Abstract
Forty-five years old male, a known case of T cell acute lymphoblastic leukemia (ALL) on maintenance therapy presented with bilateral painless progressive diminution of vision. Evaluation revealed cytomegalovirus (CMV) retinitis with low CD4 counts. CMV retinitis is usually seen in HIV disease or in post allogenic stem cell transplant recipients. CMV retinitis occurring in ALL maintenance phase is very rare. The disease is aggressive and shows incomplete response to medical therapy.
Introduction
Cytomegalovirus (CMV) or Human Herpes Virus (HHV)-5 is a DNA virus which is known to cause opportunistic infection in immuno-compromised hosts. It can involve any organ system, commonest being gastrointestinal tract. Diagnosis of CMV infection is made by the isolation of CMV virus or detection of viral protein or nucleic acid in body fluids or tissue specimen. However, CMV retinitis is diagnosed by the presence of characteristic lesions on fundoscopy in immuno-compromised patients by an experienced ophthalmologist [1]. CMV retinitis is very rare in acute lymphoblastic leukemia especially during the maintenance therapy [2, 10, 11]. There are no studies which have looked at this entity in the past and currently there are only case reports that have been published reporting CMV retinitis after high dose chemotherapy especially during the induction phase [3–5]. CMV retinitis is usually treated with IV ganciclovir [12, 13]. We present an interesting and an extremely rare case of CMV retinitis in a case of acute lymphoblastic leukemia diagnosed during the maintenance phase of chemotherapy.
Case Report
This 45 year old male patient, who was diagnosed as a case of T-cell acute lymphoblastic leukemia (ALL) on maintenance chemotherapy since March 2011, presented with insidious onset progressive blurring of vision. At presentation, he had no high risk features such as a high leucocyte counts, presence of mediastinal mass, CNS disease or testicular involvement. His post-induction bone marrow was in remission and the bone marrow cytogenetics was suggestive of normal male karyotype. Hence, he had a favorable prognosis with respect to his disease. He was given BFM-ALL 95 protocol and was on maintenance phase since March 2011. The protocol for maintenance phase included oral prednisolone 100 mg/day for 5 days in a month, IV vincristine 2.0 mg every month, oral methotrexate 35 mg once a week and 6-mercaptopurine 100 mg daily along with intrathecal methotrexate 12.5 mg every 3 months. As a part of protocol at our center, for all patients of ALL during the maintenance phase, prophylaxis with sulphamethoxazole/trimethoprim for Pneumocystis jiroveci pneumonia and oral fluconazole for invasive candidiasis is routinely given. Anti-viral prophylaxis is not included both during induction as well as the maintenance phase in this protocol. Our patient had completed 1 year and 3 months of maintenance chemotherapy when he had presented to us with progressive painless diminution of vision, which was initially in the left eye and gradually it progressed to the right eye. There was a lag period of approximately 2 months and 20 days between the involvement of left and right eye. There were no associated complaints of pain, epiphora, grittiness, diplopia or itching in the eyes, headache, vomiting, jaundice, fever, breathlessness, loose stools or pain in abdomen and any focal neurological deficits. On clinical examination there was no obvious pallor, icterus or lymphadenopathy. The systemic examination was also within normal limits. A meticulous examination of both the eyes done by the ophthalmologist revealed normal eyeball movements and normal bulbar and palpebral conjunctiva. Vision was 6/24 in the left eye and 6/30 in the right eye. His biochemical parameters revealed mild anemia with Hemoglobin of 11 g/L, normal total leukocyte count; 4.8 × 109/L and platelet count; 210 × 109/L. The peripheral smear was normal with no evidence of relapse of the disease. His HIV done thrice was negative. Considering his clinical presentation, in consultation with the ophthalmologist, the differential diagnosis that were considered were relapsed disease, optic nerve compression, retinal hemorrhage, steroid induced cataract, glaucoma, CMV retinitis, toxoplasma retinitis or a simple refractive error of the left eye. His subsequent fundoscopy showed hemorrhages in the left eye characteristic of CMV retinitis (Fig. 1). A diagnosis of CMV retinitis was hence made for which he was initiated on oral valganciclovir 900 mg twice daily, 4 days after this he underwent a session of laser photocoagulation. However, in view of continuing deterioration in his vision and lack of response even after 2 weeks of treatment he was taken for a repeat session of laser photocoagulation of the left eye and his maintenance chemotherapy was also discontinued. Despite all this, there was no improvement in vision in his left eye and the visual loss had progressed to his right eye. At this point of time, he was administered a single dose of 1,000 μg (0.1 mL) intravitreal ganciclovir and was admitted and initiated on IV ganciclovir at the recommended dose of 5 mg/kg twice daily which was continued for 2 weeks. In the meanwhile a repeat fundoscopy done showed hemorrhages with exudates in the right eye also characteristic of CMV retinitis (Fig 2). His CMV PCR copy number was 859 copies/mL at this point of time. Unfortunately, the disease showed relentless clinical progression leading to retinal detachment of the left eye for which an emergency buckling surgery combined with an anterior chamber intra-ocular lens (ACIOL) implantation was done with phaco-emulsification. Following this, he had some improvement in the vision of the left eye. He was switched back to oral valganciclovir and was restarted with the maintenance chemotherapy. On follow up, a repeat fundoscopy revealed absence of exudates and hemorrhages however residual hemorrhages were still noted in the left eye (Figs. 3, 4). He was continued on oral valganciclovir for the next 6 months however a repeat CMV copy number showed only a mild decrease and was 600 copies/mL checked on his last follow up. Till date, examination of his eyes as on his last follow up in March 2013 revealed decreased vision limited to only finger count in the left eye and 6/36 vision in the right eye. The chronological sequence of events are mentioned in Table 1.
Fig. 1.
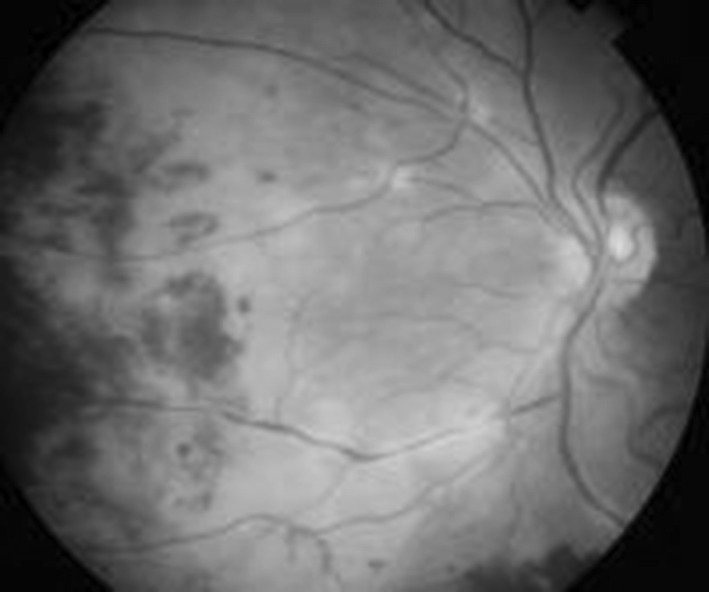
Fundoscopy image of left eye taken on 14-07-2012 showing hemorrhages and exudates present diffusely on the temporal and nasal part of the retina. Patient started having complaints of progressive painless diminution of vision in the left eye for which this fundoscopy was done. These lesions were typical of CMV retinitis
Fig. 2.
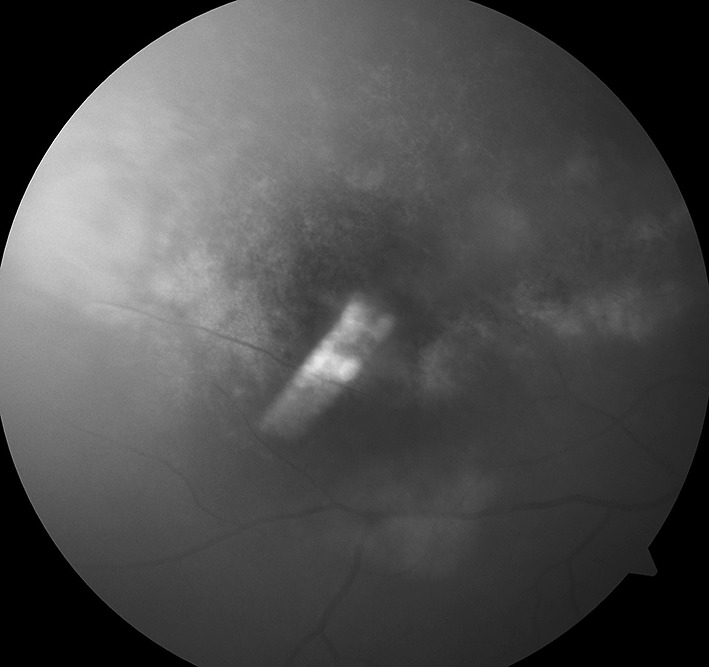
Fundoscopy image of right eye taken on 13-09-2012 showing diffuse exudates and minimal hemorrhages present on temporal and nasal part of the retina. Patient started complaining of progressive painless diminution of vision in right eye also while on Inj Ganciclovir therapy which was recently started on 08-09-2012
Fig. 3.
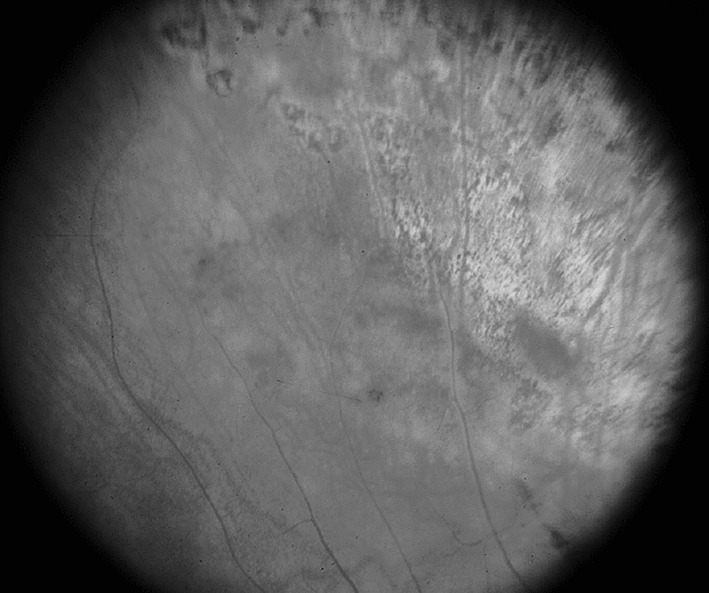
Fundoscopy image of right eye taken on 11-02-2013 showing normal retina with healed lesions seen previously and the patient also having vision of 6/36 in right eye
Fig. 4.
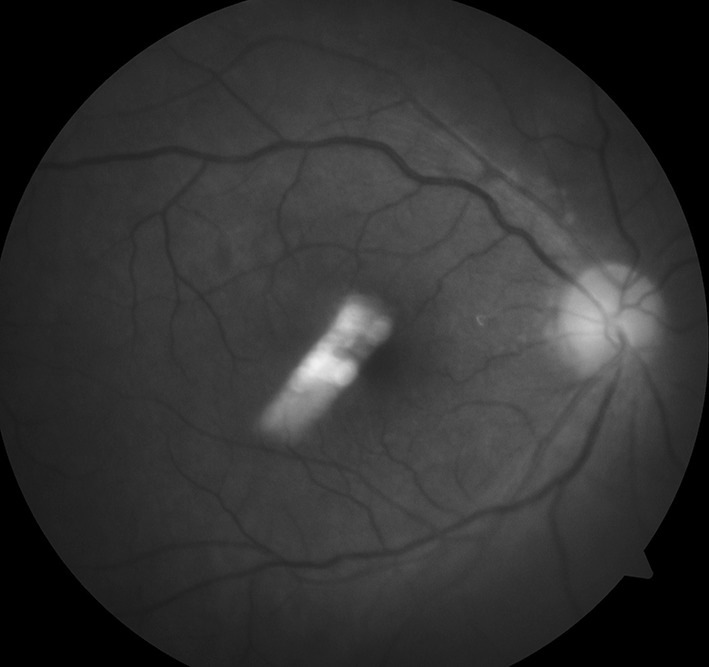
Fundoscopy image of left eye taken on 11-02-2013 still showing diffuse hemorrhages, exudates and scarring involving temporal and nasal part of the retina. There was also evidence of retinal detachment. At this point of time his vision was just finger counting at 1 m
Table 1.
Showing the sequence of events with followup of the patients in chronological order
| Date | Maintenance chemotherapy | Clinical features | Fundoscopic changes | Anti-viral therapy | CMV load |
|---|---|---|---|---|---|
| 14/07/12 | Continued | Painless progressive visual loss in left eye | Hemorrhages and exudates in the left eye | Oral valganciclovir 900 mg BD | – |
| 18/07/12 | Continued | Still present | Exudates and hemorrhages increased | Nd-YAG laser photocoagulation done in left eye | – |
| 01/08/12 | Stopped | Visual loss still present | Fundoscopic findings almost same | Repeat photocoagulation done with oral valganciclovir continued | – |
| 29/08/12 | Withheld | Developed visual loss in right eye with no improvement in left eye | Increased exudates and hemorrhages with characteristic cheese/pepper appearance seen in both eyes L > R | Oral valganciclovir continued with intra-vitreal injection given in left eye | – |
| 07/09/12 | Withheld | Same vision | – | Oral valganciclovir stopped and started on Inj Ganciclovir which was given from 08/09/12 to 24/09/12 | 859 copies/mL |
| 25/09/12 | Withheld | Same vision | – | Oral valganciclovir restarted with retinal detachment surgery and ACIOL implantation done | – |
| 08/10/12 | Restarted | Vision improved in right eye to 6/60 and only finger counting at 1 m in left eye | Healing changes seen in right eye with absence of exudates and hemorrhages In left eye residual hemorrhages are seen with absent exudates |
Oral valganciclovir continued | – |
| 05/11/12 | Continued | Same vision | – | Oral valganciclovir continued | – |
| 10/12/12 | Continued | Same vision | – | Oral valganciclovir continued | – |
| 07/01/13 | Continued | Left eye same vision with 6/36 in right eye | – | Oral valganciclovir continued | – |
| 11/02/13 | Continued | Same vision | Healing changes seen in both eyes but in left eye it was associated with retinal detachment and fibrosis around macula | Oral valganciclovir stopped | 600 copies/mL |
| 18/03/13 | Maintenance was stopped at the end of March as he completed 2 years | Same vision | – | Oral valganciclovir stopped | – |
This table also shows the phase of chemotherapy, visual acuity, fundoscopy, CMV copy number, treatment given for CMV retinitis and the details of chemotherapy patient was undergoing
Discussion
CMV causes a self limiting mononucleosis type of syndrome in immuno-competent patients, however in immuno-compromised hosts it can cause significant disease. Without any anti-viral prophylaxis, 8–39 % of solid organ transplantation and 20–35 % of allogenic HSCT recipients develop CMV infection [6]. It can involve any organ system with the commonest being gastrointestinal tract. Diagnosis of CMV infection is made by the isolation of CMV virus or detection of viral protein or nucleic acid in any body fluid or tissue specimen. “CNS disease” is defined by the identification of CNS symptoms together with the detection of CMV in CSF samples, by culture or PCR, or in brain biopsy specimens, by culture, histopathologic testing, immuno-histochemical analysis, or in situ hybridization. Other system involvement is diagnosed by detection of CMV by virus isolation, histopathologic testing, immuno-histochemical analysis, or in situ hybridization. Detection of CMV by PCR alone is insufficient for diagnosis. CMV retinitis is diagnosed by the presence of characteristic lesion on fundoscopy in immuno-compromised state as confirmed by ophthalmologist [2]. CMV disease occurring in ALL particularly during maintenance is very rare. Ocular involvement in acute leukemia most commonly involve retina (70 %) [1, 7, 8]. CMV retinitis causes necrosis, vascular sheathing, hemorrhages, and combined exudative and rhegmatogenous retinal detachment [9]. The typical fundoscopic findings in the early stages reveals 1 or 2 foci of disease as multifocal disease at presentation is uncommon. Most early lesions occur in a perivascular distribution (hematogenous spread of the virus). Lesions that present posteriorly appear along retinal vessels as large areas of thick white infiltrates accompanied by retinal hemorrhages described as “pizza pie” or “cheese pizza” in appearance. Lesions begin peripherally and spread posteriorly in a contiguous fashion. However, the prevalence of CMV antigenemia and disease in patients with hematological malignancies who are not transplant recipients or HIV infected is largely unknown and is thought to be low [4]. The patient had lymphocytopenia and decreased number of CD4-positive lymphocytes for a year. This hence suggested that cellular immunodeficiency was critical in the development of retinitis. We propose, when CD4-positive lymphocyte counts are low during maintenance chemotherapy, immediate ophthalmologic examination and CMV testing is warranted in cases with disturbances of visual acuity. Most patients with CMV retinitis will initially present with unilateral disease. CMV disease if left untreated, in an immuno-compromised patient has a 50 % risk of progression to the contralateral eye within 6 months.
Acknowledgments
Acknowledgments
No financial assistance was taken for this study.
Conflict of interest
None.
References
- 1.Ljungman Per. Definitions of CMV infections and disease in transplant recipients. Clin Infect Dis. 2002;34:1094–1097. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 2.Wakai K, Sano H, Shimada A, Shiozawa Y, Hayashi Y, Park MJ, Sotomatsu M, Yanagisawa R, Koike K, Kozawa K, Ryo A, Tsukagoshi H, Kimura H. Cytomegalovirus retinitis during maintenance therapy for T-cell acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2013;35(2):162–163. doi: 10.1097/MPH.0b013e318279e920. [DOI] [PubMed] [Google Scholar]
- 3.Taha Ruba, et al. Two ocular infections during conventional chemotherapy in a patient with acute lymphoblastic leukemia: a case report. Case Rep Oncol. 2010;3:234–239. doi: 10.1159/000318230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alemayehu W, Shamebo M, Bedri A, Mengistu Z. Ocular manifestations of leukaemia in Ethiopians. Ethiop Med J. 1996;34:217–224. [PubMed] [Google Scholar]
- 5.Libby E, Movva S, Quintana D, Abdul-Jaleel M, Das A. Cytomegalovirus retinitis during chemotherapy with rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone. J Clin Oncol. 2010;28(32):e661–e662. doi: 10.1200/JCO.2010.29.6467. [DOI] [PubMed] [Google Scholar]
- 6.Nichols WG, Boeckh MJ. Recent advances in the therapy and prevention of CMV infections. J Clin Virol. 2000;16:25–40. doi: 10.1016/S1386-6532(99)00065-7. [DOI] [PubMed] [Google Scholar]
- 7.Shibata K, Shimamoto Y, Nishimura T, Okinami S, Yamada H, Miyahara M. Ocular manifestation in adult T cell leukaemia/lymphoma. Ann Hematol. 1997;74:163–168. doi: 10.1007/s002770050276. [DOI] [PubMed] [Google Scholar]
- 8.Cogan et al DG. Immunosuppression and eye disease. Am J Ophthalmol. 1977;83:777–788. doi: 10.1016/0002-9394(77)90903-5. [DOI] [PubMed] [Google Scholar]
- 9.Meredith TA, Aaberg TM, Reeser FH. Rhegmatogenous retinal detachment complicating CMV retinitis. Am J Ophthalmol. 1979;87:793–796. doi: 10.1016/0002-9394(79)90356-8. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi R, Takanashi K, Suzuki D, Nasu T, Uetake K, Matsumoto Y. Retinitis from cytomegalovirus during maintenance treatment for acute lymphoblastic leukemia. Pediatr Int. 2012;54(2):288–290. doi: 10.1111/j.1442-200X.2011.03429.x. [DOI] [PubMed] [Google Scholar]
- 11.Moritake H, Kamimura S, Kojima H, Shimonodan H, Harada M, Sugimoto T, Nao-I N, Nunoi H. Cytomegalovirus retinitis as an adverse immunological effect of pulses of vincristine and dexamethasone in maintenance therapy for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60(2):329–331. doi: 10.1002/pbc.24298. [DOI] [PubMed] [Google Scholar]
- 12.Singh R, Trehan A, Jain R, Bhalekar S. Cytomegalovirus retinitis in an ALL child on exclusive chemotherapy treated successfully with intravitreal ganciclovir alone. J Pediatr Hematol Oncol. 2012;26(2):237–240. doi: 10.1097/MPH.0b013e31827078ad. [DOI] [PubMed] [Google Scholar]
- 13.Urakami Tomoko, Nishiuchi Ritsuo, Oda Megumi, Takemoto Chikayo, Tomiyama Yoshie, Manki Akira, Seino Yoshiki. Successful treatment of cytomegalovirus retinitis in a child with acute lymphoblastic leukemia during maintenance chemotherapy. Jpn J Pediatr Hematol. 2002;16(5):312–316. [Google Scholar]


