Abstract
Anti-M antibodies are usually of IgM, appear as cold agglutinins and are clinically insignificant. We are reporting two cases of anti-M in cases of solid tumors where the anti-M caused discrepancy in blood grouping, reacted in coombs phase of crossmatching. Anti-M in first case showed dosage effect. These antibodies can be clinical significant when detected in coombs phase, making M antigen negative coombs compatible unit transfusion imperative.
Keywords: Anti-M, Solid tumors, ABO discrepancy, Cross match incompatibility
Introduction
Anti-M is a relatively common naturally occurring predominantly IgM antibody reacting optimally at 4 °C, it usually reacts weakly or not at all at 37 °C. However in rare cases an IgG anti-M may cause hemolytic diseases of the newborn (HDN) [1–3] and delayed hemolytic transfusion reaction (HTR) [1, 4]. It shows antigen dosage effect, most anti-M react more strongly with M+N− red cells than M+N+ red cells, very weak anti-M may not react with M+N+ red cells at all, making antibody identification difficult [4, 5]. Though a commonly occurring antibody, it may occasionally, have immense clinical significance when reactive in coombs phase. We present two case reports of anti-M detected during pretransfusion compatibility testing where they caused ABO blood group discrepancy and incompatibility in cross matching.
Case Report 1
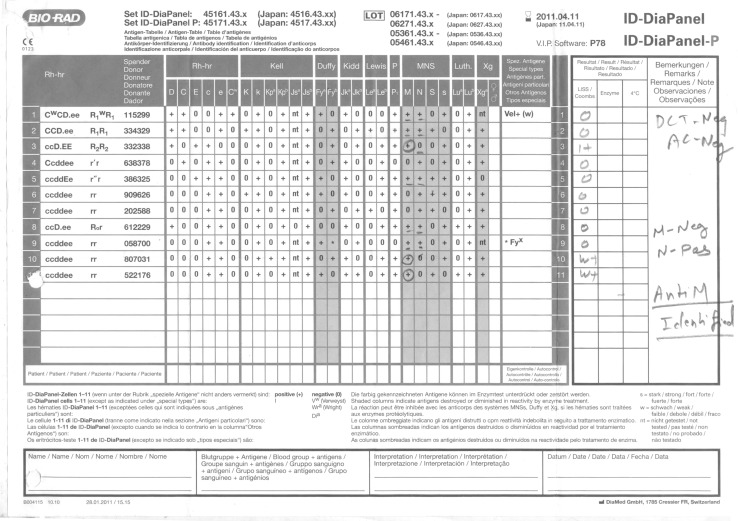
A 44 year old female patient with no previous history of blood transfusion, a case of metastatic high grade soft tissue sarcoma (NOS) of left thigh admitted in our hospital for surgery. Her hemoglobin was 9.3 g/dl and hematocrit 29 %. Request for arranging two units PRBCs was received in the blood bank. The blood group of the patient was typed as A1 Rh (D) Positive. However on crossmatching few red cell units were incompatible by gel technology (Diamed ID Microtyping System).A complete immunohematological workup of the case was initiated. Direct antiglobulin test (DAT) was performed on red cells from EDTA sample using polyspecific antiglobulin reagents (anti IgG and C3d) and was found to be negative along with negative autocontrol. Indirect antiglobulin (IAT) test using pooled O positive cells was also negative. After exclusion of the possible clerical and technical errors we performed coombs crossmatch with more units. Out of 14 units only 6 units were compatible. Antibody screening with commercially available three cell panel (ID-DiaCell I-II-III Asia), showed hemolysis with panel I and negative reaction with panel II and III cells (Fig. 1). Subsequently, antibody identification using 11 cell panel (ID-Diapanel) was carried out and anti-M antibody detected. It showed 1+ reaction with homozygous cells panel 3(M+N−) and weak reaction with panel 10 and 11 (M+N−) and no reaction was seen with heterozygous panel cells (M+N+) (Fig. 2).No reaction was seen when enzyme treated cells were used. To determine the immunoglobulin class of the antibody, serum was treated dithiothreitol (DTT). The antibody persisted after the DTT treatment, indicating presence of IgG component along with IgM. Red cell phenotyping of the patient as well as the coombs compatible units was performed. The patient was negative for M antigen, out of the 6coombs crossmatch compatible units, 2 units were found to be M antigen negative (M–N+) and four were found to be heterozygous (M+N+). Anti-M shows dosage effect and the same was also seen in this case. Reaction was seen with units homozygous for M antigen and no reaction was seen when M antigen was present in single dose M+N+ (Heterozygous). Even though the heterozygous units were coombs crossmatch compatible, they were not used for transfusion as they could lead to a delayed hemolytic transfusion reaction. Only the two M antigen negative (M–N+) units were used for transfusion. No immediate or delayed transfusion reaction occurred.
Fig. 1.
Antigram of screening 3 cell panel (case 1)
Fig. 2.
Antigram 11 cell panel used in antibody identification (case 1)
Case Report 2
A 71 year old male patient, a case of periampullary carcinoma with CKD was posted for surgery. His hemoglobin was 7.5gm/dl, hematocrit was 20 %, blood urea 424 mg/dl, and serum creatinin was 15.75 mg/dl. He was transfused with 2 units of packed red cells during hemodialysis 2 weeks prior to our receiving the sample. A request for 4 units of packed red cells was received as the patient was planned for Whipple’s procedure. There was discrepancy in ABO blood grouping and incompatibility in coombs crossmatch. Blood grouping was done by Bio-Rad ID system, cell grouping showed blood group as B Rh (D) positive while serum grouping showed panreactivity, it showed 4+ reaction with A cells and 4+ with double cell populations with B and O cells (Table 1).Direct antiglobulin test (DAT) was performed on red cells from EDTA sample using polyspecific antiglobulin reagents (anti IgG and C3d) and was found to be negative along with negative autocontrol. Indirect antiglobulin (IAT) test using pooled O positive cells was positive. Antibody screening with commercially available three cell panel (ID-DiaCell I-II-III Asia), showed 3+ reaction with panel I and III and negative reaction with Panel II (Fig. 3).The anti-M was identified in 11 cell panel (Diamed) where it showed 3+ reaction with homozygous (M+N−) as well as with heterozygous (M+N+) cell panels in coombs phase and 4+ with double cell population at room temperature (Fig. 4).The discrepancy in reverse grouping was resolved by using M antigen negative A, B and O cells and the blood group of the patient was typed as B Rh (D) Positive (Table 2).There was problem in finding the compatible units during coombs crossmatch. We performed exhaustive crossmatching with 90 units of group specific donor units but only 2 units were found to be coombs crossmatch compatible which were M antigen negative. Further patient’s family members were tested for MN antigen status and 2 more M antigen negative compatible units were found. The patient was transfused these units intraoperatively with no immediate or delayed transfusion reaction.
Table 1.
Result of ABO blood grouping using Bio-Rad ID system
| Anti-A | Anti-B | DVI | Ctl | A cells | B cells | O cells |
|---|---|---|---|---|---|---|
| 0 | 4+ | 4+ | 0 | 4+ | 4+dp* | 4+dp* |
* Double cell population
Fig. 3.
Antigram of screening 3 cell panel (case 2)
Fig. 4.
Antigram of 11 cell panel (case 2)
Table 2.
Result of blood grouping by using M antigen negative A, B and O cells
| Anti-A | Anti-B | DVI | Ctl | A cells | B cells | O cells |
|---|---|---|---|---|---|---|
| 0 | 4+ | 4+ | 0 | 4+ | 0 | 0 |
Discussion
Anti-M antibodies are usually of the IgM class and appear as saline reactive or cold agglutinins and are considered clinically insignificant [1]. An IgG Anti-M has rarely been implicated in delayed hemolytic transfusion reactions or hemolytic disease of the newborn. Anti-M causing blood group discrepancy and crossmatch incompatibility has been reported in the Indian literature [5, 7, 8]. Frequency of anti M in routine blood donors is one in 2,500 with M+N− and one in 5000 with M+N+ cells [6, 9]. Detection of anti-M antibody in pretransfusion testing is a challenging task as anti-M antibodies exhibit dosage effect. It is not sufficient to provide units that are crossmatch compatible at 37 °C without typing for M antigen. If during antibody screening anti-M is reactive at 37 °C in the AHG phase, then even if the units are compatible on coombs crossmatch, phenotyping of the donor units become important.Anti-M reacts more strongly when M antigen is present in double dose M+N− (homozygous cells) and may not react with single dose M+N+ (heterozygous cells) as seen in our case report one. If heterozygous compatible units are transfused, delayed hemolytic transfusion reaction may occur. Other notable finding in our report is that both patients had solid tumors. Literature search revealed a report in which the possible association of anti-M antibodies and neoplasm was mentioned, where 9 out of 10 cases had neoplasm [10]. Cases have been reported where anti-M was found in sera of patients suffering from neoplastic disease [4, 11, 12]. This may be a chance association. Up till now very few cases of anti-M antibody have been reported, so we cannot determine whether the association of anti-M antibody and malignancy is a coincidence or whether there is a genuine relationship between them. More cases need to be studied to establish a correlation between anti-M antibody and malignancy.
Acknowledgments
We would like to thank Mr. Sumit Gupta (Bio-Rad) for his contribution and support.
References
- 1.Brecher ME (ed) (2005) AABB Technical Manual. 15th ed. American association of blood banks, Bethesda p. 340
- 2.Duguid J, Bromilow I, Entwistle G, Wilkinson R. Haemolytic disease of the newborn due to anti-M. Vox Sang. 1995;68:195–196. doi: 10.1111/j.1423-0410.1995.tb03927.x. [DOI] [PubMed] [Google Scholar]
- 3.Thompson DJ, Stults DZ, Daniel SJ. Anti-M antibody in pregnancy. Obstet Gynecol Surv. 1989;44(9):637–641. doi: 10.1097/00006254-198909000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Sancho JM, Pujol M, Fernandez F, Soler FM, Manzano P, Felio E. Delayed hemolytic transfusion reaction due to anti M antibody. Br J Haematol. 1998;103:268–269. doi: 10.1046/j.1365-2141.1998.00970.x. [DOI] [PubMed] [Google Scholar]
- 5.Tondon R, Kataria R, Chaudhry R. Anti-M: report of two cases and review of literature. Asian J Transfus Sci. 2008;2:81–83. doi: 10.4103/0973-6247.42695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harmening D. Modern blood banking and transfusion practices. 5. Philadelphia: F.A. Davis company; 2005. p. 166. [Google Scholar]
- 7.Rangarajan K, Subramanian A, Agrawal D, Chatterjee K. Trauma patient with M-antibody. Indian J Pathol Microbiol. 2010;53:574–575. doi: 10.4103/0377-4929.68280. [DOI] [PubMed] [Google Scholar]
- 8.Khalid S, Dantes R, Varghese S, Al Hakawati I. Naturally occurring anti M complicating ABO grouping. Indian J Pathol Microbiol. 2011;54:170–172. doi: 10.4103/0377-4929.77394. [DOI] [PubMed] [Google Scholar]
- 9.Klein HG, Anstee DJ. Other red cell antigens. In: Klein HG, Anstee DJ, editors. Mollison’s blood transfusion in clinical medicine. 11. London: Blackwell; 2005. pp. 220–221. [Google Scholar]
- 10.Klein SidneyJ, Reilly EmmettB, James J. Matsushima an uncommon blood group isoantibody (anti-M) in neoplastic disease. Nature. 1963;197:393–394. doi: 10.1038/197393a0. [DOI] [PubMed] [Google Scholar]
- 11.Selseleh M (MS), Esmaili J (BS) (2006) A case study of anti-M with IgG type and clinical significance. SJIBTO 3(1): 73–78 (Abstract)
- 12.Parry-Jones N, Gore ME, Taylor J, Treleaven JG. Delayed hemolytic transfusion reaction caused by anti-M antibody in a patient receiving interleukin-2 and interferon for metastatic renal cell cancer. Clin Lab Haematol. 1999;21(6):407–408. doi: 10.1046/j.1365-2257.1999.00260.x. [DOI] [PubMed] [Google Scholar]