Abstract
Coeliac disease is a systemic autoimmune disorder with major intestinal manifestations. It has multiple hematologic associations including anaemia (mostly due to iron, folate and/or vitamin B12 malabsorption), other cytopenias, coagulation abnormalities, hyposplenism, IgA deficiency and lymphomas. Aplastic anaemia has however, only rarely been described with celiac disease in published literature. We here present a case of atypical coeliac disease in a 40 year male Indian patient, with insignificant gastrointestinal symptoms, presenting with aplastic anaemia manifested by pancytopenia with hypocellular bone marrow. On gluten free diet, his symptoms like weakness, fatigue and malaise were relieved—blood and platelet transfusion requirement also diminished.
Keywords: Coeliac disease, Aplastic Anaemia, India
Introduction
Coeliac disease (CD), or gluten-sensitive enteropathy (GSE), is an autoimmune disorder of malabsorption and is characterized by reversible small bowel mucosal inflammation with villous atrophy affecting patients with a specific genetic predisposition (HLA-DQ2 allele in 90–95 % and HLA-DQ8 in the remainder) [1]. The mucosal lesion develops after ingestion of dietary gluten and recovers when gluten containing cereals (like wheat, rye, barley and oat) are withdrawn from the diet. CD can have multiple hematologic manifestations like anaemia, thrombocytosis, thrombocytopenia, leukopenia/neutropenia, venous thromboembolism, coagulopathy, hyposplenism, IgA deficiency and lymphoma [2]. Aplastic anaemia (AA) however, is only very rarely described in association with CD. Here we present a rare association of AA with CD in an adult male Indian patient.
Case Report
A 40 year old male patient from Burdwan District of West Bengal, India came to our out patients department (OPD) with symptoms of weakness, breathlessness on exertion and purpuric rashes all over the body. He was suffering from lethargy and weakness for last 6 months and purpuric rashes for last 1 month, after an episode of short duration fever. He was advised admission in a state medical college hospital due to severe anaemia and thrombocytopenia (Hb 5.1 g% and platelet count 60,000/cmm) but he refused admission. He probably took some haematinics and antipyretics (paracetamol) prescribed by local physician for several months before attending to our OPD. The patient was admitted in our hospital attached to the School of Tropical Medicine, Kolkata. On examination, we found that he had severe pallor and purpuric spots all over the body. No organomegaly or lymphadenopathy was present. Cardiovascular, Respiratory and Neurologic examinations were found to be normal.
Laboratory investigations revealed: Hb-6.9 g/dl; RBC-2.16 million/cmm, normocytic normochromic; Total Leukocyte Count-1,700/cmm (N-2 %, L-91 %, M-5 %, E-2 %); Platelet-15,000/cmm; PCV-20.4 %, MCV-94.5 fl, MCH-31.9 pg, MCHC-33.8 g/dl; Reticulocyte count 0.5 %. LFT: total bilirubin-1 mg % (conj-0.4 and unconj-0.6),total protein-6.7 g% (albumin-3.4 and globulin-3.3), ALT-22 IU/L, AST-19 IU/L, ALP-140 IU/L; Fasting Blood Sugar, urea, creatinine, Na+, K+—normal; PT and APTT—normal; Serum folic acid-11.8 ng/ml (normal 7–14), vitamin B12-484 pg/ml (normal 200–835), iron-114 μg/dl (normal 60–150), ferritin-3,965 ng/ml (normal 15–250), TIBC-324 μg/dl (normal 250–400); Elisa for HIV, HBsAg and anti- HCV-non-reactive. Chest X-ray and USG abdomen revealed no abnormality.
Bone marrow biopsy showed severely hypocellular fatty marrow due to marked depression of normal haematopoietic elements with a few erythroblasts and megakaryocytes remaining (Figure 1). So diagnosis of very severe AA was made and several units of packed cell and platelet concentrate were transfused. As he could not afford of bone marrow transplantation or anti-thymocyte globulin therapy, we treated him with cyclosporine starting with 100 mg twice daily and then gradually increasing to a total dose of 400 mg/day with folic acid 5 mg daily along with injection nandrolone decanoate 100 mg deep intramuscular weekly. He was discharged and again readmitted four times in our hospital with an interval of eight to 13 days with anaemia and thrombocytopenia. Platelet count gradually went down below 10,000/cmm. Each time he was given several units of packed cell and platelet concentrate.
Fig. 1.

Bone marrow biopsy showing severely hypocellular fatty marrow (H & E X 10)
During his sixth hospital admission, he complained of pain abdomen after meal and on enquiry, he revealed that he had been suffering from occasional pain abdomen and diarrhea for several years before he was referred to our hospital for anaemia and purpura. Keeping in mind of atypical CD, we performed serum anti tissue transglutaminase (tTG) antibody test (by micro ELISA method) and the result value was high—21.6 U/ml (normal range up to 15 U/ml). Upper Gasro Intestinal Endoscopy showed multiple punctate haemorrhagic spots in oesophagus, stomach and duodenum. Mucosa of second part of duodenum was flattened. Duodenal biopsy showed: Subtotal villous atrophy, lymphocyte infiltration in lamina propria along with intra-epithelial infiltration (Figs. 2, 3). Diagnosis of CD with immune bone marrow hypoplasia was made.
Fig. 2.
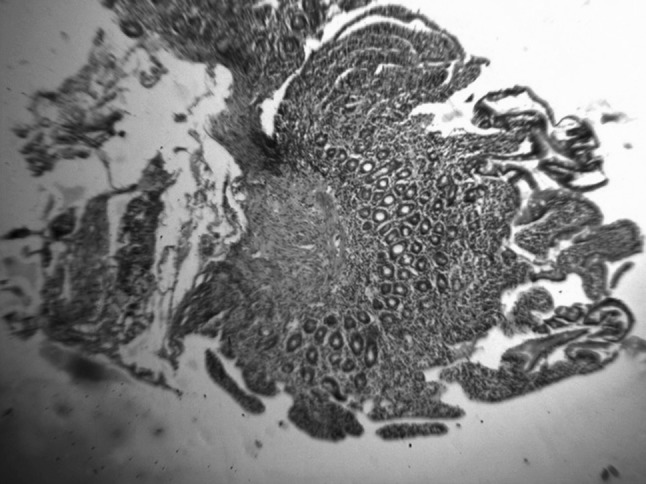
Duodenal histopathology showing subtotal villous atrophy (H & E X 10)
Fig. 3.
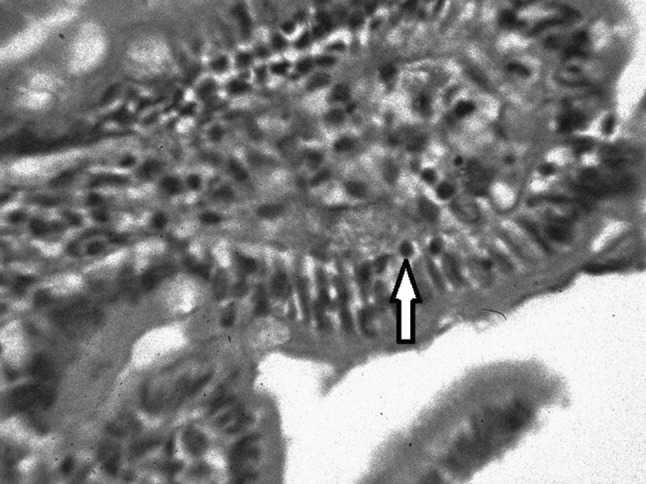
Duodenal histopathology showing intra epithelial lymphocytes (H & E X 100)
As the patient was from very poor socioeconomic condition, he could not continue cyclosporin. He was advised gluten free diet and oral prednisolone. During two months follow up of gluten free diet, he was feeling very well—weakness, breathlessness, fatigue, malaise, purpuric rashes completely disappeared. After two months of last blood transfusion, his Hb concentration was 7.4 gm %. Total leucocyte count 3,300/cmm (N-27 %, L-70 %, M-2 %, E-1 %) and platelet count rose to 30,000/cmm. The patient was kept in monthly follow up – his Hb and platelet count were increasing but he was lost to follow-up after 3 months. In the third month of follow up, his Hb was 8.8 g%, Total leucocyte count 3,400/cmm (N-31 %, L-65 %, M-2 %, E-2 %), platelet count 35,000/cmm and reticulocyte count was 1 %.
Before diagnosis of CD, he used to take blood transfusions every 2 weeks—whereas after gluten free diet and oral prednisolone, he had to take blood transfusion only once after 2 months, without any further requirement of blood or platelet concentrate in subsequent three months follow up.
Discussion
CD is not uncommon in India – it is prevalent in Bengal and Punjab provinces of India where wheat rather than rice is staple diet. Population-based screening in Chandigarh has shown the prevalence of CD is about one in 120 [3]. CD is considered an “iceberg” of a disease—“classical CD” in small number of patients, a larger number as “atypical CD” presenting with anaemia, osteopenia, infertility, neurologic symptoms; while an even larger group are essentially asymptomatic referred to as “silent CD”.
There are some case reports of anaemia with severe thrombocytopenia [4], and pancytopenia [5] in CD. Cause of pancytopenia in CD is multifactorial. Severe deficiency of Iron, folic acid, vitamin B12, and other nutrients can lead to pancytopenia. Leucopenia may be due to deficiency of folate and copper whereas thrombocytopenia may be autoimmune in nature. CD is associated with other organ-specific autoimmune disorders. A link with AA is in keeping, as it shares an underlying immune pathological mechanism, with auto-reactive T-cells mediating tissue-specific destruction.
Very few cases of association of CD with AA have been reported worldwide. First three cases of CD associated with AA were reported by Grey-Davies et al. [6]. All three patients were adult females and one of them died of severe sepsis. One patient improved, with bone marrow hypoplasia recovering, after 8 months of gluten free diet. She was also given oral cyclosporine and two courses of anti-thymoglobulin (ATG) (at 6 months interval). In another patient, CD with secondary hyperparathyroidism and osteomalacia was diagnosed. She commenced a gluten-free diet supplemented with 1-a-calciferol, calcium, vitamin B12 and folic acid. Bone pain and mobility improved but she remained pancytopenic. Six months later, her transfusion requirements had diminished and bone marrow revealed reduced fibrosis and trilineage haematopoiesis. Salmeron et al. [7] reported five patients diagnosed with AA and CD from their centre. CD was diagnosed in three patients at the same time as AA while the two other patients were diagnosed with CD before AA. All patients were treated with ATG plus cyclosporin. One patient responded to this regimen. The four other patients failed to respond to immunosuppression, three of whom underwent haematopoietic stem cell transplantation. Concerning CD, diarrhoea returned in 3 patients despite following a gluten-free diet. Histology at relapse showed the persistence of duodenal villous atrophy in one of them, which led to the clinical diagnosis of autoimmune enteropathy.
Only one case of AA–CD association has been reported from India. Maheswari et al. [8] reported a case of 13-year-old boy who presented with pain in bilateral knee and ankle joints accompanied by petechiae all over the body and hematochezia. On investigation for short stature, he was diagnosed as a case of celiac disease. His pancytopenia was evaluated to be due to aplastic anemia. He was started on gluten free diet and the counts improved over 8 months.
The cause-effect relationship in this association of AA–CD is debatable. Celiac disease is a unique autoimmune disorder in which the environmental precipitant, gluten, is involved and it may involve many organs in body including bone marrow. On the other hand, micronutrient deficiency in CD like iron, folic acid, vitamin B12, copper and others, as a result of malabsorption, may be instrumental in bone marrow hypoplasia. So gluten free diet, micronutrient supplement along with immunosuppressant therapy like cyclosporine and glucocorticoid may help recovery of bone marrow in AA–CD association, particularly in economically poor patients in developing countries who cannot afford bone marrow transplantation or ATG therapy. Cyclosporin therapy in AA may also modulate the course of CD, particularly in typical symptomatic gastrointestinal cases.
High serum ferritin with normal serum iron and TIBC observed in our patient might be the marker for acute phase reaction due to enhanced autoimmune reaction in body against gluten in diet. The explanation is hypothetical but it can be established with testing serum ferritin level in AA associated with CD in further studies.
‘Silent/atypical celiac disease’, in the absence of gastrointestinal symptoms, may be uncovered if clinicians are aware and vigilant about signs of CD in AA. Our patient was completely relieved of generalised symptoms like weakness, fatigue, lethargy and malaise which were mainly due to CD. His blood and platelet transfusion requirement also diminished. This was true for nine previously reported cases of AA–CD association of whom more than half cases did not require bone marrow transplantation.
Conclusion
In conclusion, an AA–CD association may be more frequent than previously thought. More studies are needed on this particular association, which has been largely underestimated until now. We urge clinicians to be vigilant for signs of CD in patients with aplastic anaemia.
References
- 1.Green PHR, Cellier CC. Coeliac disease. N Engl J Med. 2007;357:1731–1742. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 2.Halfdanarson TR, Litzow MR, Murray JA. Hematologic manifestations of coeliac disease. Blood. 2007;109:412–421. doi: 10.1182/blood-2006-07-031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lal S, Nain CK, Prasad KK, Thapa BR. Celiac disease in Chandigarh: a population survey. Indian J Gastroenterol. 2005;24(Supplement 1):A39. [Google Scholar]
- 4.Fisgin T, Yarali N, Duru F, Usta B, Kara A. Hematologic manifestation of childhood coeliac disease. Acta Haematol. 2004;111:211–214. doi: 10.1159/000077568. [DOI] [PubMed] [Google Scholar]
- 5.Lo W, Sano K, Lebwohl B, et al. Changing presentation of adult coeliac disease. Dig Dis Sci. 2003;48:395–398. doi: 10.1023/A:1021956200382. [DOI] [PubMed] [Google Scholar]
- 6.Grey-Davies E, Hows JM, Mars JCW. Aplastic anaemia in association with coeliac disease: a series of three cases. Br J Haematol. 2008;143:258–260. doi: 10.1111/j.1365-2141.2008.07341.x. [DOI] [PubMed] [Google Scholar]
- 7.Salmeron G, Patey N, de Latour RP, Raffoux E, Gluckman E, Brousse N, Socié G, Robin M. Coeliac disease and aplastic anaemia: a specific entity? Br J Haematol. 2009;146:122–124. doi: 10.1111/j.1365-2141.2009.07719.x. [DOI] [PubMed] [Google Scholar]
- 8.Maheshwari A, Nirupam N, Aneja S, Meena R, Chandra J, Kumar P. Association of coeliac disease with aplastic anaemia. Indian J Pediatr. 2012;79(10):1372–1373. doi: 10.1007/s12098-011-0579-6. [DOI] [PubMed] [Google Scholar]


