantly more trials to reach the task criteria. A much higher inter-individual variation in old than in young adults was revealed. Furthermore, in the PDR task, we found a significantly higher perseverance in aged compared to young adults, indicating an age-related deficit in cognitive flexibility. This study presents the first touchscreen-based data on the cognitive skills and age-related dysfunction in mouse lemurs and provides a unique basis to study mechanisms of inter-individual variation. It furthermore opens exciting perspectives for comparative approaches in aging, personality, and evolutionary research.
Introduction
In humans there is strong evidence for inter-individual variability in the decline of cognitive ability with age. Normal cognitive aging has been well described and decline is not universal among the different cognitive domains but it is found in specific domains such as processing speed and reasoning, memory, and executive functions (for review see [1]). Sensitive and reliable cognitive testing procedures may reveal such impairments. Simple discrimination and reversal learning, for instance, have been used to investigate learning and cognitive flexibility and revealed age-related impairments in humans (e.g. [2], [3]), monkeys (e.g. [4]–[6]), and rats (e.g. [7]–[10]).
Promising novel non-human primate models for aging and age-associated diseases are mouse lemurs, the world's smallest non-human primates [11], [12]. Mouse lemurs are genetically more closely related to humans than rodents. They are nocturnal, solitary foragers while, during the day, they form sleeping groups [13], [14]. In the wild, mouse lemurs have a maximum lifespan of 8 years [15]. However, the life expectancy of mouse lemurs is higher in captivity: for our colony, the maximum recorded lifespan is 15 years. Due to their mouse-like body size, the maintenance and breeding of mouse lemurs is cost-efficient [11]. Therefore, they represent a valuable exception among primates for conducting long-term research, offering an ideal opportunity for studying their aging process not only cross-sectional, but also in longitudinal studies using individuals with known life history in existing aging colonies in captivity [11], [16]. Until now, studies on mouse lemurs have shown that cerebral atrophy is found in most aged animals [17], [18]. Brain pathologies similar to those of AD-patients can be found in some aged individuals [19], [20]; for review see [11], [12]. For example, Bons, Delacourte, and colleagues described β-amyloid plaques and pathological tau protein aggregation in M. murinus [19], [20]. Dhenain and colleagues later demonstrated iron accumulations in the mouse lemur brain as a process of non-pathological aging but with the same topography as in humans [21]. Besides biological and biochemical aspects of cerebral aging, studies on age-related cognitive decline in mouse lemurs are more limited. However, while no significant difference was observed between young adults and aged animals in cognitive tasks involving odour [22] or visual discrimination of light [23], some impairments were observed in more complex tasks such as shift and spatial rule-guided discrimination tasks [23]–[25]. A recent study demonstrated that mouse lemurs seem to be the only non-human primates reproducing the link between regional cerebral atrophy and age-associated cognitive alterations [23]. A decline in executive functions in aged mouse lemurs was associated with an atrophy of the septal region while impairment in spatial memory was correlated with atrophied hippocampo-entorhinal regions [23]. Most of the above-described studies used non-automated behavioural tasks, which impedes a direct comparison in translational research. Developing sensitive, reliable, and translational tasks to assess specific cognitive domains is, therefore, crucial to understand underlying neural mechanisms during aging.
The touchscreen testing method is a common procedure to assess cognitive abilities in humans and may help to easily detect age-related impairments in specific domains (e.g. the Cambridge Neuropsychological Test Automated Battery (CANTAB), a renowned battery of neuropsychological tests, see e.g. [26], [27]). The touchscreen testing method has several advantages such as high stimulus control, minimized operator-subject interaction, and a wide variety of cognitive tasks that can be performed using standardized testing procedures. A further advantage relevant for translational research is that touchscreen-based tests are adaptable to a broad range of animal species: animals may easily give responses to stimuli displayed on a screen by touching with the nose or hand. During the last decades, batteries of tests using a touchscreen testing method have been successfully used for assessing cognitive skills in rodents [28]–[32] as well as New World (marmoset: e.g. [33]) and Old World monkeys (rhesus monkey: e.g. [34], baboon: e.g. [35]). Some studies showed that age effects on executive functions can be reliably detected in both humans [26] and monkeys [36] using an automated method. To date, a computer-assisted translational approach that allows translating human-comparable cognitive tasks to mouse lemurs is missing. On the long run, such an approach would help to behaviorally and reliably test the functionality of specific brain regions of interest and to understand the mechanisms responsible for the great inter-individual variability in performance, i.e. discriminating healthy aging from neuropathological disorders.
In this study, we applied for the first time a touchscreen-based procedure to the model mouse lemur and examined different facets of cognition using two paradigms: a visual pairwise discrimination (PD) task and reversal learning (PDR). Learning the discrimination between two visual stimuli involves perceptual learning and non-hippocampal, associative stimulus-reward learning [37]. Since the reversal learning requires inhibition of the previously learned responses and the ability to learn the new stimulus-reward contingencies, the task assesses cognitive flexibility [37]. We hypothesized that, comparable to humans, monkeys, and rodents, young and aged mouse lemurs can be trained successfully to interact with a touch-sensitive screen and to respond for food reinforcement. Furthermore, we postulated that, comparable to humans, monkeys, and rodents, in computerized touchscreen-based tasks, age will have a significant effect on performance in the two cognitive tasks. We did not expect to find significant differences in attention and motivation levels during the tasks between the age groups.
Our study revealed that mouse lemurs can be trained successfully to use a touch-screen to get a reward within an average of 24 days. Individual performances in PD and PDR tasks correlate significantly, suggesting that individual performance is unrelated to the respective task. Compared to the young, aged mouse lemurs showed strong impairments in both PD and PDR tasks and high inter-individual variation. Attention and motivation did not differ between age groups in the respective tasks. Thus, our study provides the first touchscreen-based data on the cognitive skills and age-related dysfunction in the novel primate aging model mouse lemur. Findings open exciting perspectives for comparative approaches in aging, personality, and evolutionary research.
Material and Methods
Ethical statement
Experiments are non-invasive and belong to basic research. They may also be used to improve well-being under captive conditions. Experiments were performed in accordance with the NRC Guide for the Care and Use of Laboratory Animals, the European Directive 2010/63/EU on the protection of animals used for scientific purposes, and the German Animal Welfare Act. Our non-invasive testing procedure was approved by the Animal Welfare Committee of the University of Veterinary Medicine and approved and licensed by the Animal Welfare Committee of the Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit (reference numbers: previously AZ 33.9-42502-05-10A080, now AZ 33.12-42502-04-14/1454, 28.04.2014).
All information mentioned is in accordance with the recommendations of the Weatherall report, “The use of non-human primates in research”.
We provided environmental enrichment to the mouse lemurs: within the housing cages, the animals of our colony have branches and hollow cylinders that allow them to climb and hide within their home cages. In addition, each cage is equipped with several sleeping boxes, to model the situation in nature, where mouse lemurs sleep, rest, and rear their offspring in tree holes [38].
Subjects
Thirty adult grey mouse lemurs (Microcebus murinus, see Fig. 1), 14 males and 16 females, were included in this study (see Table 1). According to the age classification in previous studies [39], [40], we formed two age cohorts: one group of 20 young adults (10 males, 10 females; mean age = 2.6 years, range: 1.1–4.1, see Table 1) and one group of 10 aged adults (4 males, 6 females; mean age = 7.9 years, range: 6.9–9.5 years, see Table 1). Prior to the study, a veterinarian had checked the health status of each animal. An ophthalmologic examination was conducted, which allowed us to discard any individual with ocular pathology (approx. 1/3rd of the aged animals from our colony; for methods see [41]).
Figure 1. The grey mouse lemur (Microcebus murinus) represents the smallest primate aging model.
Photograph taken by Dr. Christian Schopf.
Table 1. Performance of the mouse lemurs during the acquisition of the visual discrimination (PD) and the reversal learning (PDR).
| PD task | PDR task | |||||||||||||
| Subject | Sex | Age | Total number of training days | Positive image | Image bias in the first session | Number of sessions (trials) to reach the criterion | Response latency | Reward latency | Number of sessions (trials) to reach the criterion | Response latency | Reward latency | Number of perseverative errors (binomial criterion) | Number of perseverative errors (50% criterion) | Number of trials to reach 50% criterion |
| OTT | m | 2.0 | 13 | marble | - | 5 (150) | 5.156 | 1.619 | 11 (330) | 4.255 | 1.448 | 64 | 110 | 180 |
| PEA | m | 2.6 | 48 | fan | - | 19 (570) | 7.563 | 2.198 | 14 (420) | 3.040 | 1.285 | 82 | 111 | 180 |
| PED | m | 2.1 | 24 | fan | - | 8 (240) | 4.607 | 2.088 | 9 (270) | 3.352 | 1.459 | 66 | 90 | 150 |
| PEP | m | 2.3 | 14 | fan | - | 7 (210) | 2.481 | 0.919 | 15 (450) | 2.071 | 0.972 | 50 | 157 | 270 |
| PPP | m | 2.0 | 31 | marble | - | 8 (233) | 3.997 | 2.071 | 17 (426) | 14.461 | 2.609 | 189 | 219 | 306 |
| PHI | m | 2.3 | 71 | fan | marble | 22 (614)* | 12.296 | 27.673 | 25 (682) | 17.415 | 24.229 | 65 | 159 | 263 |
| PLU | m | 2.1 | 25 | marble | marble | 3 (90)* | 3.134 | 1.220 | 20 (570) | 8.104 | 1.057 | 82 | 149 | 241 |
| QUK | m | 3.2 | 35 | fan | marble | 16 (470)* | 11.559 | 4.475 | 16 (340) | 19.330 | 59.989 | 78 | 150 | 250 |
| QUN | m | 3.0 | 11 | marble | - | 13 (381) | 4.664 | 1.539 | 18 (540) | 3.629 | 1.302 | 122 | 213 | 330 |
| QUL | m | 3.5 | 36 | marble | - | 19 (570) | 3.635 | 1.410 | 26 (780) | 2.012 | 0.865 | 100 | 164 | 270 |
| NEL | f | 1.2 | 38 | marble | fan | 12 (215)* | 8.843 | 1.743 | 16 (381) | 5.679 | 1.520 | 98 | 185 | 261 |
| NOR | f | 1.1 | 20 | marble | - | 4 (120) | 5.040 | 1.286 | 13 (319) | 17.903 | 2.559 | 40 | 127 | 229 |
| PAM | f | 3.1 | 26 | fan | marble | 18 (437)* | 10.013 | 1.290 | 15 (358) | 6.954 | 1.031 | 61 | 162 | 238 |
| PAU | f | 3.0 | 9 | marble | - | 12 (360) | 3.785 | 1.802 | 32 (960) | 3.676 | 1.015 | 216 | 367 | 600 |
| PEG | f | 2.5 | 17 | fan | - | 5 (150) | 5.598 | 1.325 | 7 (210) | 7.472 | 1.428 | 58 | 87 | 180 |
| PEN | f | 2.9 | 6 | fan | - | 6 (180) | 5.433 | 2.976 | 9 (245) | 20.064 | 2.830 | 67 | 108 | 154 |
| PER | f | 3.1 | 13 | fan | - | 9 (237) | 9.946 | 1.787 | 7 (208) | 3.705 | 1.749 | 46 | 84 | 148 |
| POP | f | 2.4 | 32 | marble | - | 4 (120) | 3.117 | 1.405 | 11 (285) | 43.976 | 32.011 | 27 | 96 | 165 |
| QNI | f | 4.1 | 10 | marble | - | 7 (210) | 3.486 | 1.591 | 18 (540) | 2.887 | 1.473 | 140 | 198 | 300 |
| QEL | f | 3.9 | 17 | fan | - | 6 (180) | 7.460 | 0.935 | 13 (390) | 7.641 | 0.982 | 102 | 191 | 330 |
| ULI | m | 7.8 | 12 | fan | - | 25 (574) | 56.709 | 15.055 | 20 (600) | 4.042 | 1.210 | 228 | 274 | 360 |
| URB | m | 7.5 | 20 | marble | marble | 6 (180)* | 3.630 | 1.623 | 19 (539) | 12.569 | 1.503 | 147 | 277 | 419 |
| URI | m | 7.4 | 33 | marble | - | 14 (420) | 19.202 | 2.462 | 34 (1020) | 8.803 | 1.539 | 89 | 434 | 750 |
| VIR | m | 8.6 | 22 | fan | - | 26 (780) | 6.174 | 1.217 | 24 (617) | 9.035 | 2.108 | 100 | 195 | 350 |
| TIP | f | 6.9 | 6 | marble | - | 19 (570) | 2.844 | 3.348 | 33 (979) | 6.530 | 1.398 | 447 | 493 | 769 |
| UND | f | 7.8 | 33 | fan | - | 8 (191) | 10.928 | 2.888 | 14 (375) | 5.896 | 2.531 | 14 | 152 | 284 |
| URA | f | 7.9 | 9 | marble | - | 10 (300) | 5.037 | 1.796 | 11 (330) | 8.666 | 1.408 | 73 | 122 | 180 |
| URS | f | 7.6 | 19 | fan | - | 9 (270) | 5.379 | 1.314 | 9 (300) | 4.420 | 1.714 | 64 | 91 | 150 |
| VAN | f | 8.4 | 14 | fan | - | 11 (330) | 5.757 | 1.721 | 21 (630) | 5.955 | 1.870 | 171 | 229 | 330 |
| WIL | f | 9.5 | 63 | marble | - | 26 (780) | 3.061 | 2.678 | 60 (1800) | 4.615 | 2.852 | 797 | 1001 | 1680 |
*Discarded from the performance analysis in the PD task because of an image bias.
All mouse lemurs used in this study were bred and kept in the colony of the Institute of Zoology, University of Veterinary Medicine Hannover [16], licensed for the maintenance and breeding of mouse lemurs (Erlaubnis gemäß §11 Abs. 1 Satz 1 Nr. 1 Tierschutzgesetz in Verbindung mit § 12 Tierschutz-Versuchstierverordnung, Landeshauptstadt Hannover, reference number AZ 42500/1H, 15.01.2014). The Zimmermann's mouse lemur colony was founded more than two decades ago at the University of Stuttgart-Hohenheim and it was the first colony in which the Goodman's mouse lemur (Microcebus lehilahytsara) has successfully been bred in captivity [16]. All subjects are registered in the mouse lemur studbook. The mouse lemurs used in the described study lived under a reversed, seasonally fluctuating light cycle (LD 14∶10 during the long-day period of 8 months; LD 10∶14 during the short-day period of 4 months) and were housed in different rooms where the dark phase, i.e. activity period, started either at 10:00 a.m., 12:00 a.m., or 2:00 p.m. In all rooms, the temperature and relative humidity were controlled and set to 23–25°C and 50–60%, respectively. Three times a week, the diet of the mouse lemurs consisted of seasonally changing fresh fruits and vegetables, dried fruits, nuts, as well as mealworms or locusts. Milk porridge enriched with vitamins, minerals, and albumin was offered the other 4 days of the week. During the study period, the tested mouse lemurs were maintained either alone or in pairs in cages of at least 0.75 m3 per animal. Animal weight was controlled every day and, on average, was 63±4 g during the testing period, with no significant differences between young and aged animals (Mann-Whitney U-test, Nyoung = 20, Naged = 10, U = 85.0, p = 0.53). The weight at which mouse lemurs could be trained successfully corresponds to the weight under natural conditions (compare [16], [42], [43]). Adipose mouse lemurs had to be under a restricted diet to reach the normal weight before testing.
For this study, each mouse lemur was tested alone and on a daily basis ( = 1 session/day). Wild mouse lemurs are solitary foragers and separation from their sleeping partners during their activity phase corresponds to their natural behaviour [13], [14], [44]. Prior to the study, all animals were naïve to touch-sensitive screen devices.
Test chamber
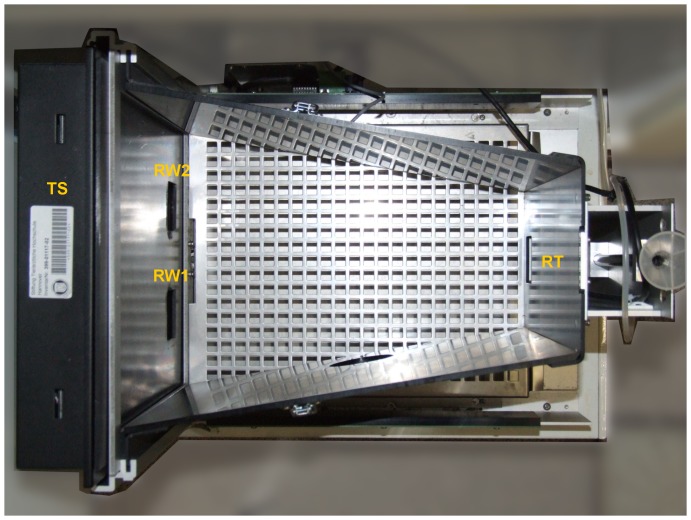
Experiments were performed in a separate testing room, containing a test chamber with a touch-sensitive screen (see Fig. 2). The test chamber was a customized version of the setup used by Bussey and colleagues [28] for rats (89540R-PD Task for rat Touch Screen System, Campden Instruments). The touch-sensitive screen could display visual stimuli in the front of the trapezoidal chamber (width: front = 245 mm, back = 130 mm; length = 330 mm; height = 95 mm), a reward tray delivering apple juice was in the rear wall of the chamber. The chamber was equipped with infrared photocells allowing the recording of entries in the reward tray. Each chamber output (touch on the screen and break of a photocell beam) was simultaneously recorded and analysed by a computer. A video camera and a video recorder were used to film and record each experiment for later offline analysis. A black Perspex mask with 2 response windows (46 mm×46 mm) allowed the mouse lemurs to give a response on one of 2 screen areas. Since mouse lemurs are nocturnal, both training and testing sessions were performed in the dark, i.e. all the visible lights that come with the basic version of the touchscreen setup (house and tray light) had permanently been deactivated.
Figure 2. Test chamber (PD+ PDR task, top view).
TS = touchscreen device; RW1, RW2 = response windows; RT = reward tray.
Training Protocol
The training and test protocols for rodents [28], [30], [31] were adapted to mouse lemurs. Mouse lemurs were trained and tested once a day ( = 1 session), 7 days a week, and within the first 2 hours of their nocturnal activity. The training stimuli were randomly drawn from a pool of 38 pictures (for a list see Fig. S1A). Each picture could only be drawn once per session. The training protocol was divided into the following 5 steps:
○ Step 1 - Habituation to the chamber: the animal was allowed to explore the chamber for 20 minutes. The reward tray was filled with 1 ml apple juice. This step lasted 1 session.
○ Step 2 – Initial training with images: two identical images were displayed on the screen for 30 seconds ( = one trial). The mouse lemur was rewarded as soon as it touched one image (75 µl apple juice), which made both images disappear, or after the two images disappeared automatically (25 µl apple juice). A reward was accompanied by the noise of the activated juice delivery pump. This noise was clearly perceived by the mouse lemurs (when first heard, all subjects showed ear movements and looked into the direction of the pump). A new trial started after a 10-second inter-trial interval (ITI). The maximal duration of a session was set to 30 minutes. We observed that mouse lemurs can adopt two different strategies in this step: either they actively interact with the screen within the 30 s and get a reward or they stay close to the reward tray to collect a reward after 30 s and wait there for the next trial/reward. We, therefore, defined two different criteria for this training step. For the first one, a given subject had to complete 30 trials in 20 minutes. This criterion could only be reached, when the subject interacted correctly with the touchscreen (1st strategy). For those animals that adopted the second strategy, to simply collect the automatic reward without touchscreen interaction, the subject had to complete 30 trials in 30 minutes in 3 consecutive sessions. The interaction with the touchscreen then had to be learned in training step 3 (see below) by the latter animals.
○ Step 3 – Must touch one image: only 1 image was displayed at a pseudo-randomly chosen position (15× on the left side; 15× on the right side; never more than three consecutive trials on the same side), the other response window contained no image ( = blank). The subject was rewarded with 25 µl apple juice as soon as it touched the image ( = correct response). A new trial started when the mouse lemur collected the reward. A response to the blank screen was not rewarded ( = incorrect response). A trial ended when the image was touched. Two consecutive trials were separated by a 10-s ITI. To reach the criterion for this step, the mouse lemur had to perform 30 trials in less than 30 minutes.
○ Step 4 – Must initiate a trial: as in step 3, only 1 image was shown at a pseudo-random position. To display the image (i.e. initiate the trial), the mouse lemur had to introduce its head into the empty reward tray. The mouse lemur was rewarded with 25 µl apple juice as soon as it gave a correct response. A trial ended when the image was touched. Two trials were separated by a 10-s ITI. To reach the criterion for this step, the mouse lemur had to perform 30 trials in less than 30 minutes.
○ Step 5 – Incorrect responses signalled by a tone: similar to step 4, but incorrect responses additionally were signalled by a 500-ms long 2000 Hz tone. In the case of incorrect responses, the trial was immediately stopped and the ITI was set to 15 s. To reach the criterion for this step, the mouse lemur had to complete 30 trials in 30 minutes with at least 80% correct responses on 2 consecutive days.
Testing Protocol
Once the training was accomplished, mouse lemurs were tested in the Pairwise Discrimination (PD) task. The aim of this task is to evaluate the ability to discriminate two unknown images (white shapes on a black background). For all individuals of M. murinus trained in the pairwise discrimination and its reversal task, we used the “marble-fan” pair of visual stimuli that was also used in many studies investigating visual discrimination in mice ([30]; Fig. S1B). One image was arbitrarily used as the positive stimulus, the other one as the negative stimulus. The arbitrary choice was counterbalanced between individuals. The mouse lemur was rewarded with 25 µl apple juice as soon as it touched the positive stimulus. A touch on the negative stimulus was signalled by the same tone as in training step 5 and the increased ITI of 15 s. The ITI after correct choices was 10 s. A mouse lemur was considered discriminating both stimuli when it reached at least 80% correct responses in 2 consecutive sessions (maximum 30 trials per session). Within a session, both images were pseudo-randomly presented 15 times on the left and 15 times on the right side. The rewarded stimulus could not appear more than three consecutive trials on the same side. Once the mouse lemurs reached the PD task criterion (for a brief example video, see Movie S1), they were tested in the reversal task (PDR). The former positive stimulus was now the negative one and vice-versa. In the PDR, the criterion was identical to that in the PD task (i.e. at least 80% correct responses in 2 consecutive daily sessions).
Data analysis
Training
The number of sessions required to complete each training step and the whole training phase were recorded for each mouse lemur. We then compared these numbers of sessions between young and aged adults using a Mann-Whitney U test. In general, we used the non-parametric Mann-Whitney U test for the comparisons made in this study, since our data has not uniformly been normally distributed (Shapiro-Wilk test).
PD task
Since visual stimulus bias may affect the data analysis and interpretation in PD [30], we assessed for each mouse lemur, whether it showed a preference or an aversion for the positive stimulus in the first session using a binomial test. Six mouse lemurs (3 young males, 2 young females and 1 aged male) showed such a stimulus bias in the first session (see Table 1). Most of them showed a positive bias for the “marble” image (3 young males, 1 young female and 1 aged male), 1 for the “fan” image (1 young female). The performance data for these animals were discarded from the performance analysis in the PD task.
The performance of a mouse lemur was assessed using a learning curve with the percentage accuracy for each session and by counting the number of sessions and trials as well as errors an animal needed to reach the task criterion. The performance of both age groups was compared using a Mann-Whitney U test. Within the group of young animals, we also tested for an influence of sex (male vs. female) using a Mann-Whitney U test.
Lastly, we controlled for differences in attention and motivation during the task between both age groups [30]. For that purpose, the average latency to respond after the stimuli display and the average latency to collect the reward after giving a correct answer was calculated for each mouse lemur and over all sessions. We compared these measurements between young and aged adults using a Mann-Whitney U test. Within the young subjects, we again tested for an influence of sex (male vs. female) using a Mann-Whitney U test.
PDR task
In the first session, using a binomial test, we checked whether mouse lemurs showed a bias for the former PD positive stimulus, as it would be expected. As for the PD task, the performance of each mouse lemur was then assessed using a learning curve with the percentage accuracy for each session and the number of sessions and trials an animal needed to reach the task criterion. Furthermore, we analysed the perseveration phase as defined by Mar and colleagues [31]. For this purpose, using a binomial test, we determined for each session, whether a mouse lemur persevered and responded significantly more to the formerly positive stimulus. The perseverative phase, therefore, included all sessions in which the mouse lemurs showed a response bias for the formerly positive stimulus. Within the perseverative phase, the number of errors, so-called perseverative errors, was counted for each animal. As a second measure for the cognitive flexibility/perseverance of the subjects during reversal learning, we counted the individual number of trials needed and errors made until reaching a criterion of 50% correct trials (chance level) in two complete, consecutive sessions. This criterion was taken to define the definite end of perseverance and the starting point of the new learning phase.
The performance and the number of perseverative errors of both age groups were compared using a Mann-Whitney U test. Within the young animals, we also tested for sex differences (male vs. female) using a Mann-Whitney U test.
Lastly, we controlled for differences in attention and motivation during the task between both age groups [31]. For that purpose, the average latency to respond after the stimuli display and the average latency to collect the reward after giving a correct answer over all sessions were calculated for each mouse lemur. We compared these measurements between young and aged adults using a Mann-Whitney U test. Within the group of the young subjects, we also tested for an influence of sex (male vs. female) using a Mann-Whitney U test.
When not indicated, results are given in Mean ±SEM. The level of statistical significance was set at p = 0.05. A trend was considered when 0.05<p≤0.1. All statistical tests were exact and calculated using STATISTICA 10 (StatSoft, Hamburg, Germany).
Results
Training
All mouse lemurs were successfully trained to interact with the touch-sensitive screen. They mostly used their snout but also both snout and one hand or only one hand to touch the screen. They needed an average of 24.2±2.9 days of training before entering the PD task (see Table 1). Training step 2 was completed in 4.1±0.7 days, step 3 in 6.0±1.4, step 4 in 2.9±0.6, and step 5 in 10.2±1.5 days. No significant differences in the total number of training days, nor in the number of days needed to complete each different training step was found between young and aged mouse lemurs (Mann-Whitney U test, U≥87, Nyoung = 20, Naged = 10, p≥0.588). Great inter-individual variability in the number of days required for training was found in both age groups (young range: 6–71; aged range: 6–63; see Table 1).
PD task
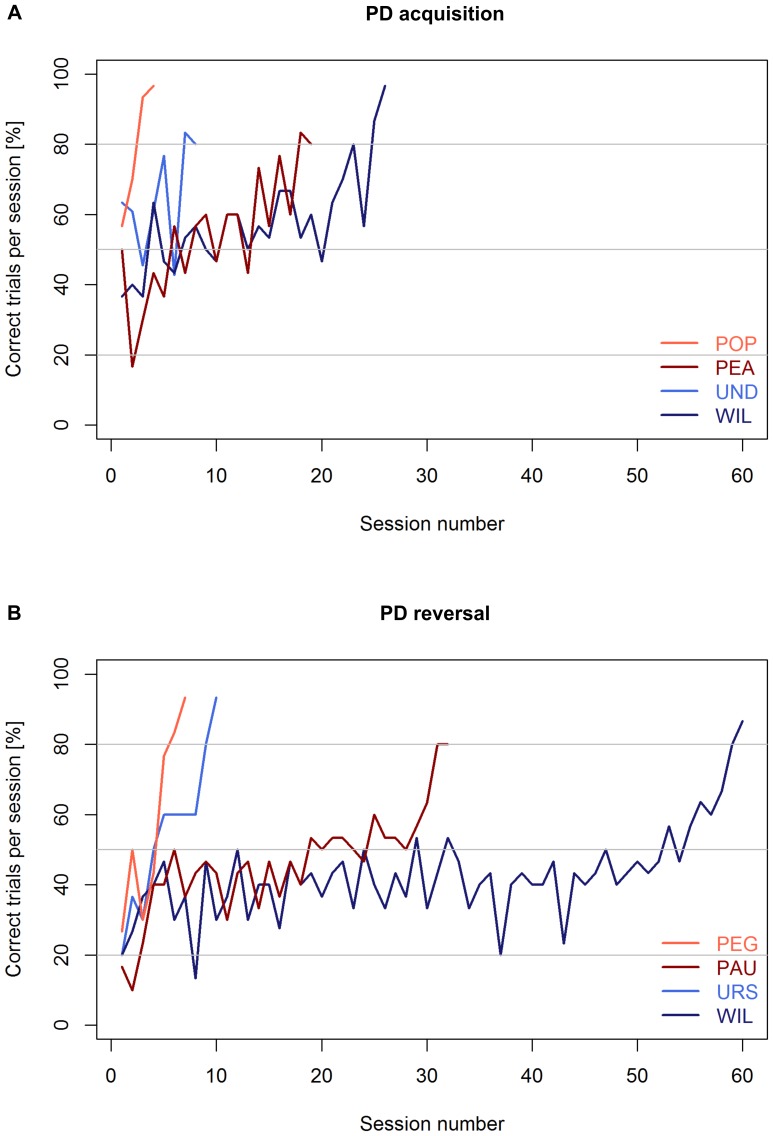
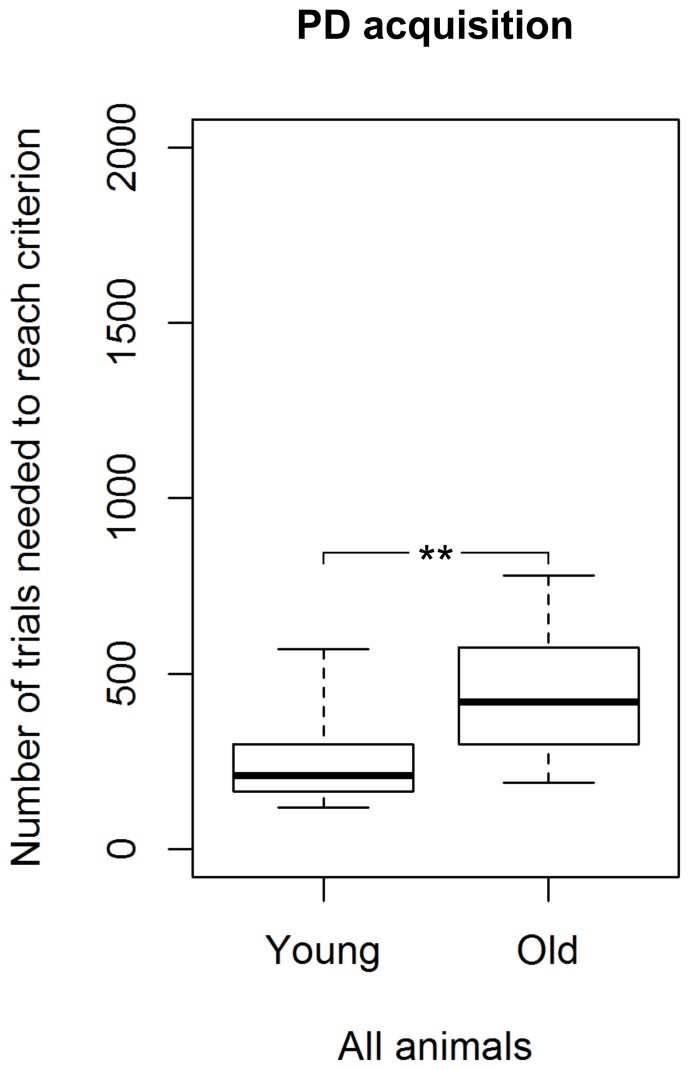
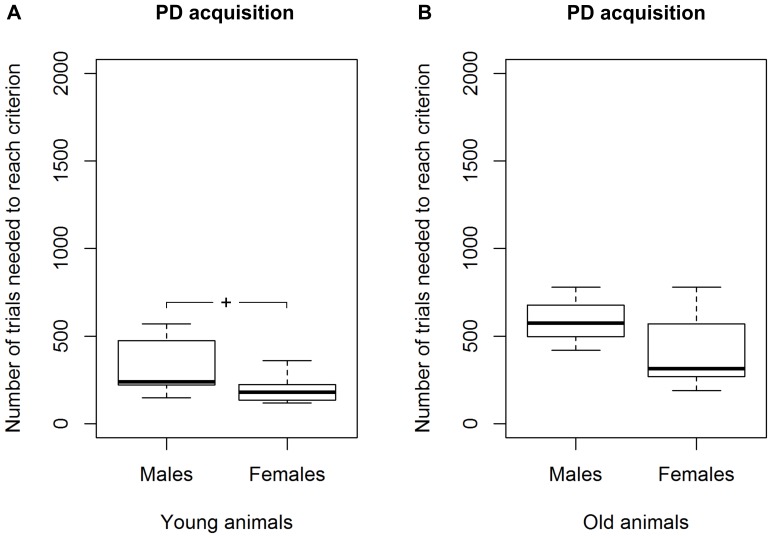
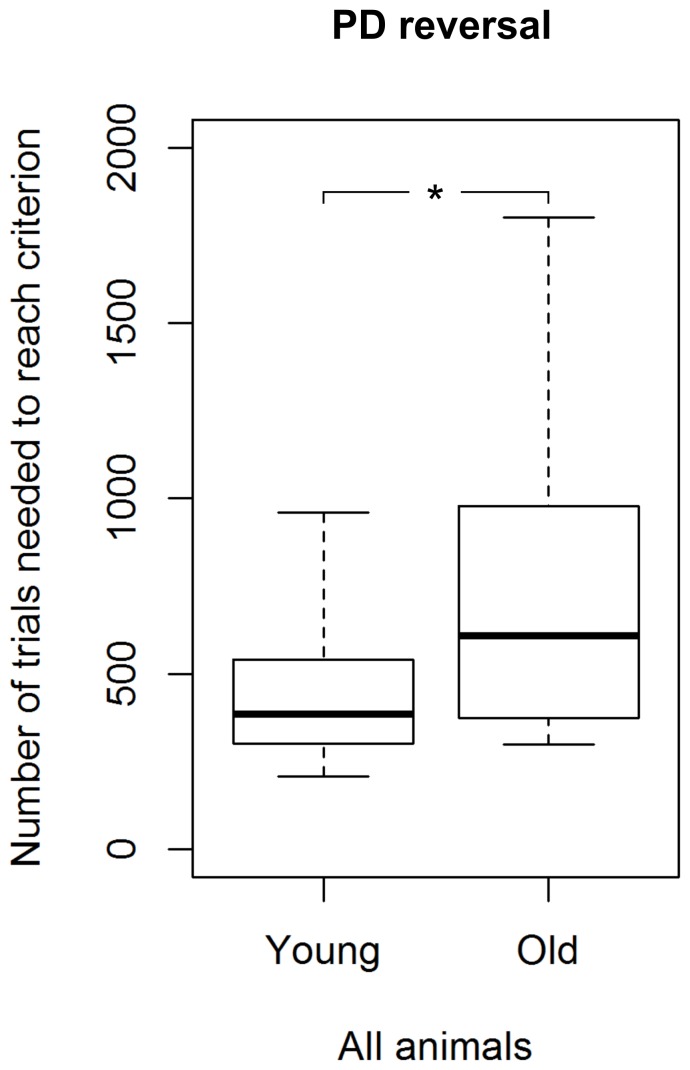
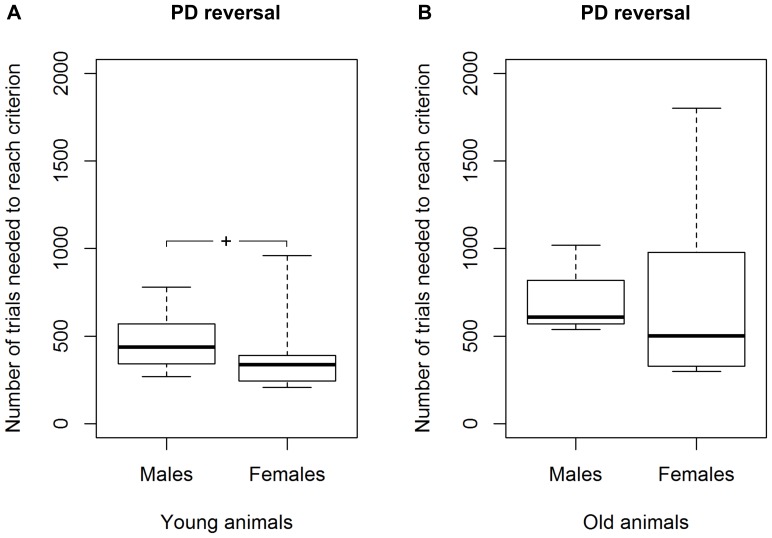
All tested mouse lemurs succeeded in acquiring the visual discrimination (see Table 1; for examples of learning curves see Fig. 3A). Among the 24 mouse lemurs that showed no spontaneous preference for an image, we found a significant difference in the number of sessions and trials required to reach the criterion between young and aged adults (Mann-Whitney U test, Nyoung = 15, Naged = 9, U≤24, p≤0.009). Young mouse lemurs needed fewer trials (median = 210) than aged ones (median = 420) to reach the criterion (Fig. 4). Great inter-individual variability in the number of trials to reach the criterion was found: the performance of young adults ranged from 120 to 570, while it was even wider (range: 191–780) for the aged ones (Fig. 4). Among young adults, we found that females tended to require fewer trials to reach the criterion than males (Mann-Whitney U test, Nmales = 7, Nfemales = 8, U = 11, p = 0.054; Fig. 5A). Due to the smaller sample size, aged adults could not reliably be statistically compared for sex differences in the median number of trials needed to reach the PD-criterion. Nevertheless, aged females also showed better values than aged males (Fig. 5B).
Figure 3. Representative learning curves of young and old mouse lemurs in the visual pair-wise discrimination task (PD) and its reversal (PDR).
A Learning curves of a good (POP, UND) and a bad (PEA, WIL) PD learner from each age category (red = young subjects; blue = old subjects). B Learning curves of a good (PEG, URS) and a bad (PAU, WIL) PDR learner from each age category (red = young subjects; blue = old subjects).
Figure 4. Performance of young and aged mouse lemurs in the visual discrimination task (PD).
** Indicates a significant difference with p<0.01. Nyoung = 15, Naged = 9. The box represents the lower quartile, median, and upper quartile, the whiskers represent the minimum and maximum values.
Figure 5. Performance of males and females in the visual discrimination task (PD).
A Results for the young mouse lemurs and B results for the aged mouse lemurs. + Indicates a trend with 0.05<p<0.1. Nyoung males = 7, Nyoung females = 8, Naged males = 3, Naged females = 6. The box represents the lower quartile, median, and upper quartile, the whiskers represent the minimum and maximum values.
Control for attention to the task
Young and aged mouse lemurs did not differ significantly in their latency to respond after the stimuli display (medianyoung = 4.7 s, range: 2.5–9.9 s; medianaged = 5.8 s, range: 2.8–56.7 s; Mann-Whitney U test, Nyoung = 15, Naged = 9, U = 46, p = 0.215). Among young adults, we found no significant differences in the latency to respond between the sexes (Mann-Whitney test, Nmales = 7, Nfemales = 8, U = 22, p = 0.536). Among aged adults, females showed a shorter median latency to respond than males (medianfemales = 5.2 s, range: 2.8–10.9 s; medianmales = 19.2 s, range: 6.2–56.7 s). However, the sample of the aged males was too small to statistically verify this effect.
Control for motivation to collect the reward
Young mouse lemurs tended to collect the reward quicker than the aged ones (medianyoung = 1.6 s, range: 0.9–3.0 s; medianaged = 2.5 s, range: 1.2–15.1 s; Mann-Whitney test, Nyoung = 15, Naged = 9, U = 39, p = 0.096), but this statistical trend was mainly caused by the contribution of one of the old males (ULI).
PDR task
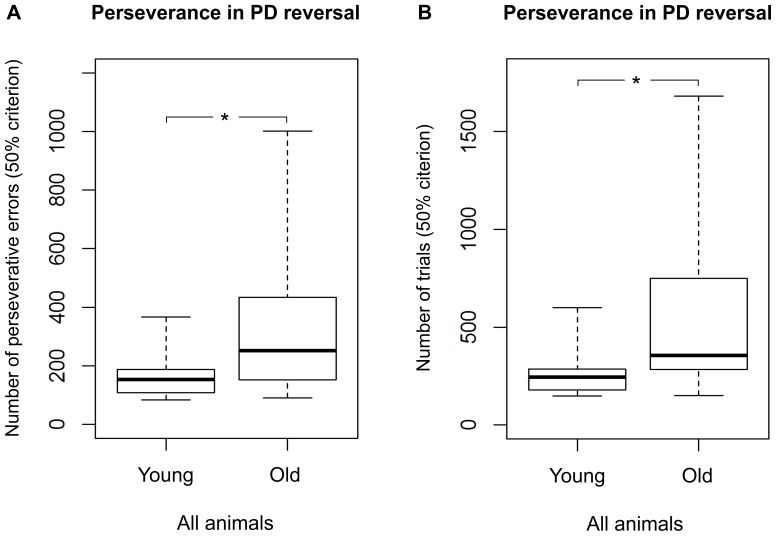
All 30 tested mouse lemurs succeeded in the reversal learning (see Table 1; for examples of learning curves see Fig. 3B). In the first session, the average accuracy was 20.3±1.8%. We found a statistical trend for a difference in the number of sessions (p = 0.061) and a significant difference in the number of trials required to reach the criterion between young and aged adults (Mann-Whitney U test, Nyoung = 20, Naged = 10, U = 54.5, p = 0.044; Fig. 6). Young mouse lemurs needed fewer trials (median = 390) than aged ones (median = 617) to reach the criterion (Fig. 6). Again, great inter-individual variability in the number of trials to reach the criterion was found: the performance of young adults ranged from 208 to 960, while it was much wider (range: 300–1800) for the aged ones. Among the young adults, we found that females tended to require fewer trials (median = 302) to reach the criterion than males (median = 426; Mann-Whitney U test, Nmales = Nfemales = 10, U = 27.5, p = 0.089; Fig. 7A; for the results of the aged animals, see Fig. 7B). Aged mouse lemurs tended to make more perseverative errors (binomial criterion) than young ones (medianyoung = 72, range: 27–216; medianaged = 123, range: 14–797; Mann-Whitney U test, Nyoung = 20, Naged = 10, U = 60, p = 0.082). Using the 50% criterion to measure perseverance revealed that aged and young adults differed significantly in both the total number of perseverative errors (medianyoung = 153.5, range: 84–367; medianaged = 251.5, range: 91–1001; Mann-Whitney U test, Nyoung = 20, Naged = 10, U = 47, p = 0.019; Fig. 8A) and the number of trials (medianyoung = 245.5, range: 148–600; medianaged = 355, range: 150–1680; Mann-Whitney U test, Nyoung = 20, Naged = 10, U = 43, p = 0.011; Fig. 8B). On the group level, aged adults needed more trials to quit following the former rule and, accordingly, made more wrong decisions before ultimately re-reaching chance performance.
Figure 6. Performance of young and aged mouse lemurs in the reversal learning task (PDR).
* Indicates a significant difference with p<0.05. Nyoung = 20, Naged = 10. The box represents the lower quartile, median, and upper quartile, the whiskers represent the minimum and maximum values.
Figure 7. Performance of males and females in the reversal learning task (PDR).
A Results for the young mouse lemurs and B results for the aged mouse lemurs. + Indicates a trend with 0.05<p<0.1. Nyoung males = 10, Nyoung females = 10, Naged males = 4, Naged females = 6. The box represents the lower quartile, median, and upper quartile, the whiskers represent the minimum and maximum values.
Figure 8. Perseverance of young and aged mouse lemurs in the reversal learning task (PDR).
A Number of trials needed to reach the 50% criterion in the PDR. B Number of perseverative errors made until reaching the 50% criterion in the PDR. * Indicates a significant difference with p<0.05. Nyoung = 20, Naged = 10. The box represents the lower quartile, median, and upper quartile, the whiskers represent the minimum and maximum values.
Within the group of the young animals, no significant sex differences in the number of perseverative errors were found for any of the criteria (Mann-Whitney U test, Nmales = Nfemales = 10, U≥40, p≥0.481).
Correlation between PD and PDR performance
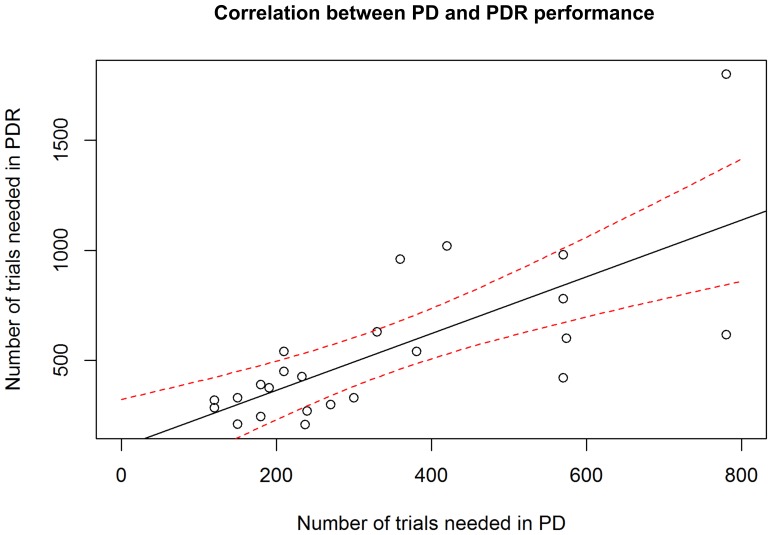
We found a highly significant, positive correlation (Spearman's rank correlation, r = 0.72, p = 0.00007) between the individual performances in the PD and PDR tasks, i.e. subjects that needed a high number of trials to reach the criterion in the visual discrimination task also needed a high number of trials to reach the criterion in the reversal task (Fig. 9)
Figure 9. Correlation between individual PD and PDR performances.
Number of trials needed to reach criterion in the reversal learning task (PDR) plotted against the number of trials needed to reach criterion in the visual discrimination task (PD) (Ntotal = 24). The solid black line represents the regression line, the dashed red lines represent the 95% confidence interval as estimated from the linear regression model. The individual performances in PD and PDR are highly significantly correlated (Spearman's rank correlation, r = 0.72, p = 0.00007).
Control for attention to the task
Young and aged mouse lemurs did not differ significantly in their latency to respond after the stimuli were displayed (medianyoung = 6.3 s, range: 2.0–44.0 s; medianaged = 6.2 s, range: 4.0–12.6 s; Mann-Whitney U test, Nyoung = 20, Naged = 10, U = 88, p = 0.619). We did not find any significant sex differences in this variable within the young animals (Mann-Whitney U test, Nmales = Nfemales = 10, U = 35, p = 0.280).
Control for motivation to collect the reward
Young and aged mouse lemurs did not significantly differ in their latency to collect the reward (medianyoung = 1.5 s, range: 0.9–60.0 s; medianaged = 1.6 s, range: 1.2–2.9 s; Mann-Whitney U test, Nyoung = 20, Naged = 10, U = 81, p = 0.422). No significant sex differences in the median reward latency were found within the young animals (Mann-Whitney U test, Nmales = Nfemales = 10, U = 44, p = 0.684).
Discussion
Our findings revealed for the first time that young and aged mouse lemurs could be successfully trained on a visual pair-wise discrimination task and its reversal using a touchscreen standardized automated system. We found age-associated cognitive decline in the acquisition of the visual discrimination as well as in the reversal learning. We, thus, demonstrated for the first time the successful use of a standardized touchscreen method and cross-species comparative approach to assess age-related cognitive impairments in mouse lemurs.
Interestingly, we found that aged mouse lemurs, as a group, were impaired in the acquisition of a visual discrimination between two images. This result supports comparable findings in humans [3] and monkeys [36] but contrasts with previous findings on mouse lemurs in a visual discrimination task [23]. While the procedures used in the human and monkey studies were comparable to our mouse lemur study (i.e. touchscreen-based testing method using discrimination between images [3], [36]), the visual discrimination task of Picq and colleagues [23] differed in two details: firstly, in the study of Picq and co-workers, mouse lemurs were placed in a work chamber where they had to choose between two corridors leading to a reinforcement chamber. The visual discrimination had to be made on the basis of the illumination of the corridors (S+ = illuminated corridor; S- = dark corridor). In the visual discrimination task used for our study, mouse lemurs were required to (1) learn to perceptually discriminate two white shapes on a black background and to (2) learn which of the two shapes was associated with the reward. Thus, differences in the difficulty of the visual discrimination may have led to the diverging results. Secondly, the main motivation for the subjects in the study of Picq and colleagues was to reach the safety of a nest box. By choosing a food reward for our study, the conditioning paradigm in our case was clearly appetitive and differences in the conditioning procedure might also have contributed to the differing observations.
The fact that all our mouse lemurs succeeded in the visual discrimination task, shows that they were able to perceptually discriminate both images. Thus, using a standardized and automated testing procedure, our results revealed for the first time that aged mouse lemurs show cognitive impairments that have so far only been found in aged humans and monkeys. Since all mouse lemurs were checked for eye diseases, corneal consistence, integrity of retina, and intraocular pressure of each eye prior to the study, we can exclude differences in the perceptual abilities between the age groups in our study [41]. We found a strong inter-individual variability in the learning performance. The oldest animal (WIL, 9.5 years old) had the worst learning performance while other aged mouse lemurs (e.g. VAN, 8.4 years old) performed just as good as young ones. This task based on a touchscreen testing method has been proven to be sensitive to dysfunctions in the perirhinal cortex of the medial temporal lobe of rats [45]. The temporal lobe, including the secondary visual area, is actually one of the regions that are the most affected by age-dependent atrophies in mouse lemurs [23]. A magnetic resonance imaging study is underway to explore whether a link between brain morphology and cognition can be confirmed in our subjects.
We found that aged mouse lemurs, as a group, were also impaired when faced with the reversed reward contingency (PDR task). Reversal learning procedures are widely used to assess flexibility or behavioral adjustment to changing rules [31]. In this task, mouse lemurs not only had to learn to extinguish the previously rewarded response but also to choose the previously unrewarded image. We found significant differences in perseverance measures in the PDR between the two age cohorts, indicating that aged subjects persevere more in their errors, i.e. they had difficulties to ignore the previously rewarded response and to flexibly adapt to the reversed stimulus-reward contingency. This result is in line with previous findings in aged mouse lemurs [23], [24], aged rats [10], aged monkeys [6], and aged humans [3] and, thus, may reflect a common pattern in brain aging across mammals. The PDR task has proven to be sensitive to the integrity and functionality of the orbitofrontal cortex in rats and monkeys [9], [46]. Orbitofrontal lesions in rats and monkeys and lesions of the dorsolateral striatum in mice significantly slow down visual reversal learning [9], [46], [47]. Lesions of the medial prefrontal cortex in rodents may also impair the reversal learning, but only when visual stimuli are difficult to discriminate [47]. Again, our current magnetic resonance imaging study in mouse lemurs will help to illuminate potential links between atrophies in these brain regions and cognition in the same subjects.
Within young mouse lemurs, we reported that females seemed to perform better than males in acquisition of the visual discrimination and reversal learning. Although the sample size was small, aged females also tended to be better than males of comparable age (see Fig. 5B). A larger sample will help to confirm sex differences in cognitive functions and to explore the potential influence of sex hormones on learning abilities (see for instance the effect of hormonal status on spatial memory in female chimpanzees [48]) in future studies.
Lastly, we provide future prospects for the study of visual acuity in nocturnal small-brained primates using the same behavioural tasks as in rodents, non-human, and human primates. We showed that mouse lemurs could solve a visual discrimination of shapes. Through the visual discrimination of white shapes on a black background, we gained behavioural insight into the visual acuity of mouse lemurs, since they were clearly able to make their choice from the rear end of the chamber. Mouse lemurs, thus, can visually discriminate white shapes of less than 4 cm2 from a distance of more than 20 cm. To our best knowledge, there is only one study that has previously reported data on visual acuity in the grey mouse lemurs: Dkhissi-Benyahya and colleagues estimated visual acuity anatomically [49], using peak retinal ganglion cell density and spacing, and found an acuity value of 4.2 cycles/degree, which is higher than for rats (about 1 cycle/degree see [50]). It confirms that visual acuity of nocturnal mammals, usually considered as poor, actually shows a great variability between species. The computer-assisted standardized method described here may also be used to conduct further behavioural investigations on the visual acuity of mouse lemurs that might provide valuable information concerning the evolution of the visual system in nocturnal mammals.
To conclude, we successfully adapted a touchscreen based testing method for the assessment of age-related impairments in non-hippocampal, associative learning and cognitive flexibility (a component of executive functions) to the use in the grey mouse lemur, M. murinus, a novel primate model for aging. Based on that, the development and adaptation of further cross-taxa touchscreen-based automated attention, learning, and memory tasks for mouse lemurs will help to assess further facets of cognition and its disorders and to embed the learning and memory capacity of these early primates into the evolution of primate intelligence.
Supporting Information
List of visual stimuli used. A 38 different stimuli have been used during the training procedure. B Pair of stimuli (“marbles” = left; “fan” = right) used for the actual visual discrimination task and its reversal.
(TIF)
Example video of a young subject in the visual discrimination task (PD). The video starts in the middle of a session and shows how the animal completes 4 trials of the 30 trial session. The touchscreen and the two response windows can be seen at the upper end of the field of view (FOV), whereas the reward tray is located outside the FOV at the opposite side of the chamber.
(WMV)
Acknowledgments
We thank Mathias Craul, Marko Dubičanac, Annette Klaus, and Barbara Mengelkamp for helping to collect parts of the data. Further, we thank the animal keepers, Achim Sauer, Lisa Früh, Iris Grages, Johanna Samtlebe, and Katharina Nitschke.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are included within the paper and its Supporting Information files.
Funding Statement
This study is part of a project which has received funding from the European Community's 7th Framework Programme (FP7/2007–2013) under grant agreement n° 278486 acronym “DEVELAGE” (http://www.develage.eu/index.html). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Deary IJ, Corley J, Gow AJ, Harris SE, Houlihan LM, et al. (2009) Age-associated cognitive decline. Brit Med Bull 92: 135–152. [DOI] [PubMed] [Google Scholar]
- 2. Boutet I, Milgram NW, Freedman M (2007) Cognitive decline and human (Homo sapiens) aging: An investigation using a comparative neuropsychological approach. J Comp Psychol 121: 270–281. [DOI] [PubMed] [Google Scholar]
- 3. Weiler JA, Bellebaum C, Daum I (2008) Aging affects acquisition and reversal of reward-based associative learning. Learn Mem 15: 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herndon JG, Moss MB, Rosene DL, Killiany RJ (1997) Patterns of cognitive decline in aged rhesus monkeys. Behav Brain Res 87: 25–34. [DOI] [PubMed] [Google Scholar]
- 5. Lai ZC, Moss MB, Killiany RJ, Rosene DL, Herndon JG (1995) Executive system dysfunction in the aged monkey - Spatial and object reversal learning. Neurobiol Aging 16: 947–954. [DOI] [PubMed] [Google Scholar]
- 6. Voytko ML (1999) Impairments in acquisition and reversals of two-choice discriminations by aged rhesus monkeys. Neurobiol Aging 20: 617–627. [DOI] [PubMed] [Google Scholar]
- 7. Barense MD, Fox MT, Baxter MG (2002) Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learn Mem 9: 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brushfield AM, Luu TT, Callahan BD, Gilbert PE (2008) A comparison of discrimination and reversal learning for olfactory and visual stimuli in aged rats. Behav Neurosci 122: 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schoenbaum G, Nugent SL, Saddoris MP, Setlow B (2002) Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport 13: 885–890. [DOI] [PubMed] [Google Scholar]
- 10. Schoenbaum G, Setlow B, Saddoris MP, Gallagher M (2006) Encoding changes in orbitofrontal cortex in reversal-impaired aged rats. J Neurophysiol 95: 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bons N, Rieger F, Prudhomme D, Fisher A, Krause KH (2006) Microcebus murinus: a useful primate model for human cerebral aging and Alzheimer's disease? Genes Brain Behav 5: 120–130. [DOI] [PubMed] [Google Scholar]
- 12. Languille S, Blanc S, Blin O, Canale CI, Dal-Pan A, et al. (2012) The grey mouse lemur: A non-human primate model for ageing studies. Ageing Res Rev 11: 150–162. [DOI] [PubMed] [Google Scholar]
- 13. Radespiel U (2000) Sociality in the gray mouse lemur (Microcebus murinus) in northwestern Madagascar. Am J Primatol 51: 21–40. [DOI] [PubMed] [Google Scholar]
- 14. Radespiel U, Cepok S, Zietemann V, Zimmermann E (1998) Sex-specific usage patterns of sleeping sites in grey mouse lemurs (Microcebus murinus) in northwestern Madagascar. Am J Primatol 46: 77–84. [DOI] [PubMed] [Google Scholar]
- 15.Zimmermann E, Radespiel U (2007) Primate life histories. In: Henke W, Tattersall I, editors.Handbook of paleoanthropology.Vol. 2. Primate evolution and human origins.Berlin Heidelberg: Springer. pp.1163–1205. [Google Scholar]
- 16. Wrogemann D, Radespiel U, Zimmermann E (2001) Comparison of reproductive characteristics and changes in body weight between captive populations of rufous and gray mouse lemurs. Int J Primatol 22: 91–108. [Google Scholar]
- 17. Dhenain M, Chenu E, Hisley CK, Aujard F, Volk A (2003) Regional atrophy in the brain of lissencephalic mouse lemur primates: Measurement by automatic histogram-based segmentation of MR images. Magnet Reson Med 50: 984–992. [DOI] [PubMed] [Google Scholar]
- 18. Dhenain M, Michot JL, Privat N, Picq JL, Boller F, et al. (2000) MRI description of cerebral atrophy in mouse lemur primates. Neurobiol Aging 21: 81–88. [DOI] [PubMed] [Google Scholar]
- 19. Bons N, Jallageas V, Silhol S, Mestre-Frances N, Petter A, et al. (1995) Immunocytochemical characterization of Tau proteins during cerebral aging of the lemurian primate Microcebus murinus . C R Acad Sci III 318: 741–747. [PubMed] [Google Scholar]
- 20. Delacourte A, Sautiere PE, Wattez A, Mourtongilles C, Petter A, et al. (1995) Biochemical characterization of Tau proteins during cerebral aging of the lemurian primate Microcebus murinus . C R Acad Sci III 318: 85–89. [PubMed] [Google Scholar]
- 21. Dhenain M, Duyckaerts C, Michot JL, Volk A, Picq JL, et al. (1998) Cerebral T2-Weighted Signal Decrease During Aging in the Mouse Lemur Primate Reflects Iron Accumulation. Neurobiol Aging 19: 65–69.. [DOI] [PubMed] [Google Scholar]
- 22. Joly M, Deputte B, Verdier JM (2006) Age effect on olfactory discrimination in a non-human primate, Microcebus murinus . Neurobiol Aging 27: 1045–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Picq JL, Aujard F, Volk A, Dhenain M (2012) Age-related cerebral atrophy in nonhuman primates predicts cognitive impairments. Neurobiol Aging 33: 1096–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Picq JL (2007) Aging affects executive functions and memory in mouse lemur primates. Exp Gerontol 42: 223–232. [DOI] [PubMed] [Google Scholar]
- 25. Trouche SG, Maurice T, Rouland S, Verdier JM, Mestre-Frances N (2010) The three-panel runway maze adapted to Microcebus murinus reveals age-related differences in memory and perseverance performances. Neurobiol Learn Mem 94: 100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Robbins TW, James M, Owen AM, Sahakian BJ, Lawrence AD, et al. (1998) A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: Implications for theories of executive functioning and cognitive aging. J Int Neuropsych Soc 4: 474–490. [DOI] [PubMed] [Google Scholar]
- 27. Sahakian BJ, Owen AM (1992) Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J Roy Soc Med 85: 399–402. [PMC free article] [PubMed] [Google Scholar]
- 28. Bussey TJ, Padain TL, Skillings EA, Winters BD, Morton AJ, et al. (2008) The touchscreen cognitive testing method for rodents: How to get the best out of your rat. Learn Memory 15: 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bussey TJ, Saksida LM, Rothblat LA (2001) Discrimination of computer-graphic stimuli by mice: A method for the behavioral characterization of transgenic and gene-knockout models. Behav Neurosci 115: 957–960. [DOI] [PubMed] [Google Scholar]
- 30. Horner AE, Heath CJ, Hvoslef-Eide M, Kent BA, Kim CH, et al. (2013) The touchscreen operant platform for testing learning and memory in rats and mice. Nat Protoc 8: 1961–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mar AC, Horner AE, Nilsson SRO, Alsio J, Kent BA, et al. (2013) The touchscreen operant platform for assessing executive function in rats and mice. Nat Protoc 8: 1985–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oomen CA, Hvoslef-Eide M, Heath CJ, Mar AC, Horner AE, et al. (2013) The touchscreen operant platform for testing working memory and pattern separation in rats and mice. Nat Protoc 8: 2006–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Spinelli S, Ballard TM, Higgins GA, Feldon J, Pryce CR (2003) CANTAB-based attention and memory studies in the common marmoset monkey. J Psychopharmacol 17: A60–A60. [Google Scholar]
- 34. Weed MR, Taffe MA, Polis I, Roberts AC, Robbins TW, et al. (1999) Performance norms for a rhesus monkey neuropsychological testing battery: acquisition and long-term performance. Cognitive Brain Res 8: 185–201. [DOI] [PubMed] [Google Scholar]
- 35. Zurcher NR, Rodriguez JS, Jenkins SL, Keenan K, Bartlett TQ, et al. (2010) Performance of juvenile baboons on neuropsychological tests assessing associative learning, motivation and attention. J Neurosci Meth 188: 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nagahara AH, Bernot T, Tuszynski MH (2010) Age-related cognitive deficits in rhesus monkeys mirror human deficits on an automated test battery. Neurobiol Aging 31: 1020–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Romberg C, Horner AE, Bussey TJ, Saksida LM (2013) A touch screen-automated cognitive test battery reveals impaired attention, memory abnormalities, and increased response inhibition in the TgCRND8 mouse model of Alzheimer's disease. Neurobiol Aging 34: 731–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Radespiel U, Ehresmann P, Zimmermann E (2003) Species-specific usage of sleeping sites in two sympatric mouse lemur species (Microcebus murinus, M. ravelobensis) in Northwestern Madagascar. Am J Primatol 59: 139–151. [DOI] [PubMed] [Google Scholar]
- 39. Perret M (1997) Change in photoperiodic cycle affects life span in a prosimian primate (Microcebus murinus). J Biol Rhythm 12: 136–145. [DOI] [PubMed] [Google Scholar]
- 40.Zimmermann E, Radespiel U, Mestre-Frances N, Verdier JM (submitted) Life history variation in mouse lemurs (Microcebus murinus, M. lehilahytsara): the effect of environmental and phylogenetic determinants. In: Lehmann S, Radespiel U, Zimmermann E, editors. Dwarf and mouse lemurs of Madagascar: Biology, behavior, and conservation biogeography of the Cheirogaleidae. Cambridge: Cambridge University Press. [Google Scholar]
- 41.Dubicanac M, Joly M, Karck J, Nolte I, Verdier JM, et al. (2013) Ocular pathologies in aging mouse lemur (Microcebus murinus) populations: cataract formation depends on the annual photoperiodic cycle. 13th Conference of the Gesellschaft für Primatologie. Hamburg. pp. 52.
- 42. Schmid J, Kappeler PM (1998) Fluctuating sexual dimorphism and differential hibernation by sex in a primate, the gray mouse lemur (Microcebus murinus). Behav Ecol Sociobiol 43: 125–132. [Google Scholar]
- 43. Zimmermann E, Cepok S, Rakotoarison N, Zietemann V, Radespiel U (1998) Sympatric mouse lemurs in north-west Madagascar: a new rufous mouse lemur species (Microcebus ravelobensis). Folia Primatol 69: 106–114. [DOI] [PubMed] [Google Scholar]
- 44. Joly M, Zimmermann E (2011) Do solitary foraging nocturnal mammals plan their routes? Biol Lett 7: 638–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Winters BD, Bartko SJ, Saksida LM, Bussey TJ (2010) Muscimol, AP5, or scopolamine infused into perirhinal cortex impairs two-choice visual discrimination learning in rats. Neurobiol Learn Mem 93: 221–228. [DOI] [PubMed] [Google Scholar]
- 46. Dias R, Robbins TW, Roberts AC (1996) Dissociation in prefrontal cortex of affective and attentional shifts. Nature 380: 69–72. [DOI] [PubMed] [Google Scholar]
- 47. Bussey TJ, Muir JL, Everitt BJ, Robbins TW (1997) Triple dissociation of anterior cingulate, posterior cingulate, and medial frontal cortices on visual discrimination tasks using a touchscreen testing procedure for the rat. Behav Neurosci 111: 920–936. [DOI] [PubMed] [Google Scholar]
- 48. Lacreuse A, Russell JL, Hopkins WD, Herndon JG (2014) Cognitive and motor aging in female chimpanzees. Neurobiol Aging 35: 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dkhissi-Benyahya O, Szel A, Degrip WJ, Cooper HM (2001) Short and mid-wavelength cone distribution in a nocturnal Strepsirrhine primate (Microcebus murinus). J Comp Neurol 438: 490–504. [DOI] [PubMed] [Google Scholar]
- 50. Prusky GT, Harker KT, Douglas RM, Whishaw IQ (2002) Variation in visual acuity within pigmented, and between pigmented and albino rat strains. Behav Brain Res 136: 339–348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of visual stimuli used. A 38 different stimuli have been used during the training procedure. B Pair of stimuli (“marbles” = left; “fan” = right) used for the actual visual discrimination task and its reversal.
(TIF)
Example video of a young subject in the visual discrimination task (PD). The video starts in the middle of a session and shows how the animal completes 4 trials of the 30 trial session. The touchscreen and the two response windows can be seen at the upper end of the field of view (FOV), whereas the reward tray is located outside the FOV at the opposite side of the chamber.
(WMV)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are included within the paper and its Supporting Information files.