Abstract
The paraventricular nucleus of the thalamus (PVT) has been shown to participate in hedonic feeding and is thought to influence drug seeking. This understudied nucleus contains anterior (aPVT) and posterior (pPVT) subregions, which receive dense projections from hypothalamic orexin/hypocretin (OX) but exhibit anatomical and functional differences. This study sought to characterize in Long-Evans rats the involvement of these PVT subregions and their OX receptor activity in consumption of the drug, ethanol. Compared to those maintained on water and chow only (Water Group), rats trained to drink pharmacologically-relevant levels of ethanol (Ethanol Group) showed increased neuronal activation in the PVT, specifically the aPVT but not pPVT, as indicated by c-Fos-immunoreactivity. Similar results were obtained in rats administered ethanol via oral gavage, indicating that this site-specific effect was due to ethanol exposure. In support of the involvement of OX, the Ethanol group also showed increased mRNA levels of this neuropeptide in the hypothalamus and of OX 2 receptor (OX2R) but not OX 1 receptor (OX1R), again in the aPVT but not pPVT. Similarly, ethanol gavage increased double-labeling of c-Fos with OX2R but not OX1R, specifically in the aPVT. Evidence directly supporting a role for aPVT OX2R in ethanol consumption was provided by results with local injections, showing ethanol intake to be enhanced by OX-A or OX-B in the aPVT but not pPVT and reduced by a local antagonist of OX2R but not OX1R. These results focus attention on the aPVT and specifically its OX2R in mediating a positive-feedback relationship with ethanol intake.
Keywords: c-Fos, emotional behavior, immunohistochemistry, intermittent access, microinjection, rat
INTRODUCTION
The paraventricular nucleus of the thalamus (PVT) is a key node in the brain, integrating homeostatic, arousal, and circadian input and relaying efferents to limbic regions to direct behavior (Vertes and Hoover, 2008). Recently, it has been proposed to play a major role in the drug-seeking circuitry (Martin-Fardon and Boutrel, 2012) and has been shown to participate in hedonic feeding (Choi et al., 2012). This evidence suggests that the PVT may be important for both the seeking and intake of rewarding substances. There is indirect evidence supporting the idea that ethanol may be one drug whose consumption is controlled by the PVT. These findings show that intraperitoneal injection of ethanol activates this nucleus, as measured by the transcription factor c-Fos (Ryabinin et al., 1997), and that the reinstatement of beer-seeking is prevented by lesions of the PVT (Hamlin et al., 2009). Whether or not the PVT participates in voluntary ethanol drinking, however, has not yet been demonstrated.
If the PVT is involved with ethanol drinking, one mechanism through which this might occur involves orexigenic neuropeptides. Transcribed predominantly in neurons of the perifornical lateral hypothalamus (PFLH) (Peyron et al., 1998), the neuropeptide orexin/hypocretin (OX) sends one of its densest projections to the PVT throughout its rostro-caudal axis (Kirouac et al., 2005; Peyron et al., 1998). Moreover, the two receptors for OX, both the orexin 1 receptor (OX1R) with high affinity for OX-A and the orexin 2 receptor (OX2R) with equal affinity for OX-A and OX-B (Sakurai et al., 1998), are found throughout the PVT (Marcus et al., 2001) and induce membrane depolarization upon OX binding (Zhang et al., 2009). Like the PVT, OX has a role in controlling the intake of drugs of abuse such as ethanol (Kim et al., 2012), with ethanol drinking stimulated by hypothalamic injection of OX (Schneider et al., 2007) and hypothalamic levels of this peptide increased by the consumption of ethanol (Morganstern et al., 2010). The possibility that OX mediates the relationship of the PVT with ethanol is suggested by evidence showing that an ethanol-associated stimulus increases the number of Fos-positive neurons in the PVT and that these activated neurons are surrounded by OX terminals (Dayas et al., 2008). Further, c-fos gene expression in the PVT is stimulated by intracerebroventricular injection of OX (Date et al., 1999).
While often considered a homogenous structure, the PVT has at least two anatomical subdivisions, an anterior (aPVT) and posterior (pPVT) subregion (Vertes and Hoover, 2008). Although the overall output of the PVT is directed to the limbic forebrain, some differences in the projections and functions of these subregions have been reported. For example, the pPVT projects more heavily than the aPVT to the dorsal striatum and amygdala, while the aPVT has a larger projection to the hypothalamic arcuate nucleus (Li and Kirouac, 2008; Vertes and Hoover, 2008). In addition, c-fos gene expression in the aPVT, but not the pPVT, is increased by a food cue with incentive motivational properties (Flagel et al., 2011), and it is elevated in the pPVT, but not the aPVT, by a short period of food deprivation in obese Zucker rats (Timofeeva and Richard, 2001). These differences in their associations with feeding behavior suggest that the PVT subregions may also have different relationships with ethanol drinking.
To understand the role of the PVT in ethanol consumption, the present report utilized Long-Evans rats which were trained to drink ethanol using the 20% intermittent-access paradigm that induces high intake (Simms et al., 2008; Wise, 1973) or were administered ethanol at comparable levels by oral gavage. These rats were first examined for ethanol-induced PVT activation using c-Fos immunohistochemistry. Then, to assess the involvement of OX in this activation, measurements after ethanol intake were taken of OX mRNA in the PFLH and also of double-labeling of the OX receptors with c-Fos in the PVT subregions. The consequences of this PFLH-to-PVT activation on ethanol drinking were then studied through microinjection of OX agonists and antagonists directly into the PVT subregions. Together, these experiments tested the hypothesis that the PVT, due in part to the local actions of OX, is important in pharmacologically-relevant ethanol intake.
MATERIALS AND METHODS (see Appendix for further details)
Subjects
Adult, male Long-Evans rats (N = 105; 250 – 275 g, Charles River Laboratories International, Inc., Wilmington, MA, USA) were individually housed in an AAALAC-accredited facility, on a 12-hour reversed light/dark cycle (lights off at 0900 h). During the acclimation week, they received ad libitum chow (LabDiet Rodent Chow 5001, St. Louis, MO, USA) and water, via two plastic 8 oz bottles (PETCO Animal Supplies, Inc, San Diego, CA, USA). Experiments were approved by the Rockefeller University Animal Care Committee and followed the NIH Guide for the Care and Use of Laboratory Animals.
Procedures
Experiment 1
To establish that rats in our laboratory will voluntarily drink pharmacologically-relevant levels of ethanol using the 20% intermittent-access paradigm, two sets of rats (N = 16/set; Set 1 and Set 2) were trained to drink ethanol (n = 8/group; Ethanol Group) or maintained on chow and water only (n = 8/group; Water Group). During Week 3 of access, after intake had stabilized, the time-course of daily ethanol intake was determined. All subsequent testing occurred 30 minutes after daily ethanol presentation. After being acclimated to an open field during Week 3, Set 1 was tested for locomotor activity during Week 4 and for elevated plus maze anxiety during Week 5. Set 2 was tested for novelty-induced locomotor activity during Week 4 and for blood ethanol concentration (BEC) from tail vein blood collected during Week 5 (Analox GM7 Alcohol Analyzer; Lunenberg, MA, USA). (See Appendix for details of ethanol drinking and behavioral testing.)
Experiment 2
To examine the impact of voluntary ethanol drinking on neuronal activation in the PVT, Ethanol and Water groups (Set 2) during Week 6 of ethanol drinking were deeply anesthetized, 90 minutes after ethanol presentation when c-Fos levels should peak (Vilpoux et al., 2009), with 20 mg/kg xylazine (LLOYD Incorporated, Shenandoah, IA, USA) and 100 mg/kg ketamine (Fort Dodge Animal Health, Overland Park, KS, USA) (i.p.). They were perfused transcardially with 60 ml of 0.9% NaCl and then 240 ml of 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. Brains were removed, post-fixed in 4% paraformaldehyde for 24 hours at 4 °C, transferred to 25% sucrose for 4 days at 4 °C, then frozen at −80 °C. Coronal brain slices containing the PVT were processed for c-Fos immunohistochemistry. (See Appendix for details of immunofluorescence histochemistry).
Experiment 3
To confirm that PVT activation was due to ethanol exposure rather than the voluntary aspect of drinking, a new set of rats (Set 3; N = 24) was divided into four groups that were orally gavaged twice-daily (at 1000 and 1500 h) on Mondays, Wednesdays, and Fridays with either 0.75 g/kg (Low Ethanol Group; to match 30-minute voluntary intake) or 1.50 g/kg (High Ethanol Group; to match daily intake) of 20% ethanol or an equivalent volume of water (Water Group), or they were handled without insertion of the gavage needle (Control Group) (n = 6/group). Tail vein blood was sampled 30 minutes following the fifth treatment for analysis of BEC. Ninety minutes after the eleventh treatment (matched to when voluntary groups consumed ethanol at stable levels), rats were anesthetized and perfused, and brains processed for immunohistochemistry as in Experiment 2.
Experiment 4
To determine the effects of ethanol drinking on OX activity within the PVT, Ethanol and Water groups (Set 1) at the end of Week 7 of drinking were sacrificed by rapid decapitation 30 minutes after daily ethanol presentation for analysis of the PFLH and PVT using quantitative real-time polymerase chain reaction (qRT-PCR). (See Appendix for details of brain dissections and qRT-PCR.)
Experiment 5
To determine the contribution of OX receptor activity to PVT activation, alternate brain sections from the PVT of High Ethanol, Low Ethanol, Water, and Control groups (Set 3) were processed for immunohistochemistry to double-label c-Fos with OX1R or with OX2R, (See Appendix for details of immunofluorescence histochemistry).
Experiment 6
To examine the impact of OX receptor activity on ethanol drinking, rats (Set 4: N = 16) were trained to drink ethanol over four weeks and then cannulated in the aPVT or pPVT (n = 8/area). To ensure that effects were specific to ethanol, an additional set of rats (Set 5: N = 20) was trained to drink 2% sucrose, known to be consumed at comparable levels to 20% ethanol in g/kg/24h (Li et al., 2011a), and was then cannulated in the aPVT or pPVT (n = 10/area). Set 4 was injected first with OX-A (1.0, 0.5 nmol; American Peptide Co. Inc, Sunnyvale, CA, USA) or vehicle, counterbalanced in a within-subject design across three ethanol days, and then, after one week of recovery, was injected with OX-B (1.0, 0.5 nmol; American Peptide) or vehicle. These agonists were dissolved in preservative-free 0.9% NaCl (Hospira Inc., Lake Forest, IL, USA) at doses shown to promote ethanol drinking with OX-A in the hypothalamus (Schneider et al., 2007). Set 5, tested with sucrose, was injected with OX-A (1.0 nmol) versus vehicle, in a within-subject design counterbalanced across two days. By comparing effects from two different PVT subregions, each subregion served as an anatomical control for the other. (See Appendix for details of sucrose drinking and microinjections.)
Experiment 7
To determine if the effects from OX agonists might be due to endogenous OX activity and also which receptor might participate in these effects, Set 6 (N = 13) was initially treated like Set 4, with n = 6 cannulated in the aPVT and n = 7 in the pPVT. They were injected with the OX2R antagonist TCS OX2 29 (10 nmol; Tocris Bioscience, Minneapolis, MN, USA) (Hirose et al., 2003) or vehicle, counterbalanced in a within-subject design across two ethanol days, and then, after one week of recovery, were injected with the OX1R antagonist SB 334867 (10 nmol; Tocris) (Smart et al., 2001) or vehicle. The OX2R antagonist was dissolved in 0.9% NaCl (Hospira), at a dose that produced behavioral effects with pPVT injection (Li et al., 2011b), while SB 334867 was dissolved in dimethyl sulfoxide (Sigma–Aldrich, St. Louis, MO, USA).
RESULTS
Experiment 1: Intermittent access induces pharmacologically-relevant ethanol drinking
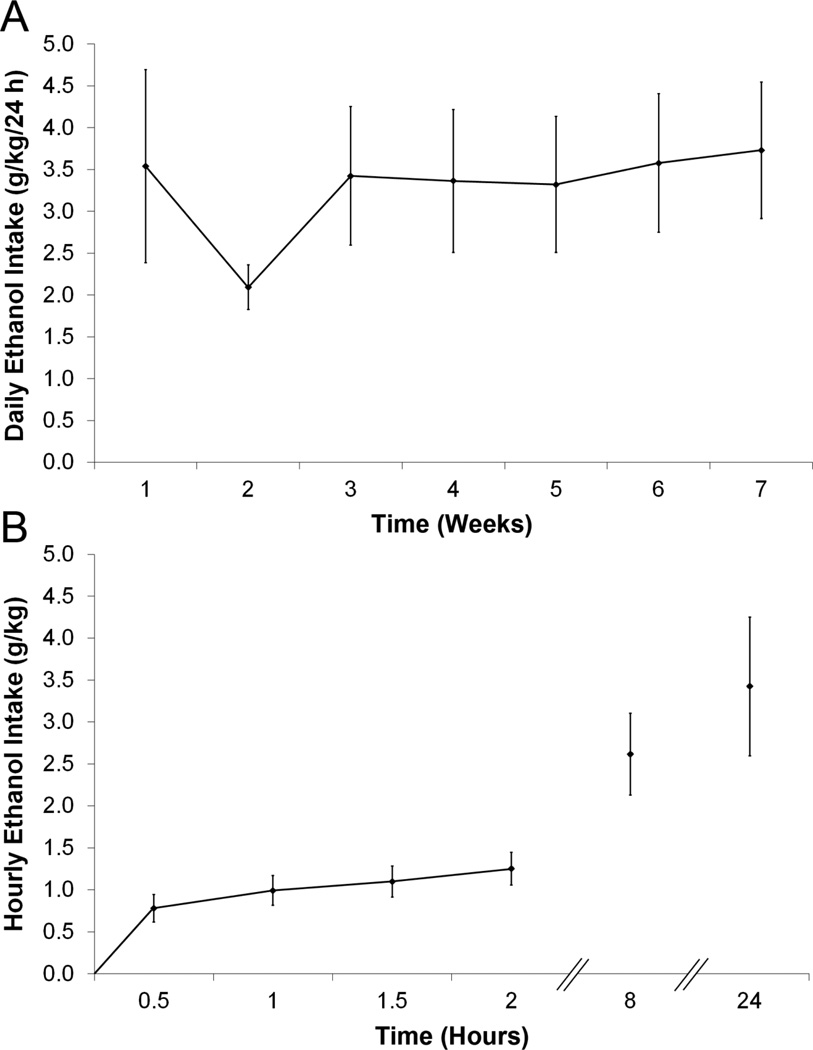
Before examining the PVT in relation to ethanol drinking, we first wanted to ensure that the rats tested in our laboratory with the 20% intermittent-access two-bottle choice paradigm would drink at pharmacologically-relevant levels. This paradigm resulted in relatively high and stable daily ethanol intake in Long-Evans rats, with a mean of 3.5 ± 0.8 g/kg/24 h in Set 1, 3.1 ± 0.4 g/kg/24 h in Set 2, and intake as high as 8.0 g/kg/24 h. Whereas weekly intake in Set 2 (but not Set 1) showed a main effect of time [F(4,28) = 10.66, p < 0.001], drinking for both sets stabilized by the third week, with subsequent week-to-week intake strongly correlated for Set 1 (r = +0.91) and Set 2 (r = +0.64) (Fig. 1A). The greatest intake occurred during the first 30 minutes, when Set 1 consumed 24.4% (0.8 g/kg) and Set 2 consumed 20.3% (0.6 g/kg) of their total daily ethanol (Fig. 1B). This 30-minute intake correlated strongly with 24-hour intake (Set 1: r = +0.81, p < 0.05; Set 2: r = +0.73, p < 0.05) and also with BEC values (Set 2: r = +0.87, p < 0.01) which averaged 53 ± 14 mg% and were as high as 134 mg%. Because of this drinking, the Ethanol groups consumed less water than the Water groups (Set 1: [F(1,14) = 37.68, p < 0.001]; Set 2: [F(1,14) = 11.07, p < 0.01]) but were similar in their daily caloric intake (Set 1: [F(1,14) = 0.34, ns]; Set 2: [F(1,14) = 0.02, ns]) and final body weight (Set 1: 543 ± 9 g vs 567 ± 9 g, not significant, ns; Set 2: 456 ± 11 g vs 465 ± 5 g, ns).
Figure 1.
Intermittent access induces high and stable levels of 20% ethanol drinking in Long-Evans rats. A. Average daily ethanol intake across the duration of the experiment (Set 1, n = 8). B. Hourly ethanol intake, assessed during Week 3 of the experiment (Set 1, n = 8). The greatest ethanol intake occurred during the first 30 minutes of access.
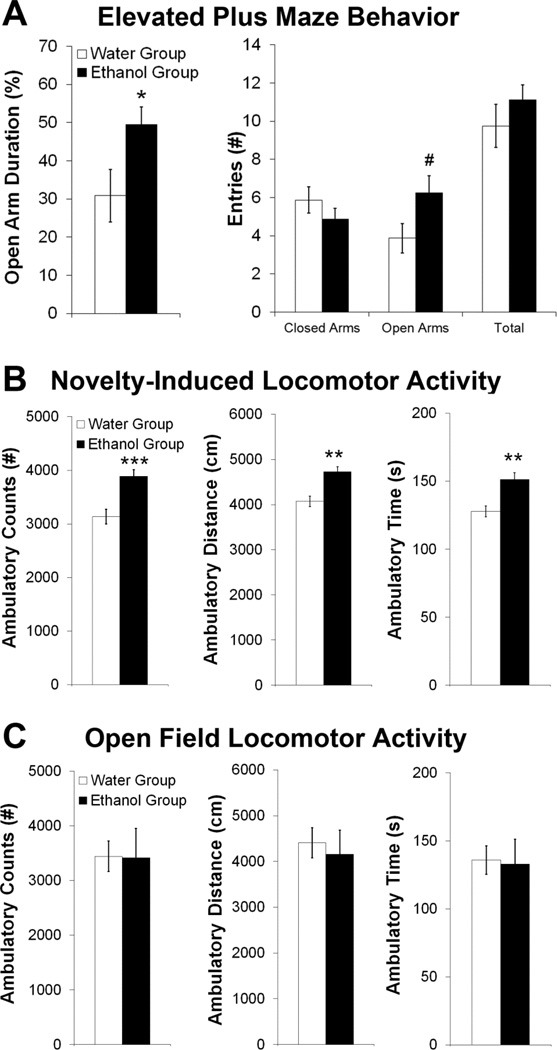
Following 30 minutes of daily ethanol (or water) access, the Ethanol and Water drinking groups showed differences in emotional behavior. In the elevated plus maze (Set 1), there was a significant main effect of group [F(1,14) = 4.51, p < 0.01] and a significant group×behavior interaction [F(3,42) = 5.05, p < 0.01]. The Ethanol rats displayed anti-anxiety behavior (Fig. 2A), as reflected by their greater percentage of time spent on the open arms (p < 0.05) and tendency toward more frequent open arm entries (p = 0.06). However, the groups exhibited no differences in spontaneous motor behavior, as assessed by closed and total arm entries. In the novel open field (Set 2), there was also a significant main effect of group [F(1,14) = 17.30, p < 0.01] and a significant group×behavior interaction [F(2,28) = 15.35, p < 0.001]. The Ethanol rats showed greater novelty-induced locomotor activity, as indicated by all three measures, ambulatory counts, distance, and time (p < 0.01) (Fig. 2B). In contrast, in a familiar open field (Set 1), there was no main effect of group [F(1,14) = 0.05, ns] nor a group×behavior interaction [F(2,28) = 0.16, ns] (Fig. 2C). Together, these findings indicate that rats, when maintained on an intermittent-access ethanol paradigm, exhibit changes in anxiety and novelty-induced locomotor activity that reflect the pharmacological actions of this drug.
Figure 2.
Intermittent-access 20% ethanol drinking alters emotional behavior after 30 minutes of access. A. The Ethanol compared to Water group showed reduced anxiety in an elevated plus maze, as indicated by increased time and number of entries in the open arm, but no difference in spontaneous locomotor behavior, as assessed by closed and total arm entries (Set 1, n = 8/group; Week 5 of ethanol access). B. The Ethanol group showed increased novelty-induced locomotor activity in an open field, as indicated by ambulatory counts, distance, and time (Set 2, n = 8/group; Week 4). C. The Ethanol and Water groups showed no significant differences in locomotor activity in a familiar open field, as assessed by ambulatory counts, distance, and time (Set 1, n = 8/group; Week 4 of ethanol access). ***p < 0.001, **p < 0.01, *p < 0.05, #p = 0.06 vs. Water Group.
Experiment 2: Anterior PVT is activated in association with ethanol drinking
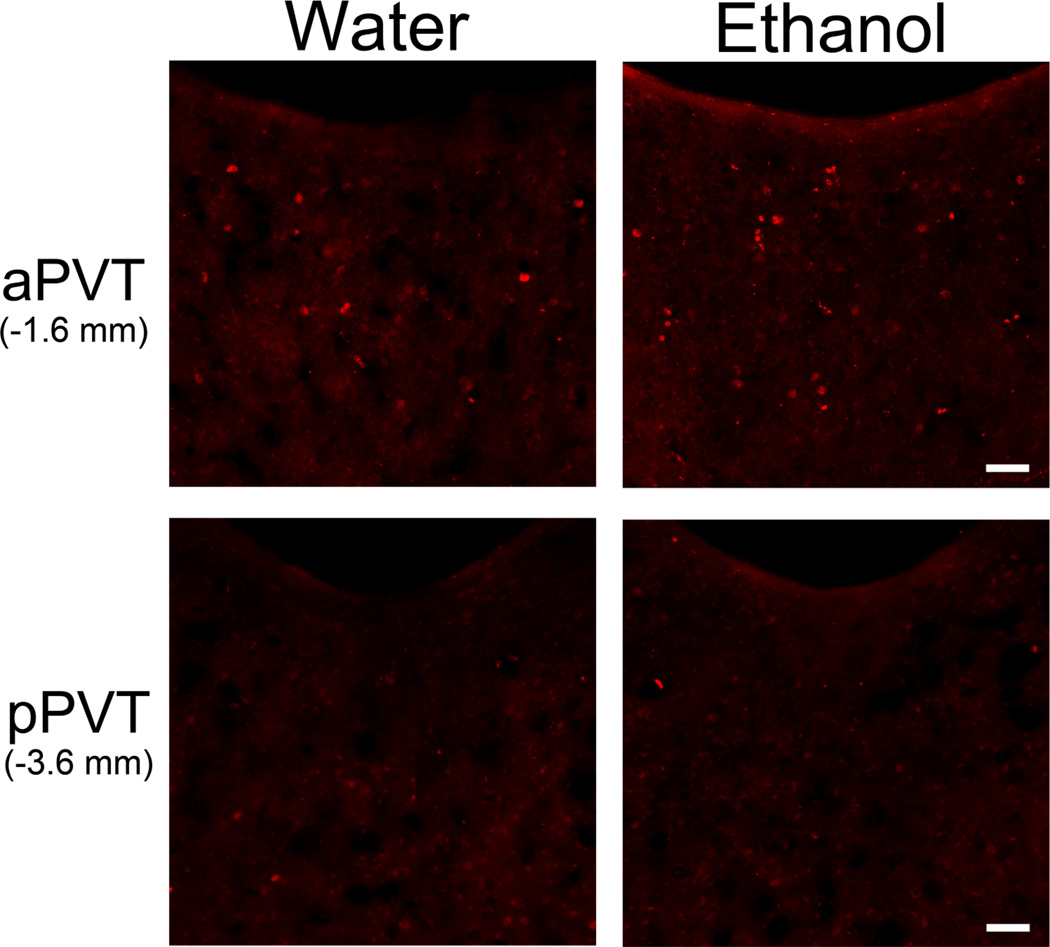
In comparing c-Fos immunoreactive (IR) neurons in Ethanol and Water drinking groups (Set 2), analysis of the PVT as a whole revealed a greater number of c-Fos-IR neurons in the Ethanol group (p < 0.05) (Table 1). Examination of PVT subregions also revealed a significant main effect of group [F(1,14) = 12.66, p < 0.01], which reflected in the Ethanol rats a small but significantly greater number of c-Fos-IR cells in the aPVT (p < 0.05) but no change in the pPVT (ns) (Table 1, Fig. 3). Ethanol intake on the day of sacrifice was significantly, positively correlated with the number of c-Fos-IR neurons, specifically in the aPVT (r = +0.60, p < 0.05) but not the pPVT (r = −0.43, ns). This result focuses attention on neuronal activity in the anterior region of the PVT in relation to voluntary ethanol drinking.
Table 1.
Intermittent access 20% ethanol drinking increases the number of c-Fos-immunoreactive (c-Fos-IR) nuclei in the paraventricular thalamus (PVT), specifically in the anterior PVT (aPVT) but not the posterior PVT (pPVT) (Set 2, n = 8/group; Week 6 of access). Numbers are average per group of total number of c-Fos-positive nuclei per rat.
| Brain Region | Number of c-Fos-IR cells | |
|---|---|---|
| Water | Ethanol | |
| PVT | 52 ± 4 | 64 ± 3* |
| aPVT | 30 ± 2 | 43 ± 5* |
| pPVT | 22 ± 2 | 21 ± 4 |
p < 0.05 vs. Water group.
Figure 3.
Photomicrographs showing increased number c-Fos-positive cells (red) in the anterior paraventricular thalamus (aPVT) but not the posterior paraventricular thalamus (pPVT) 90 minutes after the start of access to 20% ethanol compared to water (Set 2, n = 8/group; Week 6 of access). Scale bars = 50 µm.
Experiment 3: Anterior PVT is similarly activated with ethanol administration
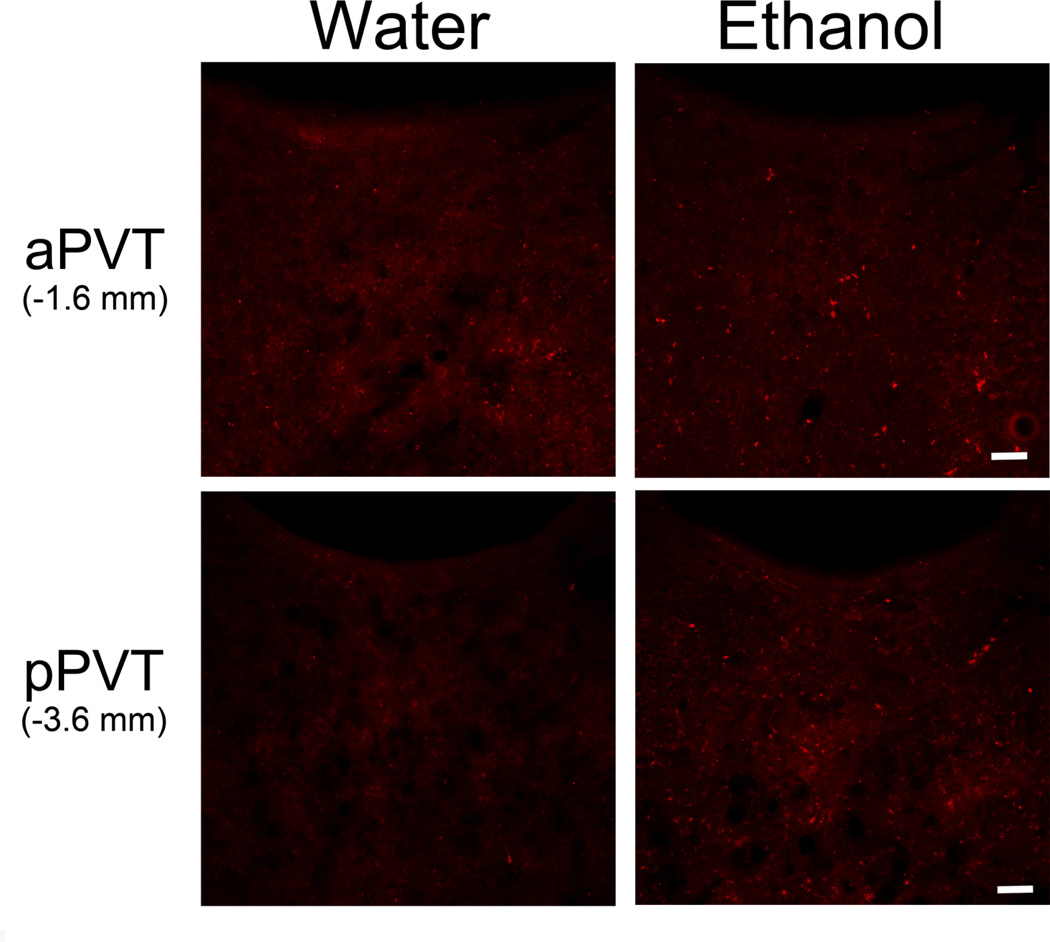
To ensure that PVT activation was in fact due to ethanol exposure, a new group of rats (Set 3) was orally gavaged with ethanol at levels matching those of voluntary intake. The Ethanol gavage rats, 30 minutes after treatment, showed BEC values within the same range as those of the ethanol drinkers. Specifically, the Low Ethanol gavage group had BEC values (54 ± 6 mg%) similar to the Ethanol drinking rats, while the High Ethanol gavage had values (92 ± 16 mg%) that were somewhat higher. In comparing c-Fos-IR neurons in Ethanol and Water gavage and Control (non-gavaged) groups, analysis of the PVT as a whole revealed a main effect of group on c-Fos-IR neurons [F(3,20) = 24.81, p < 0.001], due to a greater number of labeled neurons in the High and Low Ethanol groups compared to the Water and Control groups (p < 0.05) and also in the High compared to Low Ethanol group (p < 0.01) (Table 2). Examination of PVT subregions also revealed a significant interaction effect between group and subregion [F(3,20) = 24.28, p < 0.001], due to a significant effect in the aPVT [F(3,20) = 54.74, p < 0.001] but not pPVT [F(3,20) = 2.87, ns], similar to the findings obtained with the voluntary ethanol drinkers (Table 2, Fig. 4). Post-hoc tests revealed that, in the aPVT, both High and Low Ethanol groups had a significantly greater number of c-Fos-IR neurons than Water and Control groups (p < 0.001), while the High Ethanol group also had a greater number than the Low Ethanol group (p < 0.01). These results again focus attention on neuronal activity in the aPVT, this time in relation to oral ethanol administration, and suggest that this stimulatory effect of ethanol intake is due to ethanol itself and not specifically to the process of voluntary consumption.
Table 2.
Ethanol gavage increases the number of c-Fos-immunoreactive (c-Fos-IR) nuclei in the paraventricular thalamus (PVT), specifically in the anterior PVT (aPVT) but not the posterior PVT (pPVT) (Set 3, n = 6/group; 11 treatments). Numbers are average per group of total number of c-Fos-positive nuclei. Control = no gavage; Water = water gavage; Low Ethanol = 0.75 g/kg ethanol per gavage; High Ethanol = 1.50 g/kg ethanol per gavage.
| Brain Region | Number of c-Fos-IR Cells | |||
|---|---|---|---|---|
| Control | Water | Low Ethanol | High Ethanol | |
| PVT | 34 ± 2 | 27 ± 3 | 53 ± 5**+ | 76 ± 6***+++## |
| aPVT | 16 ± 2 | 14 ± 3 | 38 ± 2***+++ | 52 ± 3***+++## |
| pPVT | 17 ± 2 | 13 ± 1 | 15 ± 4 | 24 ± 3 |
p < 0.001 vs. Water group;
p < 0.01 vs. Water group;
p < 0.001 vs. Control group;
p < 0.05 vs. Control group;
p < 0.01 vs. Low Ethanol group.
Figure 4.
Photomicrographs showing increased number c-Fos-positive cells (red) in the anterior paraventricular thalamus (aPVT) but not the posterior paraventricular thalamus (pPVT) 90 minutes after oral 20% ethanol (1.50 g/kg/gavage) compared to water gavage (Set 3, n = 6/group; 11 treatments). Similar results were obtained when comparing the High to Low Ethanol groups (0.75 g/kg) and the Water to Control groups (images of Low Ethanol and Control groups not shown). Scale bars = 50 µm.
Experiment 4: Ethanol drinking is associated with increased orexin in hypothalamus and upregulated orexin 2 receptor in anterior PVT
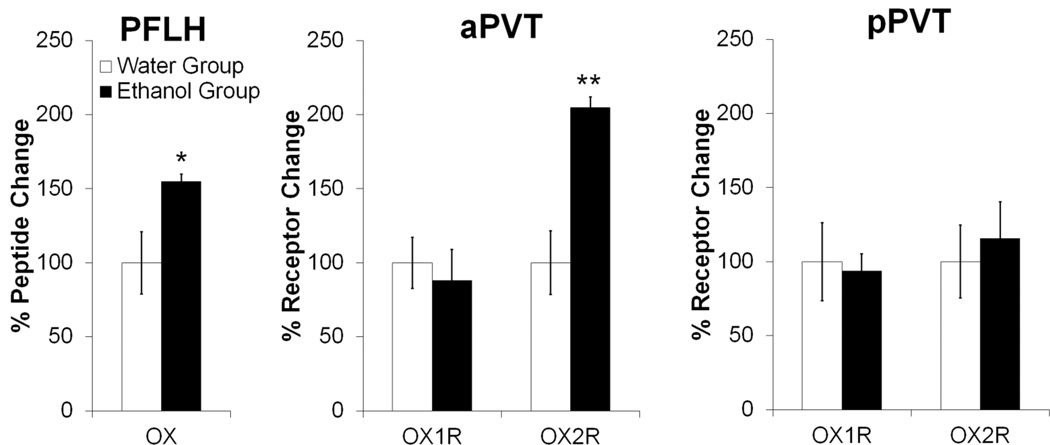
Using qRT-PCR to determine if ethanol drinking might affect the activity of OX in the PVT (Set 1), a significant increase in OX mRNA was observed in the PFLH of the Ethanol compared to Water drinking group (p < 0.05) (Fig. 5), which was positively correlated with ethanol intake on day of sacrifice (r = +0.56, p < 0.05). A similar main effect of group [F(1,14) = 8.11, p < 0.05] and a group × gene expression interaction [F(1,14) = 16.39, p < 0.01] were also observed with measurements of peptide receptors in the aPVT, but not the pPVT [F(1,14) = 0.13, ns] where Ethanol and Water groups showed no significant difference in gene expression of OX1R or OX2R (Fig. 5). This effect in the aPVT was due to an increase specifically in OX2R (p < 0.05), which was positively correlated with ethanol intake on day of sacrifice (r = +0.62, p < 0.05), with no difference detected in OX1R (ns). Thus, in addition to a greater number of Fos-IR neurons, the aPVT exhibits an increase in OX2R mRNA in association with the drinking of ethanol.
Figure 5.
Ethanol drinking increases gene expression of orexin in the perifornical lateral hypothalamus and the orexin 2 receptor in the anterior paraventricular thalamus, as assessed by quantitative real-time polymerase chain reaction 30 minutes after the start of access to 20% ethanol compared to water (Set 1, n = 8/group; Week 7 of ethanol access). Target gene expression was quantified relative to cyclophilin using the relative quantification method. Abbreviations: aPVT, anterior paraventricular thalamus; pPVT, posterior paraventricular thalamus; OX, orexin/hypocretin; OX1R, orexin 1 receptor; OX2R, orexin 2 receptor; PFLH, perifornical lateral hypothalamus. **p < 0.01, *p < 0.05 vs. Water group.
Experiment 5: Orexin 2 receptor is located on activated neurons in the PVT
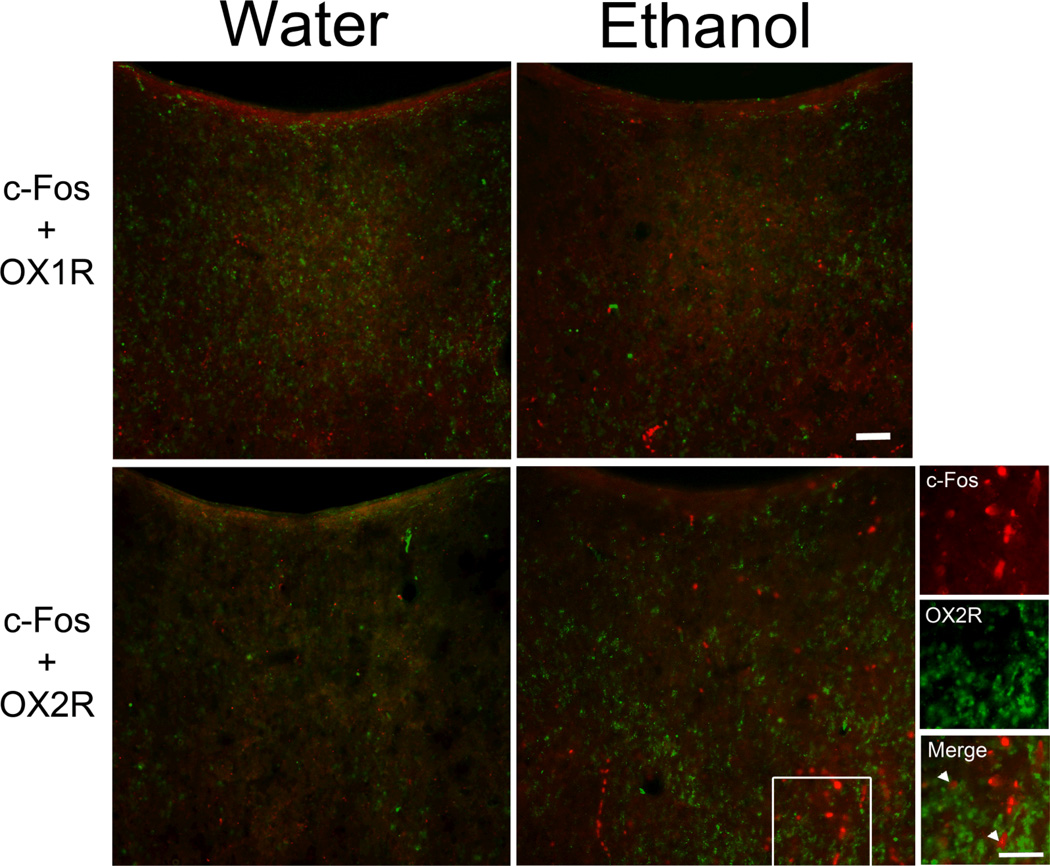
To determine if PVT neuronal activity linked to ethanol intake is related specifically to OX2R receptor function, the PVT subregions of Ethanol and Water gavage and Control groups were double-labeled for c-Fos with the OX receptors (Set 3). While the percentage of c-Fos-IR neurons double-labeled with OX1R after ethanol gavage showed no significant main effect across the PVT as a whole [F(3,20) = 1.51, ns] or a significant group × PVT subregion interaction [F(3,20) = 0.50, ns] (Table 3), the percentage of c-Fos-IR neurons double-labeled with OX2R revealed a main effect of group [F(3,20) = 15.85, p < 0.001]. Both the High and Low Ethanol groups showed greater c-Fos-IR+OX2R double-labeling in the PVT overall when compared to the Water and Control groups (p < 0.05) (Table 3). In addition, there was a significant interaction effect on OX2R double-labeling between group and PVT subregion [F(3,20) = 7.48, p < 0.01], with group differences found in the aPVT [F(3,20) = 28.02, p < 0.001] but not pPVT [F(3,20) = 0.10, ns]. Post-hoc tests revealed that, in the aPVT, both High and Low Ethanol groups had a significantly greater percentage of c-Fos-IR neurons double-labeled with OX2R compared to both the Water and Control groups (p < 0.05) (Fig. 6). Thus, PVT neurons activated in association with ethanol intake are more likely to label for OX2R, and this OX2R-induced activation is most likely to occur in the aPVT.
Table 3.
Ethanol gavage increases the percentage of c-Fos-immunoreactive (c-Fos-IR) neurons that double-label with the orexin 2 receptor (OX2R) in the anterior paraventricular thalamus (aPVT), with no such effect detected in the posterior PVT (pPVT). It does not significantly affect double-labeling of c-Fos with the orexin 1 receptor (OX1R). Control = no gavage; Water = water gavage; Low Ethanol = 0.75 g/kg ethanol per gavage; High Ethanol = 1.50 g/kg ethanol per gavage.
| Brain Region | % c-Fos+OX1R Neurons | |||
| Control | Water | Low Ethanol | High Ethanol | |
| PVT | 3.5 ± 0.4 | 5.7 ± 0.4 | 5.3 ± 1.2 | 5.3 ± 0.9 |
| aPVT | 3.5 ± 0.8 | 5.2 ± 0.7 | 4.5 ± 1.4 | 4.5 ± 0.7 |
| pPVT | 3.1 ± 1.0 | 6.3 ± 1.3 | 6.4 ± 1.6 | 7.0 ± 1.6 |
| % c-Fos+OX2R Neurons | ||||
| PVT | 6.4 ± 0.7 | 5.9 ± 0.4 | 10.1 ± 1.1**+ | 13.0 ± 1.0***+++ |
| aPVT | 6.5 ± 1.3 | 5.6 ± 1.2 | 15.9 ± 1.1***+++ | 18.0 ± 1.2***+++ |
| pPVT | 6.4 ± 1.8 | 5.7 ± 1.4 | 5.0 ± 2.5 | 5.7 ± 1.3 |
p < 0.001 vs. Water group;
p < 0.01 vs. Water group;
p < 0.05 vs. Water group;
p < 0.001 vs. Control group;
p < 0.01 vs. Control group;
p < 0.05 vs. Control group.
Figure 6.
Photomicrographs showing greater double-labeling of c-Fos immunoreactivity with the orexin 2 receptor (OX2R) but not orexin 1 receptor (OX1R) in the anterior paraventricular thalamus (aPVT) 90 minutes after oral 20% ethanol (1.50 g/kg/gavage) compared to water gavage (Set 3, n = 6/group; 11 treatments). Similar results were obtained when comparing the High to Low Ethanol groups (0.75 g/kg) and the Water to Control groups (images of Low Ethanol and Control groups not shown). c-Fos-positive cells are labeled in red, and receptor-positive cells are labeled in green. Images on the far right are higher magnifications of image marked with a white square. Arrowheads indicate double-labeled neurons. Scale bars = 50 µm.
Experiment 6: Orexin agonists in anterior PVT promote ethanol but not sucrose drinking
To investigate the behavioral function of the OX receptors in the PVT, rats were injected with OX agonists in the aPVT or pPVT. Histological examination showed that aPVT injections were made between bregma −1.56 and −2.04 mm, and pPVT injections were between −3.00 and −3.48 mm (Fig. S1). Analyses of the diffusion of 0.3 µl methylene blue dye after injection in one subregion showed that it never reached the other subregion, having a radial spread of approximately 0.5 mm.
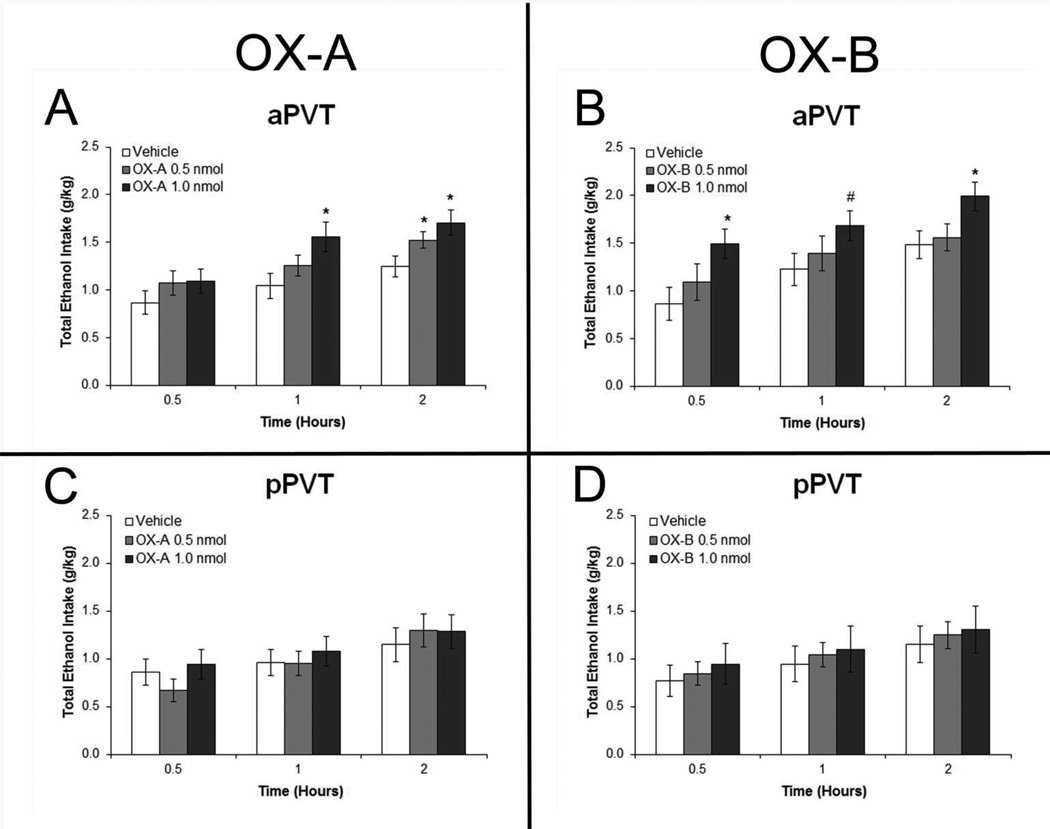
Results with ethanol drinkers injected with OX agonists in the aPVT (Set 4) revealed a significant main effect on ethanol intake from both OX-A [F(2,14) = 4.61, p < 0.05] and OX-B [F(2,14) = 4.45, p < 0.05] (Fig.7A and 7B). Within-subject contrasts revealed a significant linear trend for dose with both OX-A [F(1,7) = 12.00, p < 0.05] and OX-B [F(1,7) = 11.04, p < 0.05], indicating that the drinking responses were dose-dependent. Pairwise comparisons revealed that, with injection in the aPVT, the higher dose of OX-A significantly stimulated ethanol intake at 1 hour and 2 hours post-injection (p < 0.05), while the lower dose stimulated it only at 2 hours (p < 0.05), and also the higher dose of OX-B significantly stimulated ethanol intake at 30 minutes and 2 hours (p < 0.05), with a trend for an increase at 1 hour post-injection (p = 0.06). In contrast, with injection in the pPVT, there were no significant main effects on ethanol intake after either OX-A [F(2,12) = 0.31, ns] or OX-B [F(2,12) = 0.44, ns] (Fig.7C and 7D). There were also no significant main effects of aPVT injections of these agonists on water (OX-A: [F(2,14) = 0.47, ns]; OX-B: [F(2,14) = 0.28, ns]) or food intake (OX-A: [F(2,14) = 0.62, ns]; OX-B: [F(2,14) = 1.09, ns]) or of pPVT injections on water (OX-A: [F(2,12) = 0.50, ns]; OX-B: [F(2,12) = 2.17, ns]) or food intake (OX-A: [F(2,12) = 0.10, ns]; OX-B: [F(2,12) = 1.68, ns]) (Fig. S2).
Figure 7.
Injection of orexin-A (OX-A) and orexin-B (OX-B) in the anterior paraventricular thalamus (aPVT) but not posterior paraventricular thalamus (pPVT) increases intermittent-access 20% ethanol drinking. A. Ethanol drinking in rats injected in the aPVT with 0.3 µl OX-A (1.0, 0.5 nmol) or saline vehicle (Set 4, n = 8). B. Drinking in rats injected in the aPVT with 0.3 µl OX-B (1.0, 0.5 nmol) or saline vehicle (Set 4, n = 8). C. Ethanol drinking in rats injected in the pPVT with OX-A or saline vehicle (Set 4, n = 7). D. Drinking in rats injected in the pPVT with OX-B or saline vehicle (Set 4, n = 7). Effects were no longer evident at four hours post-injection (data not shown). *p < 0.05, #p = 0.06 vs. vehicle.
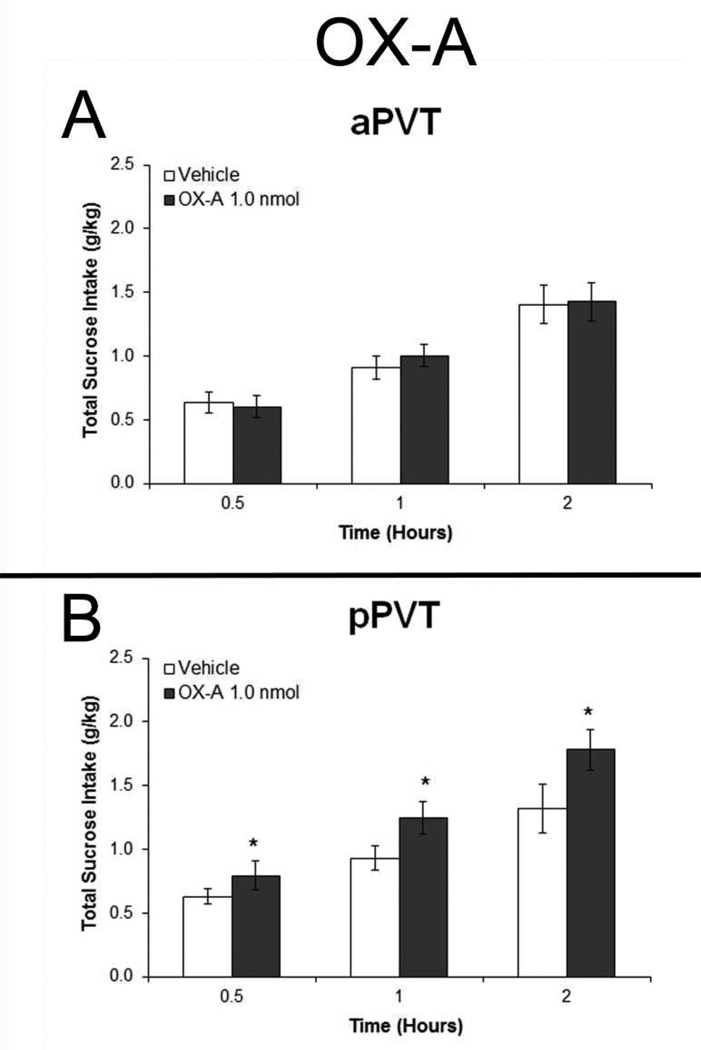
In contrast to ethanol intake, measurements in rats given sucrose to drink (Set 5) failed to reveal any significant main effects of aPVT injections of OX on the consumption of sucrose [F(1,9) = 0.54, ns], water [F(1,9) = 1.26, ns] or food [F(1,9) = 1.15, ns] (Fig. 8A and S3A). In the pPVT, however, a significant main effect of OX on sucrose drinking was observed [F(1,9) = 7.09, p < 0.05]. Pairwise comparisons showed that pPVT OX-A significantly stimulated intake at 30 minutes, 1 hour, and 2 hours after injection (p < 0.05) (Fig. 8B) but had no effect on intake of water [F(1,9) = 1.41, ns] or food [F(1,9) = 0.25, ns] (Fig. S3B). Together, these results with local injections link OX activity in the aPVT to the consumption of ethanol, but OX in the pPVT to the consumption of sucrose.
Figure 8.
Injection of orexin-A (OX-A) in the anterior paraventricular thalamus (aPVT) has no effect on intermittent-access 2% sucrose drinking but injection in the posterior paraventricular thalamus (pPVT) increases sucrose intake. A. Sucrose drinking in rats injected in the aPVT with 0.3 µl OX-A (1.0 nmol) or saline vehicle (Set 5, n = 10). B. Drinking in rats injected in the pPVT with OX-A or vehicle (Set 5, n = 10). Effects were no longer evident at four hours post-injection (data not shown). *p < 0.05 vs. vehicle.
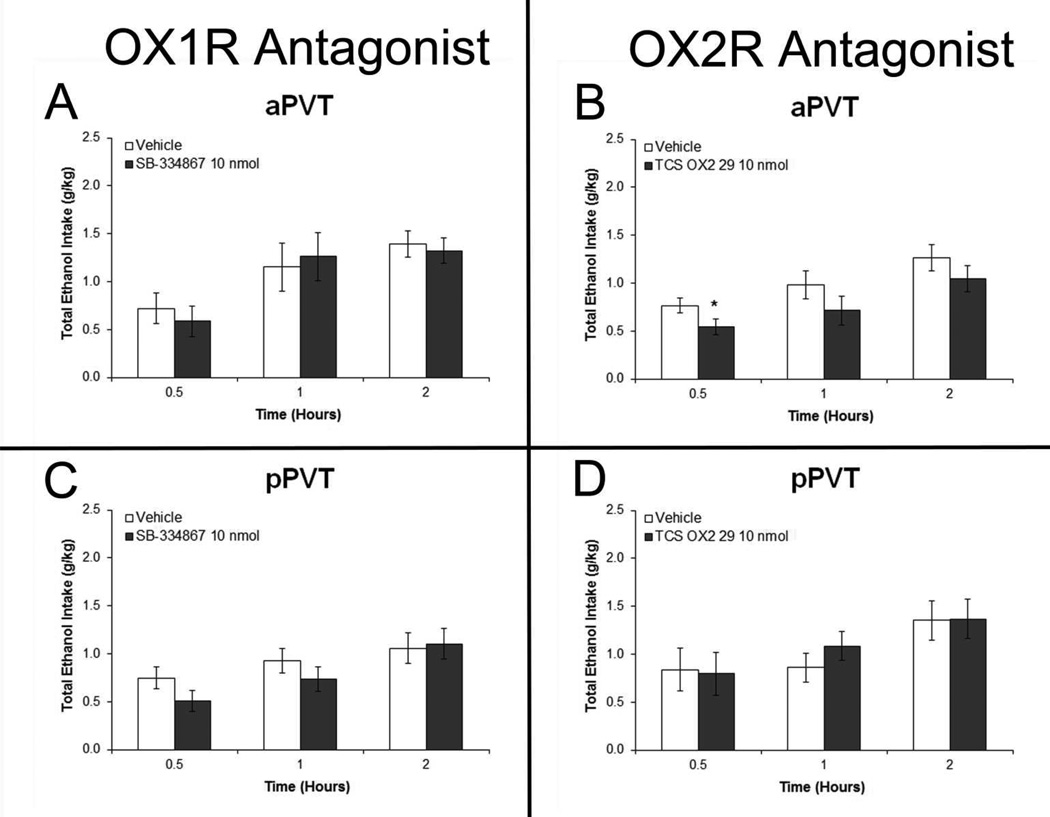
Experiment 7: Orexin 2 receptor antagonist in anterior PVT reduces ethanol drinking
In rats injected with OX antagonists (Set 6), results in the aPVT revealed a trend for a main effect of drug on ethanol intake with the OX2R antagonist TCS OX2 29 [F(1,5) = 4.92, p = 0.07] and a significant drug×time interaction [F(3,15) = 3.49, p < 0.05] (Fig. 9B). As revealed by pairwise comparisons, this effect in the aPVT was due to a significant decrease in ethanol drinking at 30 minutes after injection of TCS OX2 29 (p < 0.05). In contrast, there was no significant main effect on ethanol intake after injection of the OX1R antagonist SB 334867 in the aPVT [F(1,5) = 0.16, ns] (Fig. 9A). With injections in the pPVT, there were no significant main effects of drug on ethanol intake, after SB 334867 [F(1,5) = 0.00, ns] or TCS OX2 29 [F(1,5) = 0.11, ns] (Figs. 9C and 9D). Also, with measurements of water and food intake, there were no significant main effects after aPVT injection of SB 334867 (water: [F(1,5) = 0.25, ns]; food: [F(1,5) = 0.31, ns]) or TCS OX2 29 (water: [F(1,5) = 2.25, ns]; food: [F(1,5) = 2.40, ns]) or after pPVT injection of SB 334867 (water: [F(1,5) = 0.48, ns]; food: [F(1,5) = 0.60, ns]) or TCS OX2 29 (water: [F(1,5) = 1.54, ns]; food: [F(1,5) = 0.77, ns]) (Fig. S4). These results focus attention on OX2R rather than OX1R in the aPVT in mediating the effect of OX on the consumption of ethanol.
Figure 9.
Blockade of orexin 2 receptor (OX2R) but not orexin 1 receptor (OX1R) activity in the anterior paraventricular thalamus (aPVT) but not posterior paraventricular thalamus (pPVT) decreases intermittent-access 20% ethanol drinking. A. Ethanol drinking in rats injected in the aPVT with 0.3 µl of the OX1R antagonist SB 334867 (10 nmol) or dimethyl sulfoxide vehicle (Set 5, n = 6). B. Drinking in rats injected in the aPVT with 0.3 µl of the OX2R antagonist TCS OX2 29 (10 nmol) or saline vehicle (Set 5, n = 6). C. Ethanol drinking in rats injected in the pPVT with SB 334867 or vehicle (Set 5, n = 6). D. Drinking in rats injected in the pPVT with TCS OX2 29 or vehicle (Set 5, n = 6). Effects were no longer evident at four hours post-injection (data not shown). *p < 0.05 vs. vehicle.
DISCUSSION
Intermittent access to ethanol induces pharmacologically-relevant drinking
Under the intermittent-access 20% ethanol drinking paradigm, Long-Evans rats consumed ethanol at relatively high and stable levels, with intake sufficient to change their behavior, possibly via direct actions of ethanol on the brain. These rats showed substantial intake during the first 30 minutes of access, which increased BEC to levels considered pharmacologically-relevant (Bell et al., 2006). They also showed reduced behavioral inhibition, indicated by decreased anxiety in an elevated plus maze and increased locomotor activity in a novel open field. These findings strengthen the possibility that the rats were drinking ethanol for its reinforcing, post-ingestive effects. Similar changes in behavior have been observed in Long-Evans rats after limited access drinking (2 h/day) (Sharko et al., 2013) and in ethanol-preferring AA rats after experimenter-administered ethanol (Paivarinta and Korpi, 1993). With brain ethanol concentration known to peak around 9 minutes after oral gavage of 0.5 g/kg ethanol (Quertemont et al., 2003), the behavioral changes observed in the present study after 30 minutes of drinking approximately 0.7 g/kg ethanol may reflect its actions in the brain.
Anterior PVT is activated in association with ethanol intake
This pharmacologically-relevant ethanol intake in the present study was associated with neuronal activation within the PVT, as indicated by a greater number of neurons labeled for the immediate early gene c-Fos 90 minutes after ethanol access or administration. This is consistent with previous findings that four days of injections of ethanol increases the expression of c-Fos in the PVT (Ryabinin and Wang, 1998), although this effect is diminished after 14 days of treatment (Ryabinin et al., 1997). The present results provide the first evidence that voluntary drinking of ethanol can similarly activate the PVT and that this effect, as with oral ethanol gavage at comparable levels, is regionally-specific, with ethanol increasing c-Fos in the aPVT but not the pPVT. The apparently greater increase in c-Fos labeling observed after ethanol gavage compared to ethanol drinking may be due either to a rapid PVT activation produced by the ethanol bolus in the gavage Ethanol Groups or to a higher baseline produced by the act of drinking in the voluntary Water Group. Of interest is that the difference between the subregions in their responsiveness to ethanol may be related to their behavioral functions. Supporting its role in ethanol reinforcement, studies of the aPVT suggest that it participates in incentive salience (Flagel et al., 2011) and circadian rhythm-induced drinking (Salazar-Juarez et al., 2002). In contrast, the pPVT is suggested to play a role in negative emotional arousal (Heydendael et al., 2011; Li et al., 2009).
The orexin 2 receptor may mediate ethanol-induced activation of the anterior PVT
The present results support the involvement of the OX system in the ethanol-induced enhancement of c-Fos in the aPVT. The increased gene expression of OX in the PFLH of the 20% intermittent Ethanol group, consistent with previous studies of chronic ethanol drinking in alcohol-preferring iP rats (Lawrence et al., 2006) and acute ethanol gavage in Sprague-Dawley rats (Morganstern et al., 2010), is associated here with elevated gene expression of OX2R in the aPVT, but not the pPVT. With evidence showing OX receptors to be upregulated after repeated stimulation by OX (Zhang et al., 2007), this increased OX2R mRNA in the aPVT after ethanol drinking might reflect the release of OX peptide into this PVT subregion. The possibility that PVT neurons are activated to a greater extent by stimulation of the OX2R than the OX1R is partially supported by our own findings, in addition to published evidence using PVT slices which shows that the application of OX-B alone, which targets OX2R, can increase neuronal firing rate to about the same extent as OX-B together with OX-A, which additionally targets OX1R (Kolaj et al., 2007). The present result, revealing increased double-labeling of c-Fos with OX2R protein in the aPVT of the ethanol groups, further supports the idea that OX2R binding in this PVT subregion can activate local neurons following ethanol intake.
Activation of orexin receptors in the anterior PVT specifically promotes ethanol drinking
With OX from the PFLH possibly binding to its receptors in the aPVT after ethanol drinking, the next question is what effect this OX-induced neuronal activation has on subsequent drinking. The idea that OX receptor binding in this PVT subregion stimulates further ethanol intake is supported by our finding that this behavior occurs after injection of either OX-A or OX-B, specifically in the aPVT but not the pPVT. The additional finding, that an antagonist against the OX2R but not the OX1R in the aPVT significantly reduces ethanol drinking, strengthens the idea that these effects are due to endogenous OX2R activation. The relatively short duration of this OX2R antagonist effect is likely due to the fast dissociation rate of this particular compound (Mould et al., 2014). The possibility that the enhanced OX2R activity in the aPVT induced by ethanol promotes further ethanol consumption is consistent with published evidence showing that ventricular injection of this same OX2R antagonist reduces the self-administration of ethanol but not sucrose (Brown et al., 2013). Although these behavioral changes produced by microinjections may result in part from leakage into the dorsal third ventricle adjacent to the PVT, the finding that they occurred only with one subregion but not the other suggests that leakage was minimal, providing support for anatomical specificity.
With the effect of OX receptor activation in the aPVT being specific to the drinking of ethanol and not evident with the intake of food or water, it is particularly notable that OX receptor activation in the pPVT, while having no relation to ethanol drinking, has a specific stimulatory effect on the drinking of sucrose. This unexpected phenomenon in the pPVT parallels a recent finding that PVT OX1R knockdown, apparently occurring primarily in the pPVT, has the opposite effect of OX injection, significantly reducing the consumption of a high-fat diet (Choi et al., 2012). Future research should determine if sucrose drinking or fat consumption also increases c-Fos expression in the pPVT rather than the aPVT. Together, the present findings support a role for the OX1R in the pPVT in hedonic feeding, in contrast to the OX2R in the aPVT which has a particular role in stimulating intake of drugs of abuse like ethanol.
Summary and conclusions
The intermittent-access 20% ethanol two-bottle-choice paradigm resulted in pharmacologically-relevant drinking, reflected in behavioral changes related to reinforcement. This ethanol drinking or administration at comparable levels was associated with increased OX gene expression in the PFLH and increased neuronal activation in the aPVT along with upregulated OX2R mRNA. The evidence, that OX agonists in the aPVT in contrast to the pPVT specifically increase ethanol drinking while an OX2R antagonist reduces it, suggests that endogenous ligand binding at this particular receptor in this PVT subregion has a role in promoting voluntary ethanol consumption. We propose that the aPVT shows a positive-feedback relationship with ethanol drinking, with ethanol drinking inducing hypothalamic OX to act in the aPVT at the OX2R that stimulates neurons in this PVT subregion and further perpetuates the ethanol drinking cycle.
Supplementary Material
Acknowledgements
This research was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Numbers R01AA12882 (SFL) and K99AA021782 (JRB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We extend gratitude to Wilson Ng, Shawn Fagan, Karen Chen and Sherry Liang for technical assistance and to The Rockefeller University’s Bioimaging and Genomics Resource Centers for use of their equipment.
Footnotes
Authors Contribution
J.R.B and S.F.L. were responsible for the study concepts and design. J.R.B. and H.T.H. collected and analyzed the data. J.R.B. drafted the manuscript and prepared the figures. J.R.B and S.F.L. edited and revised the manuscript. All authors critically reviewed the content and approved the final version for publication.
REFERENCES
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Brown RM, Yon-Seng Khoo S, Lawrence AJ. Central orexin (hypocretin) 2 receptor antagonism reduces ethanol self-administration, but not cue-conditioned ethanol-seeking, in ethanol-preferring rats. Int J Neuropsychopharmacol. 2013:1–13. doi: 10.1017/S1461145713000333. [DOI] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Magrisso IJ, Fitzgerald ME, Lipton JW, Benoit SC. Orexin signaling in the paraventricular thalamic nucleus modulates mesolimbic dopamine and hedonic feeding in the rat. Neuroscience. 2012;210:243–248. doi: 10.1016/j.neuroscience.2012.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry. 2008;63:152–157. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Cameron CM, Pickup KN, Watson SJ, Akil H, Robinson TE. A food predictive cue must be attributed with incentive salience for it to induce c-fos mRNA expression in cortico-striatal-thalamic brain regions. Neuroscience. 2011;196:80–96. doi: 10.1016/j.neuroscience.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, Choi EA, McNally GP. Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. Eur J Neurosci. 2009;29:802–812. doi: 10.1111/j.1460-9568.2009.06623.x. [DOI] [PubMed] [Google Scholar]
- Heydendael W, Sharma K, Iyer V, Luz S, Piel D, Beck S, Bhatnagar S. Orexins/hypocretins act in the posterior paraventricular thalamic nucleus during repeated stress to regulate facilitation to novel stress. Endocrinology. 2011;152:4738–4752. doi: 10.1210/en.2011-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose M, Egashira S, Goto Y, Hashihayata T, Ohtake N, Iwaasa H, Hata M, Fukami T, Kanatani A, Yamada K. N-acyl 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline: the first orexin-2 receptor selective non-peptidic antagonist. Bioorg Med Chem Lett. 2003;13:4497–4499. doi: 10.1016/j.bmcl.2003.08.038. [DOI] [PubMed] [Google Scholar]
- Kim AK, Brown RM, Lawrence AJ. The role of orexins/hypocretins in alcohol use and abuse: an appetitive-reward relationship. Front Behav Neurosci. 2012;6:78. doi: 10.3389/fnbeh.2012.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirouac GJ, Parsons MP, Li S. Orexin (hypocretin) innervation of the paraventricular nucleus of the thalamus. Brain Res. 2005;1059:179–188. doi: 10.1016/j.brainres.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Kolaj M, Doroshenko P, Yan Cao X, Coderre E, Renaud LP. Orexin-induced modulation of state-dependent intrinsic properties in thalamic paraventricular nucleus neurons attenuates action potential patterning and frequency. Neuroscience. 2007;147:1066–1075. doi: 10.1016/j.neuroscience.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Bian W, Dave V, Ye JH. Blockade of GABA(A) receptors in the paraventricular nucleus of the hypothalamus attenuates voluntary ethanol intake and activates the hypothalamic-pituitary-adrenocortical axis. Addict Biol. 2011a;16:600–614. doi: 10.1111/j.1369-1600.2011.00344.x. [DOI] [PubMed] [Google Scholar]
- Li S, Kirouac GJ. Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol. 2008;506:263–287. doi: 10.1002/cne.21502. [DOI] [PubMed] [Google Scholar]
- Li Y, Li S, Sui N, Kirouac GJ. Orexin-A acts on the paraventricular nucleus of the midline thalamus to inhibit locomotor activity in rats. Pharmacol Biochem Behav. 2009;93:506–514. doi: 10.1016/j.pbb.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang H, Qi K, Chen X, Li S, Sui N, Kirouac GJ. Orexins in the midline thalamus are involved in the expression of conditioned place aversion to morphine withdrawal. Physiol Behav. 2011b;102:42–50. doi: 10.1016/j.physbeh.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Boutrel B. Orexin/hypocretin (Orx/Hcrt) transmission and drug-seeking behavior: is the paraventricular nucleus of the thalamus (PVT) part of the drug seeking circuitry? Front Behav Neurosci. 2012;6:75. doi: 10.3389/fnbeh.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganstern I, Chang GQ, Barson JR, Ye Z, Karatayev O, Leibowitz SF. Differential effects of acute and chronic ethanol exposure on orexin expression in the perifornical lateral hypothalamus. Alcohol Clin Exp Res. 2010;34:886–896. doi: 10.1111/j.1530-0277.2010.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould R, Brown J, Marshall FH, Langmead CJ. Binding kinetics differentiates functional antagonism of orexin-2 receptor ligands. Br J Pharmacol. 2014;171:351–363. doi: 10.1111/bph.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paivarinta P, Korpi ER. Voluntary ethanol drinking increases locomotor activity in alcohol-preferring AA rats. Pharmacol Biochem Behav. 1993;44:127–132. doi: 10.1016/0091-3057(93)90289-6. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quertemont E, Green HL, Grant KA. Brain ethanol concentrations and ethanol discrimination in rats: effects of dose and time. Psychopharmacology (Berl) 2003;168:262–270. doi: 10.1007/s00213-003-1437-7. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Criado JR, Henriksen SJ, Bloom FE, Wilson MC. Differential sensitivity of c-Fos expression in hippocampus and other brain regions to moderate and low doses of alcohol. Mol Psychiatry. 1997;2:32–43. doi: 10.1038/sj.mp.4000206. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Wang YM. Repeated alcohol administration differentially affects c-Fos and FosB protein immunoreactivity in DBA/2J mice. Alcohol Clin Exp Res. 1998;22:1646–1654. [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Salazar-Juarez A, Escobar C, Aguilar-Roblero R. Anterior paraventricular thalamus modulates light-induced phase shifts in circadian rhythmicity in rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R897–R904. doi: 10.1152/ajpregu.00259.2002. [DOI] [PubMed] [Google Scholar]
- Schneider ER, Rada P, Darby RD, Leibowitz SF, Hoebel BG. Orexigenic peptides and alcohol intake: differential effects of orexin, galanin, and ghrelin. Alcohol Clin Exp Res. 2007;31:1858–1865. doi: 10.1111/j.1530-0277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Sharko AC, Kaigler KF, Fadel JR, Wilson MA. Individual differences in voluntary ethanol consumption lead to differential activation of the central amygdala in rats: relationship to the anxiolytic and stimulant effects of low dose ethanol. Alcohol Clin Exp Res. 2013;37(Suppl 1):E172–E180. doi: 10.1111/j.1530-0277.2012.01907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart D, Sabido-David C, Brough SJ, Jewitt F, Johns A, Porter RA, Jerman JC. SB-334867-A: the first selective orexin-1 receptor antagonist. Br J Pharmacol. 2001;132:1179–1182. doi: 10.1038/sj.bjp.0703953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeeva E, Richard D. Activation of the central nervous system in obese Zucker rats during food deprivation. J Comp Neurol. 2001;441:71–89. doi: 10.1002/cne.1398. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB. Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J Comp Neurol. 2008;508:212–237. doi: 10.1002/cne.21679. [DOI] [PubMed] [Google Scholar]
- Vilpoux C, Warnault V, Pierrefiche O, Daoust M, Naassila M. Ethanol-sensitive brain regions in rat and mouse: a cartographic review, using immediate early gene expression. Alcohol Clin Exp Res. 2009;33:945–969. doi: 10.1111/j.1530-0277.2009.00916.x. [DOI] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- Zhang GC, Mao LM, Liu XY, Wang JQ. Long-lasting up-regulation of orexin receptor type 2 protein levels in the rat nucleus accumbens after chronic cocaine administration. J Neurochem. 2007;103:400–407. doi: 10.1111/j.1471-4159.2007.04748.x. [DOI] [PubMed] [Google Scholar]
- Zhang L, Renaud LP, Kolaj M. Properties of a T-type Ca2+channel-activated slow afterhyperpolarization in thalamic paraventricular nucleus and other thalamic midline neurons. J Neurophysiol. 2009;101:2741–2750. doi: 10.1152/jn.91183.2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.