Abstract
Wiskott-Aldrich syndrome (WAS) is an X linked rare primary immunodeficiency syndrome with an increased propensity for infection, autoimmunity and malignancy. Here we report a male child, who was diagnosed with WAS at 1 year of age following evaluation for symptomatic thrombocytopenia and eczematous skin lesions. He presented later with lymphadenopathy, which was consistent with diffuse large B cell lymphoma on histopathology. He received 6 cycles of R-CHOP chemotherapy for the same and is presently in remission after 6 months. We review the major publications of lymphoma in WAS and discuss the pathological findings, treatment and prognosis of lymphoma in WAS.
Keywords: Diffuse large B cell lymphoma, Wiskott-Aldrich syndrome, Primary immunodeficiency syndrome
Introduction
Wiskott-Aldrich syndrome (WAS) is one of the rarer primary immunodeficiency syndromes, presenting in 1–10 per 1,00,00,00 population [1]. Patients usually present with bleeding manifestations secondary to microthrombocytopenia, eczema and recurrent infections [2, 3].Malignancies are commoner in patients with WAS, and these patients usually have a poorer prognosis with a median overall survival (OS) of around 1 year [4]. A male child who was diagnosed with WAS on the basis of family history, clinical presentation, blood parameters and genotypic analysis later went on to develop diffuse large B cell lymphoma (DLBCL) 10 years after initial diagnosis of WAS. This to our knowledge, is the first report from India of a patient with WAS who developed a DLBCL.
Case Report
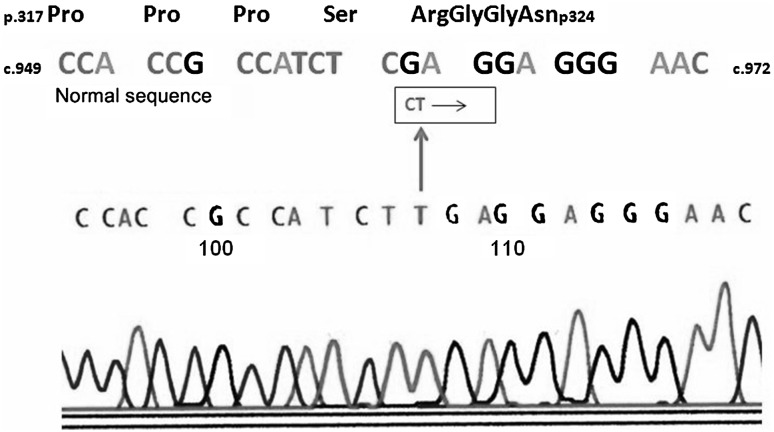
A one year old male child, born to non-consanguineous parents, with no significant antenatal, natal or neonatal history, presented with history of purpuric lesions all over the body with occasional bleeding from gums and recurrent respiratory tract infections from six months of age. Clinical examination was insignificant except for eczematous lesions over the scalp and post auricular region and multiple ecchymotic patches over the upper and lower limbs. A similar history was elicited from his parents about his sibling, who had similar complaints and was diagnosed as immune thrombocytopenia elsewhere, had undergone splenectomy for the same and succumbed to post splenectomy sepsis at 6 years of age. With a clinical suspicion of Wiskott-Aldrich syndrome (WAS), he was investigated further. At presentation he had a hemoglobin of 10.6 gm/dl with a total leukocyte count of 9,600/mm3 (neutrophils-35 %, lymphocytes-45 %).His platelet counts were 15,000/mm3 with a mean platelet volume of 6 fl. Serum quantitative Immunoglobulin analysis showed normal levels of IgG and IgM with mildly decreased levels IgA of 112 mg % (Ref normal range: 140–420). Mutation analysis revealed a nonsense mutation in exon 10 of the WAS gene at c.961C>T (p.Arg321*) that predicts premature termination of translation of WAS protein confirming the diagnosis (Fig. 1). Genomic DNA from the patient’s EDTA anti-coagulated blood was isolated by standard phenol-chloroform method. The human WAS gene exonic and flanking intronic regions were amplified by six pairs of primers as described previously [5]. Nucleotide changes in the amplified fragments were screened by a conformation sensitive gel electrophoresis (CSGE) and DNA sequencing strategy [6]. Samples displaying abnormal CSGE patterns were sequenced by the Big Dye Terminator cycle sequencing kit (Applied Biosystems, Warrington, UK) on an ABI 3130 genetic analyzer (PE Applied Biosystems, Foster City, CA, USA). The patient was offered stem cell transplantation but they deferred the treatment because of the absence of a matched family donor. The patient subsequently was lost to follow up.
Fig. 1.
Electropherogram of WASP gene in the patient demonstrating a c.961C>T mutation. This nucleotide change predicts a p.Arg321* nonsense mutation in the patient
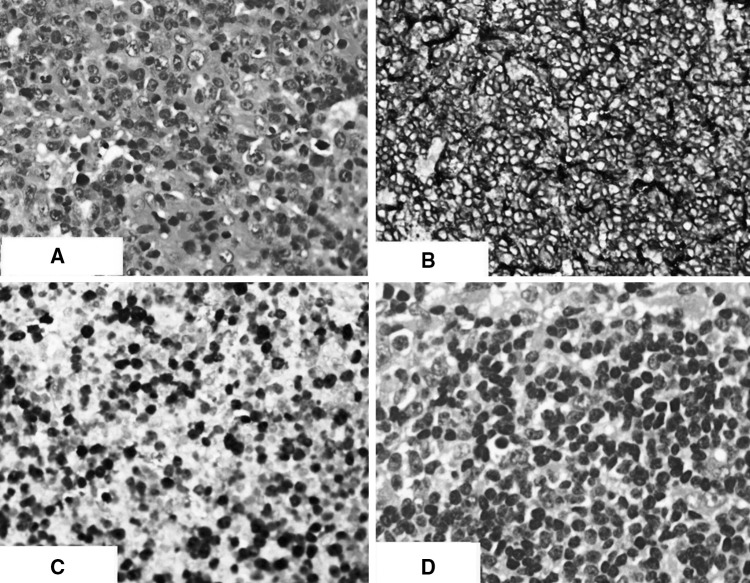
He presented 9 years later with left sided neck swelling of 8 months duration. Clinical examination revealed left lower cervical and submandibular lymph nodes (1 × 1 cm), right axillary lymph nodes (3 × 3 cm) and bilateral superficial inguinal lymph nodes (2 × 2 cm). Rest of the clinical examination was unremarkable. His blood investigations showed a hemoglobin of 11.7 gm/dl, a total leukocyte count of 9,900/mm3 (neutrophils-19 %, lymphocytes-25 %, eosinophils-55 %) and a platelet count of 10,000/mm3. Abdominal imaging revealed multiple, large, coalescent nodes in the periportal, peri pancreatic and upper para aortic region, upto 3 cm in size. The patient subsequently underwent a right axillary lymph node biopsy which was reported as Diffuse large B cell lymphoma (Large cells: CD 20 positive, CD 30 and CD 15 negative, EBV LMP negative, MIB 1 index-70 %). Atrophic germinal centres typical of Wiskott-Aldrich syndrome were found (Fig. 2). Bone marrow showed reactive eosinophilia with no evidence of lymphoma. He was staged as Ann Arbor IIIA and was started on R-CHOP 21 chemotherapy. He received a total of 6 cycles of chemotherapy with a good response to treatment and a PET CT scan done 3 months after completion of chemotherapy shows him to be in complete remission. He is presently in remission at 6 months of follow up.
Fig. 2.
Axillary lymph node biopsy specimen showing: a (×400) Large cells on H & E stain; b (×200) CD 20 positive large cells; c (×200) MIB 1 staining; d (×400) H & E staining characteristic atrophic lymph node follicles in WAS
Discussion
WAS is a rare X linked primary immunodeficiency syndrome characterized by micro thrombocytopenia, eczematous skin lesions, and recurrent infections [2, 3, 7, 8]. The protein involved, WASP, is encoded by the WAS gene [9]. It is an important regulator of actin polymerization in response to signals from the cell membrane, and is predominantly expressed in hematopoietic cells [1, 3, 8]. WAS gene mutations resulting in mutated WASP can cause full blown WAS to X linked thrombocytopenia (XLT) [10] which is associated with thrombocytopenia without significant immune impairment, and considered an attenuated version of WAS [1, 3, 8]. A recently described gain of function missense mutation at the Cdc42-binding site is associated with X linked severe congenital neutropenia [1, 8, 11].
WASP mutations in WAS and XLT result in micro thrombocytopenia, with a 100 % incidence. Though the mechanism has not been completely delineated, peripheral destruction of platelets is supposed to play a major role [1, 3]. Bleeding is thus the most common manifestation. Eczematous skin lesions are present in around 60–80 % patients [2, 3, 12]. Autoimmune hemolytic anemia is the most common autoimmune manifestation followed by arthritis, cutaneous vasculitis, Henoch Schonlein purpura [1–3] .
Patients with WAS have a high incidence (13–22 %) of tumors [2, 12, 13]. WAS gene mutation affects cells of both innate and acquired immunity, involving NK, B and T cells [1, 3, 7, 8]. Almost 90 % malignancies are of lymphoreticular origin, and have a poorer prognosis than in those with normal WASP protein [2, 3, 8]. As in other primary and acquired immunodeficiency syndromes EBV associated tumors are commoner in WAS patients [14, 15]. Abnormal immune surveillance in WAS patients underlies the increased chance of neoplastic transformation [1, 3, 7, 8]. There is decreased synapse between NK cells and tumor cells in WAS subjects [1] due to decreased actin accumulation at the synapse. This impairs NK cell mediated lysis of tumour cells.
Lymphoma has been widely described in patients with WAS [4, 15–19]. This to our knowledge is the first reported case of a diffuse large B cell lymphoma in a patient with WAS from the Indian subcontinent. Non Hodgkin’s lymphoma is the commonest tumor in WAS [4, 19]. The median age of diagnosis is 9.5 years [2]. Response to therapy directed towards the lymphoma is poor in these patients and median survival is around 1 year. Long term survival in WAS patients with lymphoma who has received appropriate chemotherapy have also been reported [20, 21]. Use of anti CD 20 monoclonal might improve the prognosis but awaits larger studies to validate it. Our patient received 6 cycles of R-CHOP chemotherapy after disease staging, and remains in remission at 6 months of follow up. Table 1 shows the major publications of lymphoma in patients with WAS. Allogeneic stem cell transplantation (SCT) from a suitable donor is the only curative therapy available for WAS. SCT has been successfully carried out in a patient with WAS and Non Hodgkin’s lymphoma with complete remission of both for over 2 years [22].
Table 1.
Review of major publications of lymphoma in WAS patients
| Author | Type of study | Described lymphoreticular malignancies | EBV status | Presentation | Treatment | Outcome |
|---|---|---|---|---|---|---|
| Cotelingam et al. [4] | Retrospective analysis | N = 24 NHL = 8 (7-Diffuse large cell immunoblastic) HL = 1 (Nodular sclerosis) | N/A | Median age-7.8 years NHL-Diffuse disease HL-Cervical and inguinal lymphadenopathy | Chemotherapy based | Median survival <1 year |
| Perry III et al. [23] | Retrospective analysis | N = 301 Lymphoreticular malignancies = 23 | N/A | Data not available | Data not available | Data not available |
| Frizzera et al. [14] | Retrospective analysis | N = 31 (Lymphoreticular disorders) WAS = 10 NHL = 5 HL = 1 Unclassified = 1 | N/A | Data not available | Data not available | Data not available |
| Gilson et al. [21] | Case report | DLBCL Ann Arbor IAE | – | 16, Male Orbital mass | Chemotherapy Presentation-UKCCSG for localised NHL Relapse-VCE × 6 cycles | Alive till 9 years of last follow up |
| Palenzuala et al. [24] | Case report | DLBCL Ann Arbor IAE | – | 15, Male Acute laryngitis: Laryngeal growth | CVP followed by local RT followed by Laser debulking | Died: Acute respiratory failure following Laser debulking |
| Nakanishi et al. [25] | Case report | DLBCL Ann Arbor IVB | + | 14, Male Aggressive lymphadenopathy, 20 years-Multiple CNS lesions | Local RT | Succumbed to Pseudomonas sepsis |
| Coccia et al [20] | Case report | DLBCL Ann Arbor IAE | – | 15 years, Male Pharyngeal growth | Chemotherapy-LNH 97 with Rituximab at 75 % dose | Alive and in remission at 3 years of last follow up |
| Kawakami et al. [26] | Case report | Follicular lymphoma with large cell transformation (Heterogeneous histology) | – | 21, Male Generalized lymphadenopathy | Not available | Died at 1 year of diagnosis |
| Kroft et al [17] | Case report | Follicular large cell lymphoma with immnoblastic features t(14,18), c-myc + Ann Arbor IB | – | 15, Male B symptoms, submandibular lymphadenopathy | Chemotherapy-CCG, Protocol 5911(regimen 1-Orange arm) MUD transplant in CR | Alive and in remission at 90 days post transplant |
| Faraci et al. [18] | Case report | Unclassified lymphoma | – | 8, Male Abdominal pain, distension. RLQ peritoneal mass involving distal jejunum | Surgical resection with distal jejunectomy -Intestinal obstruction after 6 weeks-Ileal resection (No residual disease) | Died after 4 ½ months-Massive subarachnoid haemorrhage. No residual lymphoma at autopsy |
| Pasic et al. [16] | Case report | Burkitt’s lymphoma | – | 12, Male Ileo-colic Intussusception | Terminal ileal resection-Diagnosis on surgical specimen biopsy: Rituximab based chemotherapy | In remission with chemotherapy |
| Periman et al. [27] | Case report | Hodgkin’s lymphoma: Nodular sclerosis | – | 16, Male Generalised lymphadenopathy with B symptoms | MOPP chemotherapy-Complete remission: Relapsed after 1 year-RT | Died 19 months after diagnosis of lymphoma |
| Yoshida et al. [15] | Case report | Hodgkin’s lymphoma: Lymphocyte depleted | + | 8, Male Bilateral pulmonary hilar lymphadenopathy | ABVD 4 courses followed by local fractionated RT (20 Gy) | In remission at 50 months of follow up |
| Sebire et al. [28] | Case report | Cutaneous lymphomatoid granulomatosis | + | Isolated, non healing, ulcerated skin lesion | Rituximab for 4 cycles | In remission at 18 months of follow up |
NHL Non Hodgkin lymphoma, HL Hodgkin lymphoma, N/A Not available, WAS Wiskott-Aldrich syndrome, DLBCL Diffuse large B cell lymphoma, UKCCSG United Kingdom Children’s Cancer Study Group, VCE Vincristine, Cisplatin, Etoposide, CVP Cyclophosphamide, Vincristine, Prednisolone, RT Radiation therapy, CNS Central nervous system, MUD Matched unrelated donor, CR Complete remission, RLQ Right lower quadrant, MOPP Mustine (Nitrogen mustard), Oncovin (Vincristine), Procarbazine, Prednisolone, ABVD Adriamycin, Bleomycin, Vinblastine, Dacarbazine
Conclusion
WAS is a rare primary immunodeficiency which can present in a myriad of clinical forms. Poor immunological response leads to an increased chance of malignancy, with NHL being the commonest. Prognosis of NHL in WAS remains poor, but the use of anti CD 20 monoclonal can improve the outcome in B cell neoplasms. Transplant remains the only curative therapy presently for WAS.
References
- 1.Ochs HD, Thrasher AJ. The Wiskott-Aldrich syndrome. J Allergy Clin Immunol. 2006;117(4):725–738. doi: 10.1016/j.jaci.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan KE, Mullen CA, Blaese RM, Winkelstein JA. A multiinstitutional survey of the Wiskott-Aldrich syndrome. J Pediatr. 1994;125(6, Part 1):876–885. doi: 10.1016/S0022-3476(05)82002-5. [DOI] [PubMed] [Google Scholar]
- 3.Bosticardo M, Marangoni F, Aiuti A, Villa A, Grazia Roncarolo M. Recent advances in understanding the pathophysiology of Wiskott-Aldrich syndrome. Blood. 2009;113(25):6288–6295. doi: 10.1182/blood-2008-12-115253. [DOI] [PubMed] [Google Scholar]
- 4.Cotelingam JD, Witebsky FG, Hsu SM, Blaese RM, Jaffe ES. Malignant lymphoma in patients with the Wiskott-Aldrich syndrome. Cancer Invest. 1985;3(6):515–522. doi: 10.3109/07357908509039813. [DOI] [PubMed] [Google Scholar]
- 5.Kwan SP, Hagemann TL, Radtke BE, Blaese RM, Rosen FS. Identification of mutations in the Wiskott-Aldrich syndrome gene and characterization of a polymorphic dinucleotide repeat at DXS6940, adjacent to the disease gene. Proc Natl Acad Sci. 1995;92(10):4706–4710. doi: 10.1073/pnas.92.10.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jayandharan G, Viswabandya A, Baidya S, Nair SC, Shaji RV, George B, et al. Six novel mutations including triple heterozygosity for Phe31Ser, 514delT and 516T→ G factor X gene mutations are responsible for congenital factor X deficiency in patients of Nepali and Indian origin. J Thromb Haemost. 2005;3(7):1482–1487. doi: 10.1111/j.1538-7836.2005.01339.x. [DOI] [PubMed] [Google Scholar]
- 7.Remold-O’Donnell E, Rosen FS, Kenney DM. Defects in Wiskott-Aldrich syndrome blood cells. Blood. 1996;87(7):2621–2631. [PubMed] [Google Scholar]
- 8.Orange JS, Stone KD, Turvey SE, Krzewski K (2004) The Wiskott-Aldrich syndrome. Cell Mol Life Sci [Internet] 61(18):2361–2385. Available from: http://link.springer.com/10.1007/s00018-004-4086-z. Accessed 29 Oct 2013 [DOI] [PMC free article] [PubMed]
- 9.Derry JMJ, Ochs HD, Francke U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell. 1994;78(4):635–644. doi: 10.1016/0092-8674(94)90528-2. [DOI] [PubMed] [Google Scholar]
- 10.Villa A, Notarangelo L, Macchi P, Mantuano E, Cavagni G, Brugnoni D, et al. X–linked thrombocytopenia and Wiskott-Aldrich syndrome are allelic diseases with mutations in the WASP gene. Nat Genet. 1995;9(4):414–417. doi: 10.1038/ng0495-414. [DOI] [PubMed] [Google Scholar]
- 11.Devriendt K, Kim AS, Mathijs G, Frints SG, Schwartz M, Van den Oord JJ, et al. Constitutively activating mutation in WASP causes X-linked severe congenital neutropenia. Nat Genet. 2001;27(3):313–317. doi: 10.1038/85886. [DOI] [PubMed] [Google Scholar]
- 12.Imai K. Clinical course of patients with WASP gene mutations. Blood. 2004;103(2):456–464. doi: 10.1182/blood-2003-05-1480. [DOI] [PubMed] [Google Scholar]
- 13.Ten Bensel RW, Stadlan EM, Krivit W. The development of malignancy in the course of the Aldrich syndrome. J Pediatr. 1966;68(5):761–767. doi: 10.1016/S0022-3476(66)80450-X. [DOI] [PubMed] [Google Scholar]
- 14.Frizzera G, Rosai J, Dehner LP, Spector BD, Kersey JH. Lymphoreticular disorders in primary immunodeficiencies: new findings based on an up-to-date histologic classification of 35 cases. Cancer. 1980;46(4):692–699. doi: 10.1002/1097-0142(19800815)46:4<692::AID-CNCR2820460410>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida K, Minegishi Y, Okawa H, Yata J, Tokoi S, Kitagawa T, et al. Epstein-Barr virus-associated malignant lymphoma with macroamylasemia and monoclonal gammopathy in a patient with Wiskott-Aldrich syndrome. Pediatr Hematol Oncol. 1997;14(1):85–89. doi: 10.3109/08880019709030889. [DOI] [PubMed] [Google Scholar]
- 16.Pasic S, Vujic D, Djuricic S, Jevtic D, Grujic B (2006) Burkitt Lymphoma-Induced Ileocolic Intussusception in Wiskott-Aldrich Syndrome. J Pediatr Hematol Oncol [Internet] 28(1):48–49. Available from: http://journals.lww.com/jpho-online/Fulltext/2006/01000/Burkitt_Lymphoma_Induced_Ileocolic_Intussusception.11.aspx [PubMed]
- 17.Kroft S, Finn W, Singleton T, Ross C, Sheldon S, Schnitzer B. Follicular large cell lymphoma with immunoblastic features in a child with Wiskott-Aldrich syndrome: an unusual immunodeficiency-related neoplasm not associated with Epstein-Barr virus. Am J Clin Pathol. 1998;110(1):95–99. doi: 10.1093/ajcp/110.1.95. [DOI] [PubMed] [Google Scholar]
- 18.Faraci RP, Hoffstrand HJ, Witebsky FG, Blaese R, Beazley RM. Malignant lymphoma of the jejunum in a patient with Wiskott-Aldrich syndrome: surgical treatment. Arch Surg. 1975;110(2):218–220. doi: 10.1001/archsurg.1975.01360080084016. [DOI] [PubMed] [Google Scholar]
- 19.Du S, Scuderi R, Malicki DM, Willert J, Bastian J, Weidner N. Hodgkin’s and non-Hodgkin’s lymphomas occurring in two brothers with Wiskott-Aldrich syndrome and review of the literature. Pediatr Dev Pathol. 2010;14(1):64–70. doi: 10.2350/10-01-0787-CR.1. [DOI] [PubMed] [Google Scholar]
- 20.Coccia P, Mastrangelo S, Ruggiero A, Scalzone M, Rosolen A, Maurizi P, et al. Treatment of pharyngeal non-Hodgkin lymphoma in a patient with Wiskott-Aldrich syndrome. Pediatr Blood Cancer. 2012;59(2):318–319. doi: 10.1002/pbc.23393. [DOI] [PubMed] [Google Scholar]
- 21.Gilson D, Taylor R. Long-term survival following non-Hodgkin’s lymphoma arising in Wiskott-Aldrich syndrome. Clin Oncol. 1999;11(4):283–285. doi: 10.1053/clon.1999.9066. [DOI] [PubMed] [Google Scholar]
- 22.Tavil B, Erdem AY, Azik F, Isik P, Metin A, Emir S, et al. Successful allogeneic hemopoietic stem cell transplantation in a case of Wiskott-Aldrich syndrome and non-Hodgkin lymphoma. Pediatr Transplant. 2013;17(6):E146–E148. doi: 10.1111/petr.12114. [DOI] [PubMed] [Google Scholar]
- 23.Perry GS, III, Spector BD, Schuman LM, Mandel JS, Anderson VE, McHugh RB, et al. The Wiskott-Aldrich syndrome in the United States and Canada (1892–1979) J Pediatr. 1980;97(1):72–78. doi: 10.1016/S0022-3476(80)80133-8. [DOI] [PubMed] [Google Scholar]
- 24.Palenzuela G, Bernard F, Gardiner Q, Mondain M. Malignant B cell non-Hodgkin’s lymphoma of the larynx in children with Wiskott-Aldrich syndrome. Int J Pediatr Otorhinolaryngol. 2003;67(9):989–993. doi: 10.1016/S0165-5876(03)00155-1. [DOI] [PubMed] [Google Scholar]
- 25.Nakanishi M, Kikuta H, Tomizawa K, Kojima K, Ishizaka A, Okano M, et al. Distinct clonotypic Epstein-Barr virus-induced fatal lymphoproliferative disorder in a patient with Wiskott-Aldrich syndrome. Cancer. 1993;72(4):1376–1381. doi: 10.1002/1097-0142(19930815)72:4<1376::AID-CNCR2820720437>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 26.Kawakami K, Yamaguchi M, Watanabe Y, Murata T. Development of Diffuse Large Cell Lymphoma from Follicular Lymphoma with Multiple Immunoglobulin Heavy Chain Gene Rearrangement Occurring in a Patient with Wiskott-Aldrich Syndrome. Int J Hematol. 2002;76(2):196–198. doi: 10.1007/BF02982585. [DOI] [PubMed] [Google Scholar]
- 27.Periman P, Callihan TR, Lessin L, King GW, Blaese M. Hodgkin’s disease occurring in a patient with the Wiskott-Aldrich syndrome. Cancer. 1980;45(2):372–376. doi: 10.1002/1097-0142(19800115)45:2<372::AID-CNCR2820450230>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 28.Sebire NJ, Haselden S, Malone M, Davies EG, Ramsay AD. Isolated EBV lymphoproliferative disease in a child with Wiskott-Aldrich syndrome manifesting as cutaneous lymphomatoid granulomatosis and responsive to anti-CD20 immunotherapy. J Clin Pathol. 2003;56(7):555–557. doi: 10.1136/jcp.56.7.555. [DOI] [PMC free article] [PubMed] [Google Scholar]