Abstract
Human parvovirus B19 is highly tropic to human bone marrow and replicates only in erythroid progenitor cells. It is causative agent of transient aplastic crisis in patients with chronic haemolytic anemia. In immunocompromised patients persistent parvovirus B19 infection may develop and it manifests as pure red cell aplasia and chronic anaemia. Bone marrow is characterised morphologically by giant pronormoblast stage with little or no further maturation. We encountered a case of 6 year old HIV positive male child presented with pure red cell aplasia due to parvovirus B19 infection. Bone marrow aspiration cytology revealed giant pronormoblast with prominent intranuclear inclusions led to suspicion of parvovirus B19 infection which was confirmed by DNA PCR. This case is presented to report classical morphological features of parvovirus B19 infection rarely seen on bone marrow examination should warrant the suspicion of human parvovirus B19 infection in the setting of HIV positive patient with repeated transfusions and confirmation should be done by PCR.
Keywords: Parvovirus, Pure red cell aplasia, HIV
Background
Parvovirus B19 was first associated with human disease in 1981 when it was connected with aplastic crisis in children with haemolytic anaemia [1]. Parvovirus B19 is single stranded DNA virus [2]. The main target for parvovirus B19 infection is erythroid progenitor cell in the bone marrow. Erythroid tropism is based on tissue distribution of parvovirus B19 cellular receptor globoside (blood group P antigen) [3]. Human parvovirus B19 causes cell cycle arrest of human erythroid progenitors via dysregulation of E2F family of transcription factors [4]. Acute parvovirus B19 infection is thought to confer protective lifelong immunity. In immunologically normal individuals parvovirus B19 causes an acute self limited (4–8 days) cessation of RBC production. In individuals with haemolytic anaemia this can lead to acute aplastic crisis. In immunodeficient patients failure to produce neutralising antibodies results in persistent parvovirus B19 infection [5]. Bone marrow aspiration cytology smears reveals marked erythroid hypoplasia with large vacuolated proerythroblast (Lantern cells) with cytoplasmic pseudopods [6]. In a large cohort study of 50 patients with AIDS parvovirus DNA was found in only 1 out of 191 (0.5 %) HIV positive homosexuals. However Human parvovirus B19 DNA was found in 5 out of 30 (17 %) transfusion dependant HIV positive individuals [7].
Case History
A 6 year old male child presented with fever, cough and breathlessness. He was treated with repeated blood transfusions due to anaemia. On examination patient had marked pallor with generalised anasarca. Hematological investigations revealed: Hemoglobin—2.7 gm/dl, T.L.C.—2,500/cmm, R.B.C. count—1.01 million/cmm, platelet count—1.10 lakh/cmm. Peripheral smear findings did not reveal features of sickle cell anemia or thalassemia. Reticulocyte count was 0.1 %. Patient was HIV positive. CD4 cell count of patient was 72 cells/cmm and CD4:CD8 ratio was 0.11.
Patient was labeled as severe anemia with pancytopenia. Bone marrow aspiration cytology was carried out from sternum. Cytological smears were stained with Leishman stain. Bone marrow aspiration cytology smears revealed marked erythroid hypoplasia with giant proerythroblast with cytoplasmic pseudopods (Lantern cells). Nuclei showed ground glass appearance with large nucleoli like eosinophilic intranuclear inclusions (Figs. 1, 2). Myeloid series was unremarkable. Megakaryocytes were adequate. These bone marrow cytological findings raised the suspicion of human parvovirus B19 infection. Our findings were confirmed by positive DNA PCR for parvovirus B19. Patient was treated with intravenous immunoglobulin.
Fig. 1.
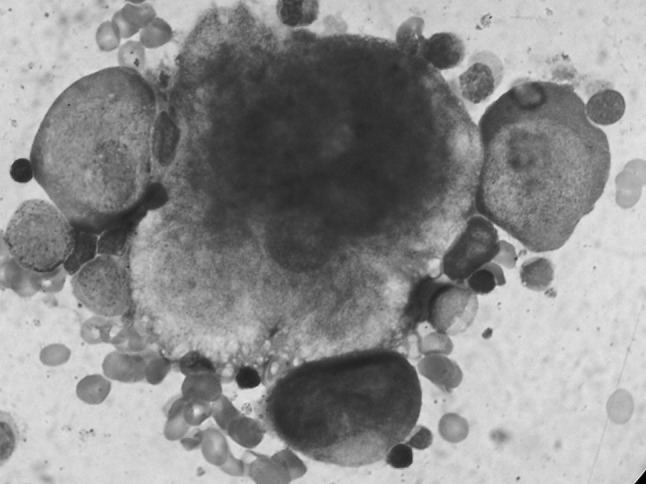
Bone marrow cytology: Leishman stain (1000×) comparing size of giant proerythroblast with megakaryocyte
Fig. 2.
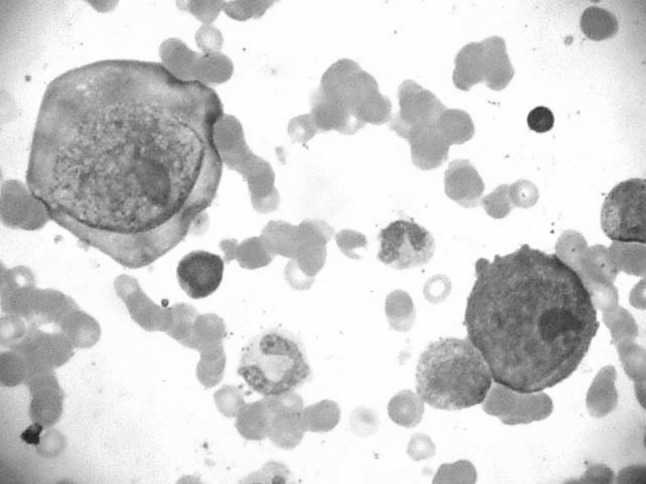
Bone marrow cytology: Leishman stain (1000×) giant proerythroblast with intranuclear inclusions and cytoplasmic pseudopods
Conclusion
The presence of large atypical erythroblasts in bone marrow aspiration cytology in the setting of HIV positive patients with transfusion dependent anaemia with classical morphological features on bone marrow examination should warrant the suspicion of Human parvovirus B19 infection and confirmation should be done by PCR.
Contributor Information
Rane Sharada Raju, Email: sharadarane22@gmail.com.
Kadgi Nalini Vinayak, Email: nalini.kadgi@gmail.com.
Vishnuprasad Madhusudan Bapat, Email: vmbapat@gmail.com.
Agrawal Preeti Balkisanji, Email: piyushdmch@yahoo.co.in.
Puranik Shaila Chandrakant, Email: puranikshaila@gmail.com.
References
- 1.Brown KE, Young NS. Parvovirus B19 infection and hematopoiesis. Blood Rev. 1995;9:176–182. doi: 10.1016/0268-960X(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 2.Cossart YE, Field AM, Cant B, Widdow D. Parvovirus like particles in human sera. Lancet. 1975;1(7898):72–73. doi: 10.1016/S0140-6736(75)91074-0. [DOI] [PubMed] [Google Scholar]
- 3.Brown KE, Anderson SM, Young NS. Erythrocyte P antigen: cellular receptor for B19 parvovirus. Science. 1993;262:114–117. doi: 10.1126/science.8211117. [DOI] [PubMed] [Google Scholar]
- 4.Wan Z, Zhi N, Wong S, Keyvanfar K, Liu D, Raghavachari N, Munson P, Su S, Malide D, Kajigaya S, Young N. Human parvovirus B19 causes cell cycle arrest of human erythroid progenitors via deregulation of the E2F family of transcription factors. J Clin Invest. 2010;120(10):3530–3544. doi: 10.1172/JCI41805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galel SA, Malone JM, Vivele MK.Transfusion Medicine. In: Greer JP, Rodgers GM, Foerster J, Paraskevas F, Lukens JN, Glader B.Wintrobe′s clinical hematology. 11th ed. Philadelphia:Lippincott Williams & Wilkins;2004. p.869-870
- 6.Brown KE, Young NS. Parvovirus and bone marrow failure. Stem cells. 1996;14:151–163. doi: 10.1002/stem.140151. [DOI] [PubMed] [Google Scholar]
- 7.Abkowitz JL, Brown KE, Wood RW, Kovach NL, Green SW, Young NS. Clinical relevance of parvovirus B19 as a cause of anemia in patients with Human Immunodeficiency virus infection. J Infect Dis. 1997;176:269–273. doi: 10.1086/517264. [DOI] [PubMed] [Google Scholar]


