Summary
Objective
To [1] compare the frequency and severity of ultrasound (US) features in people with normal knees (controls), knee pain (KP), asymptomatic radiographic OA (ROA), and symptomatic OA (SROA), [2] examine relationships between US features, pain and radiographic severity, [3] explore the relationship between change in pain and US features over a 3-month period.
Method
Community participants were recruited into a multiple group case–control study. All underwent assessment for pain, knee radiographs and US examination for effusion, synovial hypertrophy, popliteal cysts and power Doppler (PD) signal within the synovium. A 3-month follow-up was undertaken in over half of control and SROA participants.
Results
243 participants were recruited (90 controls; 59 KP; 32 ROA; 62 SROA). Effusion and synovial hypertrophy were more common in ROA and SROA participants. Severity of effusion and synovial hypertrophy were greater in SROA compared to ROA (P < 0.05). Severity of US effusion and synovial hypertrophy were correlated with radiographic severity (r = 0.6 and r = 0.7, P < 0.01) but the relationship between pain severity and US features was weak (r = 0.3, P < 0.01). In SROA participants, pain severity did not change in tandem with a change in synovial hypertrophy over time.
Conclusion
US abnormalities are common in OA. Effusion and synovial hypertrophy were moderately correlated with radiographic severity but the relationship with pain is less strong. The degree to which these features reflect “active inflammation” is questionable and they may be better considered as part of the total organ pathology in OA. Further studies are warranted to confirm these findings.
Keywords: Ultrasound, Knee osteoarthritis, Inflammation, Pain, Synovitis
Introduction
Pain is the major stimulus for people with knee osteoarthritis (OA) to seek medical attention but the causes of pain are complex and radiographs which are the standard for clinical imaging in OA are often discordant with symptoms1, 2. In recent years there has been increasing interest in the role of the synovium in painful OA. Although nowhere as florid or extensive as the inflammation observed in rheumatoid arthritis, clinical effusions and capsular thickening can be clinically evident in some joints with knee OA, and are more frequently observed using sensitive measures such as ultrasound (US) and MRI3, 4, 5, 6, 7, 8, 9, 10. Synovial changes in OA are regarded by many as a secondary response to the degradation of cartilage11 though there are others who advocate them as a primary driver for OA which may be partly responsible for pain and disease progression12, 13, 14, 15, 16.
US allows the direct and indirect evaluation of synovial abnormalities, namely the presence of grey-scale features (effusion, synovial hypertrophy and bursitis) which are widely considered to be features of inflammation in OA. In addition the presence of increased power Doppler signal (PDS) within the synovium is purported to represent more active inflammation17. Synovial abnormalities are more common in those with painful knee OA compared to those with asymptomatic OA or normal knees but the association between individual US features and pain is not conclusive6, 9, 10, 18, 19, 20. Indeed, no single US feature has been consistently associated with knee pain (KP) and it has been suggested that the presence of effusion and synovial hypertrophy may be a marker for structural damage as opposed to inflammation4, 5. Additionally, most US studies have been conducted in secondary care settings and the same may not apply to people with knee OA in the community where the vast majority of patients are managed.
The primary aim of this study was to compare the frequency and severity of synovial abnormalities in people with normal knees, KP, radiographic OA and symptomatic OA from the community. Secondary aims were to examine the relationships between US features, pain and radiographic severity and to observe whether temporal change in pain severity over a 3-month period correlated with a change in US findings.
Methods
A case–control design was used to compare four groups: people with normal knees (controls – without pain and without radiographic OA), KP without radiographic OA, asymptomatic radiographic OA (ROA) and symptomatic ROA (SROA). KP was classified according to the worst item score on any of the five Western Ontario and McMaster Osteoarthritis Index (WOMAC) pain items. Those reporting at least moderate pain were classified as pain positive and those reporting none or mild pain were classified as pain negative. Radiographic OA was defined as Kellgren & Lawrence (K/L) grade ≥221.
Participants were recruited from previous community studies of KP or knee OA (as either cases or controls) where they had consented to being approached for future research. The primary source of study participants was a cohort study of incident KP in the community22. Additional participants were recruited from a randomised controlled trial of non-prescription analgesics for people with chronic KP23 and a population based case–control study (Genetics of OA and Lifestyle (GOAL)) study24. Participants were purposefully recruited with the aim of attaining fifty participants in each of the four comparison groups. Sample size was based on a best estimate from the limited published data for prevalence of knee effusion for each group5, 9, 10. Assuming a prevalence of 60% in the SROA group, 30% in the KP and ROA group and 5% in the control group, 50 participants were required in each group (200 in total) to detect the minimum difference between groups with 90% power and <5% type 1 errors. Control and SROA participants were invited to attend a follow-up US and pain assessment at 3 months.
Participants were excluded if they had a clinical history of inflammatory arthritis, clinical hip OA, knee joint replacement, knee joint injury or surgery in the previous 3 months, steroid injection to either knee in the previous 3 months, a diagnosis of Fibromyalgia or chronic widespread pain or severely impaired mobility (Steinbrocker Grade IV). Participants were asked to refrain from taking any non-steroidal anti-inflammatory drugs (NSAID) for 48 h prior to the assessment to allow an adequate wash-out period; paracetamol could be taken for rescue pain-relief up to 12 h before.
Study approval was granted by the Derbyshire Research Ethics Committee and all participants gave written informed consent. All participants underwent a clinical assessment, US and radiographic evaluation between April 2010 and March 2012.
Assessments
A range of data was collected including age, gender, body mass index (BMI), duration of early morning stiffness (EMS) (minutes) and the presence of a moderate clinical knee effusion. The WOMAC was also used to evaluate knee stiffness and function25.
Pain was assessed using three measures, a visual analogue scale (VAS) from 0 to 100 mm for current KP severity, the pain subscale of the WOMAC questionnaire25 and the Measure of Intermittent and Constant Osteoarthritis Pain questionnaire (ICOAP)26.
Standardised, weight-bearing, semi-flexed tibio-femoral and skyline 30° patello-femoral radiographs were scored by a single reader (SD) who was blinded to US features and pain. Radiographs were scored using the Nottingham logically derived Line Drawing Atlas (LDA)27, 28. This scoring system uses mathematically calculated intervals for grading joint space width (JSW) and size of osteophyte for all three compartments of the knee, to produce an ordinal summated score. Intra-observer reproducibility for scoring using the LDA has been established as good (kappa = 0.82 (95%, CI 0.78–.089) for JSW and 0.68 (95% CI, 0.63–0.71) for size of osteophyte)27. An overall K/L grade (0–4) was also given to each knee.
US assessment
US was performed by a single assessor (MH) on the same day as clinical assessments using a Toshiba Aplio SSA-770A machine with a multi-frequency (7–12 MHz) linear array transducer. The assessor was blind to the radiographic scores but not the clinical findings.
A standardised protocol reflecting current definitions and guidelines was followed5, 29. Knees were scanned in longitudinal and transverse planes with the joint supported in 30° flexion for ventral and lateral scans and in extension for dorsal scans. The supra-patellar pouch was scanned widely (including the lateral and medial recesses). The following features and measurements were recorded:
-
(1)
Effusion: maximal depth was measured in mm and dichotomised as absent if <4 mm and present if ≥4 mm5.
-
(2)
Synovial hypertrophy: maximal depth was measured in mm and dichotomised as absent if <4 mm and present if ≥4 mm5.
-
(3)
Baker's cyst: the diameter was measured in the transverse plane and dichotomised as absent if <4 mm or present if ≥4 mm10.
-
(4)
Bursitis: bursae at the infra-patellar tendon and the insertion of the pes-anserinus site were measured and dichotomised as absent if <4 mm or present if ≥4 mm infra-patellar bursae, and absent if <2 mm or present if ≥2 mm for the pes-anserine bursa10.
-
(5)
PDS: areas of hypertrophic synovium were scanned. A pulse repetition frequency of 1000–1300 Hz with a medium wall filter was used and the gain was adjusted so the background signal was removed. Increased signal was observed in both longitudinal and transverse planes and was scored using a semi-quantitative system grade 0–3, (0 = absent, 1 = mild, 2 = moderate, 3 = marked or severe)7.
Intra-observer reliability for US measures was tested by performing a second scan within 1 week on 28 knees by the same assessor (MH). Intra-class correlation coefficients (ICC) were calculated for continuous measures of effusion 0.93 (95% CI, 0.75–0.98), synovial hypertrophy 0.89 (95% CI, 0.64–0.97) and popliteal cysts 0.79 (95% CI, 0.61–0.90). Intra-observer reliability for PDS was evaluated using a weighted kappa and was statistically perfect, kappa = 1.0 (P < 0.001), but will have been influence by the low occurrence of PDS.
Statistical analysis
Our primary analysis was to compare the differences between groups. Analyses were carried out on data for the index knee (the most symptomatic, or randomly chosen knee) using IBM SPSS Statistics 19. All analyses were tested at the significance level P < 0.05. For nominal or frequency data the Chi-square test was used, Fisher's exact test was reported where the expected frequencies were less than five. Post-hoc comparisons were made using the z-test with adjusted P values (the Bonferroni method was used when making multiple paired comparisons to control for Type I error rates). The distributions of continuous variables were tested using the Kolmogorov–Smirnov test. Differences between groups were then compared using the one-way ANOVA and post-hoc Bonferroni tests for normally distributed data and the Kruskal–Wallis test and post-hoc Mann–Whitney tests with a Bonferroni correction for non-normally distributed data.
Secondary analysis examined the relationships between continuous US measures, pain VAS scores and radiographic severity (Nottingham LDA scores) using Pearson's correlation coefficients or Spearman's rho. Change in pain VAS scores and US measures at 3-month follow-up was examined using a paired t-test or Wilcoxon Signed Ranks Test, and correlation analysis was used to examine relationships between change in pain severity and change in US measures.
Results
Characteristics of study groups
Baseline assessments were performed on 243 participants, 65% were women, mean age was 70 years and BMI was 28.1 kg/m2. The four study groups were made up of 90 controls, 59 KP, 32 ROA, and 62 SROA participants. Recruited participants were representative of their original studies in terms of age though more females than males were recruited. Final recruitment to each group was unbalanced, with the ROA group under represented and SROA group over represented in the final analysis.
Baseline characteristics for each group are presented in Table I. The gender distribution between each group was similar but the KP group was younger (P < 0.05) and the control group had a lower BMI (P < 0.05) compared to other groups. Pain variables did not differ between the KP and SROA groups. Radiographic severity (LDA scores) were higher in the SROA group compared to ROA group (P = 0.05). Clinical effusions were rare among controls (2.2%) and KP participants (3.4%) but were more common in ROA (15.6%) (P < 0.05) and again in SROA (50%), (P = 0.05). Morning stiffness ≥30 min duration was exclusively reported by those with pain.
Table I.
Clinical characteristics of each group
| Characteristics | Controls (N = 90) | KP (N = 59) | ROA (N = 32) | SROA (N = 62) | P |
|---|---|---|---|---|---|
| Age (years) mean (SD) | 71 (7.9) | 63.8 (8.8)** | 73.1 (7.9) | 73.9 (7.8) | <0.001 |
| BMI (kg/m2) mean (SD) | 26.5 (4.4)** | 28.5 (4.0) | 29.6 (5.3) | 29.2 (4.1) | =0.001 |
| Women n (%) | 63 (70%) | 33 (55.9%) | 19 (59.3%) | 42 (67.7%) | =0.29 |
| Nottingham LDA scores | |||||
| Global score (0–60) mean (SD) | 1.1 (1.5) | 0.5 (1.1) | 11.9 (7.1)* | 17.5 (8.0)** | <0.001 |
| Pain characteristics | |||||
| Pain VAS (mm) mean (SD) | 6.6 (11.0) | 48.9 (22.0)* | 7.2 (14.4) | 48.2 (24.6)* | <0.001 |
| WOMAC pain (0–20) mean (SD) | 1.0 (1.51) | 8.0 (3.34)* | 0.9 (1.34) | 8.1 (3.23)* | <0.001 |
| ICOAP subscales | |||||
| Constant (0–20) mean (SD) | 0.5 (1.2) | 5.9 (4.8)* | 0.2 (.59) | 6.9 (5.2)** | <0.001 |
| Intermittent (0–24) mean (SD) | 1.6 (2.5) | 10.2 (4.1)* | 2.0 (3.1) | 10.6 (5.5)** | <0.001 |
| Clinical | |||||
| Clinical effusion n (%) | 2 (2.2%) | 2 (3.4%) | 5 (15.6%)* | 31 (50%)** | <0.001 |
| EMS ≥ 30 min n (%) | 0 (0%) | 20 (37.7%)* | 0 (0%) | 15 (27.3%)* | <0.001 |
| WOMAC stiffness (0–8) mean (SD) | 0.8 (1.1) | 3.4 (1.6)* | 0.9 (0.9) | 4.0 (1.8)* | <0.001 |
| WOMAC function (0–68) mean (SD) | 4.2 (6.5) | 25.8 (12.3)* | 5.5 (6.6) | 29.6 (11.8)* | <0.001 |
P value represents significant difference between the four groups using Χ2 tests for frequency data and one-way ANOVA for continuous data.
*group differs significantly from control P < 0.05 after applying post-hoc Bonferroni test.
**group differs significantly from all groups P < 0.05 after applying post-hoc Bonferroni test.
ROA, radiographic OA; SROA, symptomatic OA; EMS ≥ 30 min, a dichotomous variable was created for EMS lasting longer than 30 min.
US findings at baseline
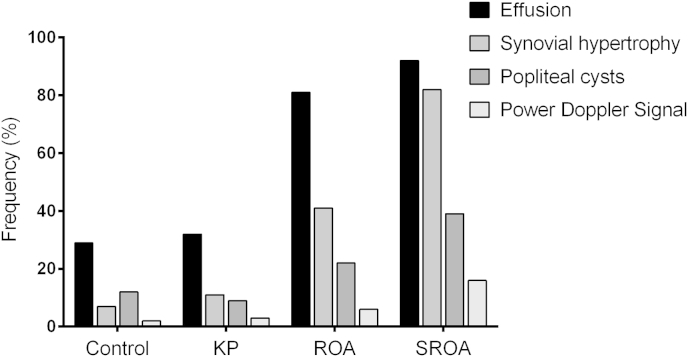
Table II summarises the US findings for each group. We found no difference in the frequency or severity of US features between control and KP participants. Effusions, synovial hypertrophy and popliteal cysts were more frequently observed in the ROA and SROA groups compared to KP and control groups (P < 0.05) (Fig. 1). Synovial hypertrophy was more common again in the SROA compared to ROA group (P < 0.05). The frequency of popliteal cysts increased in ROA (21.9%, P < 0.05) and SROA (39%, P < 0.05) groups compared to controls but were not different to each other. PD signal was more frequently observed in of SROA participants (16%) than control (2%, P < 0.05) and KP (3%, P < 0.05) groups. Grade of PD signal was not subject to analysis due to the low frequency observed. Infra-patellar bursae and pes-anserine bursae were rare and did not differ between groups.
Table II.
Frequency and severity of Ultrasound (US) features
| US features | Controls (n = 90) | KP (n = 59) | ROA (n = 32) | SROA (n = 62) | P |
| Effusion n (%) | 26 (28.9) | 19 (32.2) | 26 (81.3)* | 57 (91.9)* | <0.001 |
| Synovial hypertrophy n (%) | 7 (7.8) | 7 (11.9) | 13 (40.6)* | 51 (82.3)** | <0.001 |
| Popliteal cysts n (%) | 11 (12.4) | 5 (8.6) | 7 (21.9)* | 23 (39.2)* | <0.001 |
| Infra-pat bursitis n (%) | 3 (3.3) | 4 (6.8) | 0 (0) | 5 (8.1) | 0.28 |
| Pes-anserine bursitis n (%) | 0 (0) | 0 (0) | 0 (0) | 4 (6.5) | ns |
| PDS n (%) | 2 (2.2) | 2 (3.4) | 2 (6.3) | 10 (16.2)* | =0.005 |
| Grade 1 n (%) | 2 (2.2) | 1 (1.7) | 2 (6.3) | 5 (8.1) | |
| Grade 2 n (%) | 0 (0) | 0 (0) | 0 (0) | 5 (8.1) | |
| Grade 3 n (%) | 0 (0) | 1 (1.7) | 0 (0) | 0 (0) | |
| Continuous US features | |||||
| Effusion (mm) mean (SD) | 2.6 (2.7) | 3.4 (3.2) | 6.0 (2.8)* | 8.1 (4.0)** | <0.001 |
| Synovial hypertrophy (mm) mean (SD) | 0.7 (1.5) | 1.0 (1.9) | 3.9 (3.9)* | 6.7 (3.3)** | <0.001 |
| Popliteal cysts (mm) mean (SD) | 1.0 (2.6) | 0.8 (2.2) | 1.8 (3.6) | 3.5 (4.7)* | =0.001 |
P value represents significant difference between the four groups using Χ2 tests for frequency data and one-way ANOVA for continuous data.
*differs significantly from control and KP group P < 0.05.
**differs significantly from all groups P < 0.05.
ROA, radiographic OA; SROA, symptomatic OA.
Fig. 1.
Bar chart showing frequency (%) of US features within each comparison group.
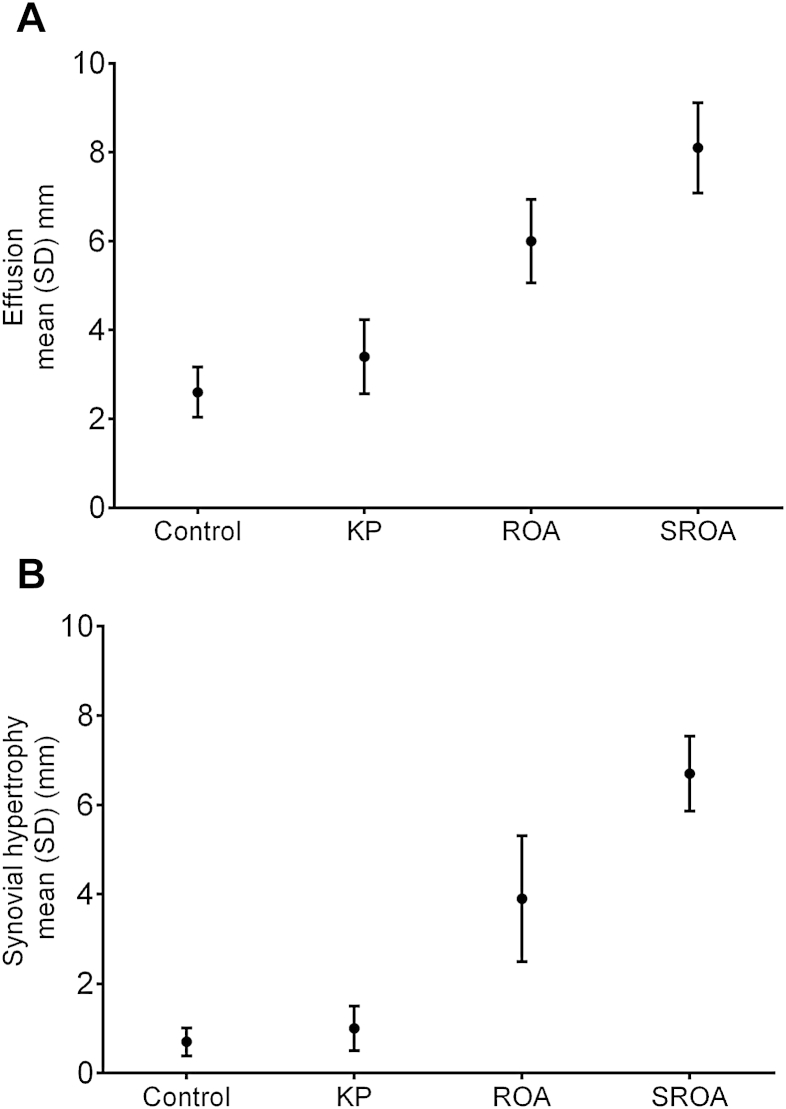
Continuous measures of effusions and synovial hypertrophy were greater in ROA (mean depth (SD) = 6.0 mm (2.8) and 3.9 mm (3.9) respectively) compared to controls and KP groups (P < 0.05), and were greater again in the SROA group (mean depth (SD) = 8.1 mm (4.0) and 6.7 mm (3.3), P < 0.05 respectively) (Fig. 2).
Fig. 2.
US measures of (A) effusion and (B) synovial hypertrophy for each group.
Relationships between US features, pain and radiographic severity
We found moderate correlations between radiographic scores derived from the Nottingham LDA and direct US measures of effusion and synovial hypertrophy (r = 0.6 and r = 0.7 respectively, P ≤ 0.01) (Table III). Correlations between pain VAS scores and US measures were weak but significant for effusion and synovial hypertrophy (r = 0.3, P < 0.01) (Table III). The strength of the correlation was similar for pain assessed using the VAS, WOMAC and ICOAP scores.
Table III.
Correlations between continuous US measures, pain severity and radiographic severity
| Effusion (mm) | Synovial hypertrophy (mm) | Popliteal cyst (mm) | |
|---|---|---|---|
| Pain VAS (mm) | 0.3** | 0.3** | 0.1 |
| WOMAC pain subscale | 0.3** | 0.3** | 0.1 |
| ICOAP intermittent subscale | 0.3** | 0.3** | 0.1 |
| ICOAP constant subscale | 0.3** | 0.3** | 0.1 |
| Radiographic severity – Nottingham LDA score (0–60) | 0.6** | 0.7** | 0.3** |
**Correlation is significant at the 0.01 level.
mm, millimeters.
Change in pain and US measures at 3 months
At 3 months, follow-up assessments for pain and US measures were carried out in 45 (72.5%) of SROA participants and 57 (63%) of controls (Table IV). Pain VAS scores and US measures did not change within the control group. In the SROA group, there was a statistically significant reduction in mean depth of synovial hypertrophy (mean difference = −1.4 mm SD (3.0), P = 0.003) after 3 months but there was no change in mean effusion, popliteal cyst measures or pain measures. We found no correlation between change in pain and change in US measures for either group (data not shown) though a change in US effusion was strongly correlated with a change in synovial hypertrophy that was statistically significant for both control (r = 0.4, P < 0.05) and SROA groups (r = 0.6, P < 0.01).
Table IV.
Pain and US measures in control and SROA participants at baseline and 3 months
| Controls N = 57 |
P | SROA N = 45 |
P | |||
|---|---|---|---|---|---|---|
| Baseline | 3 months | Baseline | 3 months | |||
| Pain VAS (mm) | 3.1 (5.6) | 5.8 (13.6) | 0.33 | 52.5 (23.0) | 54.0 (25.5) | 0.65 |
| Effusion (mm) | 2.3 (2.4) | 2.2 (2.4) | 0.80 | 8.6 (4.6) | 8.6 (4.9) | 0.96 |
| Synovial hypertrophy (mm) | 0.6 (1.5) | 0.7 (1.9) | 0.67 | 7.1 (4.0) | 5.7 (4.3) | 0.003* |
| Popliteal cysts (mm) Median (range) | 0 (0–11.6) | 0 (0–12.4) | 0.53 | 0 (0–14.3) | 1.15 (0–13.4) | 0.45 |
Data represent mean (SD) unless stated.
P value represents significant difference between the baseline and 3 months using paired t test for normally distributed and Wilcoxon signed rank test for non-normally distributed data. *difference is significant at <0.05.
SROA, symptomatic OA.
Discussion
This first community-based US study has confirmed that US features are more common in those with radiographic OA compared to those without and that severity of effusion and synovial hypertrophy are greater in those with painful OA. We found that direct measures of US features were moderately correlated with radiographic severity but had a weaker relationship with pain severity. Over a 3-month period, SROA participants showed a reduction in mean synovial hypertrophy but there was no parallel reduction in KP suggesting that, in the community at least, US features accompany structural changes of knee OA but do not readily explain the severity of pain.
Previous studies have shown grey-scale US features to be common in SROA though prevalence rates vary between 33 and 86% for effusion8, 30 17–93% for synovial hypertrophy5, 8 and 6–43% for popliteal cysts7, 8. Our frequency data are higher than some studies which may be attributable to variations in scanning protocols. We included the medial and lateral recesses of the supra-patellar pouch and joint lines when scanning and our findings are more comparable with those who scanned the joint widely8. PD signal is frequently cited as a surrogate for active synovitis but is not commonly reported in US studies of knee OA6, 31, 32. Our study is one of only a few that has reported using PDS and though we found it more frequently in SROA participants (16%, P < 0.05) compared to all other groups the severity was graded as only mild-moderate.
Few studies have directly investigated the relationship between radiographic severity and US features in knee OA. D'agostino et al. reported the presence of grey-scale US features was associated with higher radiological scores (K/L grade 3–4)5, and MRI studies have also reported a significant relationship between synovial hypertrophy and radiographic severity33. Using the Nottingham LDA we were able to correlate direct measures of US features with ordinal radiographic scores. We found moderate correlations between radiographic severity and US measures of synovial hypertrophy and effusion, which to us suggests that direct and indirect changes of the synovium are a reflection of the overall structural damage and reparative attempts of the osteoarthritic joint.
Previous conclusions on the relationship between US features and pain is knee OA are inconsistent. De Miguel et al. reported US effusion to increase the risk of KP by 6.5 times and Baker's cysts by 5.5 times but found no association between US features and pain severity10. Others have reported positive associations between US effusion with higher pain VAS scores on motion and at rest9, 34. However, two recent cross-sectional studies reported no association between US features and either the presence of KP or pain severity6, 20. We examined correlations between different pain measures (pain VAS, WOMAC, ICOAP intermittent and constant subscales) and direct measures of grey-scale features and found that both effusions and synovial hypertrophy were both correlated with pain severity but only modestly so. There was also no difference between the strength of the relationship for intermittent KP and constant KP, which may be thought of as more mechanical and inflammatory type pain, respectively. Little has been reported on the temporal changes of US features relative to change in KP. MRI studies have shown that while an increase in synovitis correlates modestly with increased pain severity or more frequent pain, a reciprocal decrease is not observed when pain decreases35, 36. We found that in SROA participants mean measures of synovial hypertrophy decreased over a 3-month period but this was not accompanied by a parallel reduction in pain severity.
This suggests that perhaps synovial hypertrophy as observed on grey-scale US is not as “inflammatory” as we would like to believe. Previous studies have shown that grey-scale US cannot differentiate between synovial hypertrophy and synovitis, tissue debris and fibrosis are known to mimic some US features of synovial proliferation but these features do not exhibit PD signal37, 38, 39. We found hypertrophy to be common but PD signal much less so. This raises the question that if effusion and synovial hypertrophy detected by US are not “inflammatory” in nature then what do they represent? The reduction in lymphatic vessels that occurs in knee OA synovium could cause increased synovial thickening and effusion through altered dynamics of fluid drainage rather than from “inflammation”40, and it has been suggested that altered joint biomechanics may permanently modify the baseline volume of synovial fluid41. In knee OA, the precipitating event is almost always mechanical in nature resulting either from acute injury, repetitive micro-trauma, increased focal stresses from abnormal anatomy or a combination thereof11. Capsular tissues are dynamic in terms of synthesis and orientation and respond to the biomechanical forces acting on them. Importantly, synovial thickening is localised rather than diffuse in OA and this may be reflective of damage in the adjacent areas of cartilage, bone or entheses but may also represent a cellular response to biomechanical stresses within the joint capsule. Different patterns of synovial thickening on US and MRI have been identified but whether they are associated with joint biomechanics has not yet been investigated8, 42, 43. It seems likely from our data that effusion and synovial hypertrophy mainly reflect the overall changes that occur in knee OA.
There are some caveats to this study. Firstly, recruitment to the study was not random; participants were purposefully recruited to each study group with the aim of comparing four groups with a balanced number of 50 participants. Participants were drawn from previous community studies of KP for whom a variable amount of time (between 3 and 10 years) had passed between participation in the original and current study22, 23, 24. As such, previous radiographic and pain status may have changed for some potential participants i.e., there would be an incidence of new radiographic OA and new KP, as well as KP having resolved in others. The prevalence of asymptomatic ROA lies between 27 and 44% of the general population44 but identifying those participants is inherently difficult as they are asymptomatic and require radiographs to confirm their status. Consequently, recruitment to each group was unbalanced, with the ROA group under represented and SROA group over represented in the final analysis. Secondly, the study design was primarily intended for the comparison of the four study groups. Secondary analyses examining the relationships between pain, structural change and US features were derived from the four different groups and the correlations may not be representative of the general population. Thirdly, the population for this study was drawn from the community and differences in population demographics limits direct comparisons with hospital-based US studies where SROA participants are often younger, with less severe radiographic changes but higher levels of reported pain5. Comparisons may also be limited by variations in definitions of US pathology and scanning protocols which vary between studies. Finally, issues around defining KP and knee OA are well documented45, 46, 47, 48. We chose our defining criteria as it enabled all participants to be allocated to a group. As a consequence the comparison groups are not completely distinct, that is some control/ROA participants had mild symptoms and some control/KP participants had minor structural change (K/L grade1).
In conclusion, this study highlights the correlation between grey-scale US features and radiographic severity. However, it questions the general assumption that synovial abnormalities are “inflammatory” in nature and is responsible for driving pain in OA, and suggests instead that they are a part of the overall structural pathology reflecting the biomechanical adaptations of the OA joint. PD signal which is widely asserted to represent a more “active inflammation” was more common in painful OA compared to controls but did not differ significantly from asymptomatic OA but this may not be the case for hospital referred patients. US features, particularly synovial hypertrophy, may well have a role to play in the development of painful OA but given the multi-factorial nature of pain this is unlikely to be straight forward. Further longitudinal studies are required to demonstrate whether US features are important in the development and progression of structural change and symptoms.
Contributions
All authors were involved in drafting and critically reading the manuscript for important content and all authors approved the final version. MH had full access to the all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. MH, SD, PC, KL, WZ and MD.
Acquisition of data. MH, SD, KL.
Analysis and interpretation of data. MH, SD, PC, KL, WZ and MD.
Funding
We are grateful to Arthritis Research UK for funding this study (AHP Training Fellowship Grant no. 18860).
Competing interests
All authors declare they have no competing interests.
Contributor Information
M. Hall, Email: michelle.hall@nottingham.ac.uk.
S. Doherty, Email: sally.doherty@nottingham.ac.uk.
P. Courtney, Email: philip.courtney@nuh.nhs.uk.
K. Latief, Email: khalid.latief@nuh.nhs.uk.
W. Zhang, Email: weiya.zhang@nottingham.ac.uk.
M. Doherty, Email: michael.doherty@nottingham.ac.uk.
References
- 1.Hannan M.T., Felson D.T., Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27:1513–1517. [PubMed] [Google Scholar]
- 2.Bedson J., Croft P.R., Bedson J., Croft P.R. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116. doi: 10.1186/1471-2474-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill C.L., Gale D.G., Chaisson C.E., Skinner K., Kazis L., Gale M.E. Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. J Rheumatol. 2001;28:1330–1337. [PubMed] [Google Scholar]
- 4.Hayashi D., Roemer F.W., Katur A., Felson D.T., Yang S.-O., Alomran F. Imaging of synovitis in osteoarthritis: current status and outlook. Semin Arthritis Rheum. 2011;41:116–130. doi: 10.1016/j.semarthrit.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 5.D'Agostino M.A., Conaghan P., Le Bars M., Baron G., Grassi W., Martin-Mola E. EULAR report on the use of ultrasonography in painful knee osteoarthritis. Part 1: prevalence of inflammation in osteoarthritis. Ann Rheum Dis. 2005;64:1703–1709. doi: 10.1136/ard.2005.037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mermerci B.B., Garip Y., Uysal R.S., Dogruel H., Karabulut E., Ozoran K. Clinic and ultrasound findings related to pain in patients with knee osteoarthritis. Clin Rheumatol. 2011;30:1055–1062. doi: 10.1007/s10067-011-1701-x. [DOI] [PubMed] [Google Scholar]
- 7.Iagnocco A., Meenagh G., Riente L., Filippucci E., Delle Sedie A., Scire C.A. Ultrasound imaging for the rheumatologist XXIX. Sonographic assessment of the knee in patients with osteoarthritis. Clin Exp Rheumatol. 2010;28:643–646. [PubMed] [Google Scholar]
- 8.Wu P.T., Shao C.J., Wu K.C., Wu T.T., Chern T.C., Kuo L.C. Pain in patients with equal radiographic grades of osteoarthritis in both knees: the value of gray scale ultrasound. Osteoarthritis and Cartilage. 2012;20:1507–1513. doi: 10.1016/j.joca.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Naredo E., Cabero F., Palop M.J., Collado P., Cruz A., Crespo M. Ultrasonographic findings in knee osteoarthritis: a comparative study with clinical and radiographic assessment. Osteoarthritis and Cartilage. 2005;13:568–574. doi: 10.1016/j.joca.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 10.de Miguel Mendieta E., Cobo Ibáñez T., Usón Jaeger J., Bonilla Hernán G., Martín Mola E. Clinical and ultrasonographic findings related to knee pain in osteoarthritis. Osteoarthritis and Cartilage. 2006;14:540–544. doi: 10.1016/j.joca.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Felson D.T. Osteoarthritis as a disease of mechanics. Osteoarthritis and Cartilage. 2013;21:10–15. doi: 10.1016/j.joca.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayral X., Pickering E.H., Woodworth T.G., Mackillop N., Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis – results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis and Cartilage. 2005;13:361–367. doi: 10.1016/j.joca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Benito M.J., Veale D.J., Fitzgerald O., van den Berg W.B., Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64:1263–1267. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aspden R.M. Osteoarthritis: a problem of growth not decay. Rheumatology. 2008;47:1452–1460. doi: 10.1093/rheumatology/ken199. [DOI] [PubMed] [Google Scholar]
- 15.Samuels J., Krasnokutsky S., Abramson S.B. Osteoarthritis: a tale of three tissues. Bull NYU Hosp Joint Dis. 2008;66:244–250. [PubMed] [Google Scholar]
- 16.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!) Osteoarthritis and Cartilage. 2013;21:16–21. doi: 10.1016/j.joca.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Porta F., Radunovic G., Vlad V., Micu M.C., Nestorova R., Petranova T. The role of Doppler ultrasound in rheumatic diseases. Rheumatology. 2012;51:976–982. doi: 10.1093/rheumatology/ker433. [DOI] [PubMed] [Google Scholar]
- 18.Tarhan S., Unlu Z. Magnetic resonance imaging and ultrasonographic evaluation of the patients with knee osteoarthritis: a comparative study. Clin Rheumatol. 2003;22:181–188. doi: 10.1007/s10067-002-0694-x. [DOI] [PubMed] [Google Scholar]
- 19.Chatzopoulos D., Moralidis E., Markou P., Makris V., Arsos G., Chatzopoulos D. Baker's cysts in knees with chronic osteoarthritic pain: a clinical, ultrasonographic, radiographic and scintigraphic evaluation. Rheumatol Int. 2008;29:141–146. doi: 10.1007/s00296-008-0639-z. [DOI] [PubMed] [Google Scholar]
- 20.Bevers K., Bijlsma J.W., Vriezekolk J.E., van den Ende C.H., den Broeder A.A. Ultrasonographic features in symptomatic osteoarthritis of the knee and relation with pain. Rheumatology. 2014 Apr 4 doi: 10.1093/rheumatology/keu030. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Altman R., Asch E., Bloch D., Bole G., Borenstein D., Brandt K. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 22.Ingham S.L., Zhang W., Doherty S.A., McWilliams D.F., Muir K.R., Doherty M. Incident knee pain in the Nottingham community: a 12-year retrospective cohort study. Osteoarthritis and Cartilage. 2011;19:847–852. doi: 10.1016/j.joca.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Doherty M., Hawkey C., Goulder M., Gibb I., Hill N., Aspley S. A randomised controlled trial of ibuprofen, paracetamol or a combination tablet of ibuprofen/paracetamol in community-derived people with knee pain. Ann Rheum Dis. 2011;70:1534–1541. doi: 10.1136/ard.2011.154047. [DOI] [PubMed] [Google Scholar]
- 24.Limer K.L., Tosh K., Bujac S.R., McConnell R., Doherty S., Nyberg F. Attempt to replicate published genetic associations in a large, well-defined osteoarthritis case–control population (the GOAL study) Osteoarthritis and Cartilage. 2009;17:782–789. doi: 10.1016/j.joca.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Bellamy N., Buchanan W., Goldsmith C., Campbell J., Stitt L. Validation study of WOMAC: a health status instrument for measuring clinically-important patient-relevant outcome following total hip or knee arthroplasty in osteoarthritis. J Orthop Rheumatol. 1988;1:95–108. [PubMed] [Google Scholar]
- 26.Hawker G.A., Davis A.M., French M.R., Cibere J., Jordan J.M., March L. Development and preliminary psychometric testing of a new OA pain measure – an OARSI/OMERACT initiative. Osteoarthritis and Cartilage. 2008;16:409–414. doi: 10.1016/j.joca.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagaosa Y., Mateus M., Hassan B., Lanyon P., Doherty M. Development of a logically devised line drawing atlas for grading of knee osteoarthritis. Ann Rheum Dis. 2000;59:587–595. doi: 10.1136/ard.59.8.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilkinson C.E., Carr A.J., Doherty M. Does increasing the grades of the knee osteoarthritis line drawing atlas alter its clinimetric properties? Ann Rheum Dis. 2005;64:1467–1473. doi: 10.1136/ard.2004.033282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wakefield R.J., Balint P.V., Szkudlarek M., Filippucci E., Backhaus M., D'Agostino M.A. Musculoskeletal ultrasound including definitions for ultrasonographic pathology.[erratum appears in J Rheumatol. 2006 Feb;33(2):440 Note: Bruyn, George [corrected to Bruyn, George AW]] J Rheumatol. 2005;32:2485–2487. [PubMed] [Google Scholar]
- 30.Kristoffersen H., Torp-Pedersen S., Terslev L., Qvistgaard E., Holm C.C., Ellegaard K. Indications of inflammation visualized by ultrasound in osteoarthritis of the knee. Acta Radiol. 2006;47:281–286. doi: 10.1080/02841850600551508. [DOI] [PubMed] [Google Scholar]
- 31.Joshua F., Edmonds J., Lassere M. Power Doppler ultrasound in musculoskeletal disease: a systematic review. Semin Arthritis Rheum. 2006;36:99–108. doi: 10.1016/j.semarthrit.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Song I.H., Burmester G.R., Backhaus M., Althoff C.E., Hermann K.G., Scheel A.K. Knee osteoarthritis. Efficacy of a new method of contrast-enhanced musculoskeletal ultrasonography in detection of synovitis in patients with knee osteoarthritis in comparison with magnetic resonance imaging. Ann Rheum Dis. 2008;67:19–25. doi: 10.1136/ard.2006.067462. [DOI] [PubMed] [Google Scholar]
- 33.Baker K., Grainger A., Niu J., Clancy M., Guermazi A., Crema M. Relation of synovitis to knee pain using contrast-enhanced MRIs. Ann Rheum Dis. 2010;69:1779–1783. doi: 10.1136/ard.2009.121426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu C., Quan A., Hose K., Chang J., Tam S., May S. The response to intra-articular steroids in knee osteoarthritis as defined by ultrasonographic outcomes: an analysis of the reliability of sequential ultrasound measurements. Arthritis Rheum. 2005;52:4090. [Google Scholar]
- 35.Hill C.L., Hunter D.J., Niu J., Clancy M., Guermazi A., Genant H. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66:1599–1603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y., Nevitt M., Niu J., Lewis C., Torner J., Guermazi A. Fluctuation of knee pain and changes in bone marrow lesions, effusions, and synovitis on magnetic resonance imaging. Arthritis Rheum. 2011;63:691–699. doi: 10.1002/art.30148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt W.A., Volker L., Zacher J., Schlafke M., Ruhnke M., Gromnica-Ihle E. Colour Doppler ultrasonography to detect pannus in knee joint synovitis. Clin Exp Rheumatol. 2000;18:439–444. [PubMed] [Google Scholar]
- 38.Fiocco U., Cozzi L., Rubatelli L., Rigon C., De Candia A., Tregnaghi A. Long-term sonographic follow-up of rheumatoid and psoriatic proliferative knee joint synovitis. Rheumatology. 1996;35:155–163. doi: 10.1093/rheumatology/35.2.155. [DOI] [PubMed] [Google Scholar]
- 39.Walther M., Harms H., Krenn V., Radke S., Faehndrich T.P., Gohlke F. Correlation of power Doppler sonography with vascularity of the synovial tissue of the knee joint in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 2001;44:331–338. doi: 10.1002/1529-0131(200102)44:2<331::AID-ANR50>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 40.Walsh D.A., Verghese P., Cook G.J., McWilliams D.F., Mapp P.I., Ashraf S. Lymphatic vessels in osteoarthritic human knees. Osteoarthritis and Cartilage. 2012;20:405–412. doi: 10.1016/j.joca.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 41.Simkin P.A., Bassett J.E. Cartilage matrix molecules in serum and synovial fluid. Curr Sci. 1995:346–351. doi: 10.1097/00002281-199507000-00014. [DOI] [PubMed] [Google Scholar]
- 42.de Lange-Brokaar B., Ioan-Facsinay A., Yusuf E., Visser W., Kroon H., van Osch G. Different patterns of synovitis as seen on CE-MRI in patients with knee osteoarthritis (Abstract) Osteoarthritis and Cartilage/OARS, Osteoarthritis Research Society. 2013;21:S16. [Google Scholar]
- 43.Roemer F.W., Kassim Javaid M., Guermazi A., Thomas M., Kiran A., Keen R. Anatomical distribution of synovitis in knee osteoarthritis and its association with joint effusion assessed on non-enhanced and contrast-enhanced MRI. Osteoarthritis and Cartilage. 2010;18:1269–1274. doi: 10.1016/j.joca.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Felson D.T., Naimark A., Anderson J., Kazis L., Castelli W., Meenan R.F. The prevalence of knee osteoarthritis in the elderly. The Framingham osteoarthritis study. Arthritis Rheum. 1987;30:914–918. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 45.Spector T.D., Hart D.J., Byrne J., Harris P.A., Dacre J.E., Doyle D.V. Definition of osteoarthritis of the knee for epidemiological studies. Ann Rheum Dis. 1993;52:790–794. doi: 10.1136/ard.52.11.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Felson D.T., McAlindon T.E., Anderson J.J., Naimark A., Weissman B.W., Aliabadi P. Defining radiographic osteoarthritis for the whole knee. Osteoarthritis and Cartilage. 1997;5:241–250. doi: 10.1016/s1063-4584(97)80020-9. [DOI] [PubMed] [Google Scholar]
- 47.O'Reilly S.C., Muir K.R., Doherty M. Screening for pain in knee osteoarthritis: which question? Ann Rheum Dis. 1996;55:931–933. doi: 10.1136/ard.55.12.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lanyon P., O'Reilly S., Jones A., Doherty M. Radiographic assessment of symptomatic knee osteoarthritis in the community: definitions and normal joint space. Ann Rheum Dis. 1998;57:595–601. doi: 10.1136/ard.57.10.595. [DOI] [PMC free article] [PubMed] [Google Scholar]