Abstract
Primary hepatic lymphoma (PHL) is a very rare sub-type of non-Hodgkin’s lymphoma and hepatitis C infection may be a contributory factor. The association of hepatitis C infection and PHL causes difficulties in management since safety of rituximab in such situations is unclear due to lack of evidence. The role of anti-viral therapy in combination with chemotherapy is also uncertain. We describe the diagnostic and therapeutic challenges posed by a patient who was diagnosed with PHL and concurrent hepatitis C infection.
Keywords: Primary hepatic lymphoma, Hepatitis C
Introduction
Even though hepatic involvement occurs secondarily in about 20 % of patients diagnosed with non-Hodgkin’s lymphoma, primary hepatic lymphoma (PHL) is very rare in NHL. Hepatitis C infection is one of the known causes for PHL and in patients with PHL and hepatitis C infection the clinical and radiological presentation may resemble hepatocellular carcinoma (HCC). Treatment induced immunosuppression can alter the balance between hepatitis C viral proliferation and host immune response with an increased incidence of severe hepatotoxicity. Management of patients with PHL and co-existent hepatitis C infection may be difficult since the role of anti-viral therapy in combination with chemotherapy is unclear and there is a lack of evidence about safety of rituximab in such situations.
Case Report
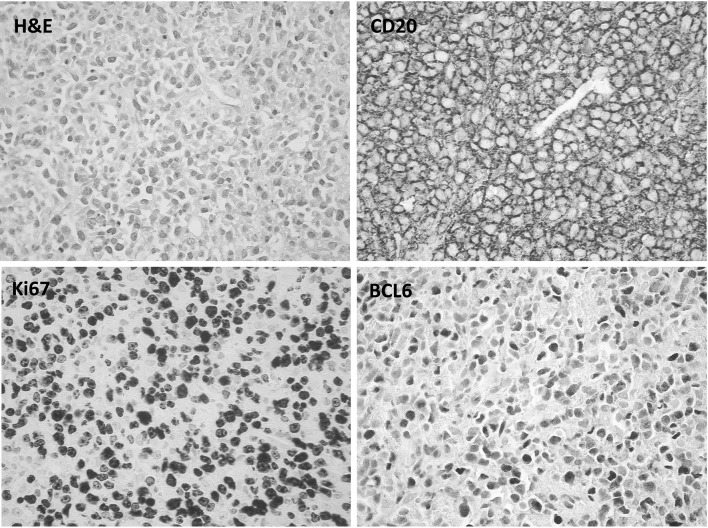
A 48-year-old Caucasian female patient presented to emergency in June 2011 with abdominal pain of 2 weeks duration and weight loss of 8 kg over 2 months. She was a past intravenous drug user with a history of hepatitis C virus (HCV) infection for 5 years prior. However there were no history of acute or chronic liver impairment and had not received any treatment for hepatitis-C infection previously. Clinical examination revealed mild scleral icterus and enlarged tender liver 5 cm below right costal margin. There was no palpable lymphadenopathy. Her performance status on ECOG scale was 2. At presentation the full blood counts showed normal values of haemoglobin (122 g/l), white cell count (5.4 × 109/l) and platelet count (182 × 109/l). Her liver function tests were moderately deranged—bilirubin level was elevated (52 mmol/l, normal range: 0–21 mmol/l); alanine transferase (ALT) and aspartate transferase (AST) levels were 1.5 times the upper limit of normal; gamma glutaryl transferase (GGT) and alkaline phosphatase (ALP) levels were approximately two times the upper limit of normal. Lactate dehydrogenase (LDH) level was moderately elevated (1,145 U/l, normal range: 125–243 U/l) and uric acid level was high (0.48 mmol/l, normal range: 0.15–0.36 mmol/l). The genotype for hepatitis C was 1b and the viral load was high (691,513 copies/ml, log10 value was 5.840 copies/ml). However, hepatitis B, HIV and Epstein Barr Virus (EBV) serologies were negative and alpha fetoprotein (AFP) levels were not increased. Triphasic liver CT scan showed hepatomegaly with multiple intra-hepatic masses of varying sizes, measuring up to 8 cm in size. Most of masses had irregular central hypodensity due to central necrosis and there was no associated biliary tract dilatation (Fig. 1a, b). Based on the CT scan picture of large liver masses with central necrosis and the background of active hepatitis C infection, a provisional diagnosis of multifocal HCC was made. She underwent emergency surgical wedge biopsy of liver and the frozen section was examined. Histopathological examination demonstrated large sheets of pleomorphic lymphoid cells. Majority of these cells were large sized with minimal cytoplasm and the nucleoli showed variable prominence. Immunohistochemical staining performed on fixed, paraffin-embedded tissue sections demonstrated strong positive membranous staining of CD45 and CD20 antigen on majority of the cells, whereas CD5, CD10 and CD30 stains were negative. Ki67 proliferative index was positive in 80 % of nuclei, bcl-6 stain was nuclear positive in 80 % and bcl-2 was negative. IRF/MUM1 was positive and in situ hybridization for EBV was negative. The histopathological and immunohistochemical features were consistent with diffuse large B cell lymphoma (DLBCL), non-germinal centre B cell like (non GCB) sub-type (Fig. 2). Staging CT scans did not show evidence of lymphoma elsewhere and bone marrow was not involved. The diagnosis was PHL and the revised-International Prognostic Index (R-IPI) score was 3, which indicated poor prognosis at presentation.
Fig. 1.
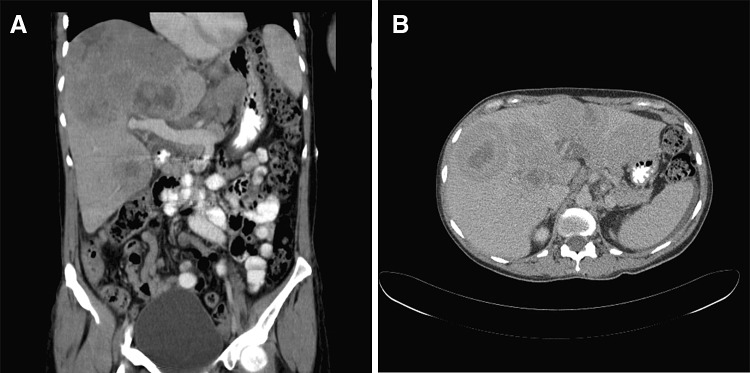
a, b CT scan coronal and transverse view findings at diagnosis in June 2011
Fig. 2.
Histopathological examination of the wedge biopsy specimen from liver mass showing features consistent with diffuse large B-cell lymphoma. H&E stain (magnification ×400). Extensive infiltration by large malignant lymphoid cells. CD20 immunohistochemical stain (magnification ×400). Strong and uniform cytoplasmic membrane positivity in the lymphoid cells. Ki-67 immunohistochemical stain (magnification ×400). Nuclear positivity in approximately 80 % of the lymphoid cells. BCL-6 immunohistochemical stain (magnification ×400). Nuclear positivity in approximately 80 % of the lymphoid cells
The derangement of her liver function tests were initially deemed to be related to hepatic lymphoma masses rather than hepatitis due to active hepatitis C infection. Hence the hepatologists recommended monitoring her liver function parameters and hepatitis C viral load closely while on chemotherapy without immediate need for antiviral therapy. When informed about the risk of severe hepatotoxicity caused by rituximab therapy in patients with hepatitis C infection, our patient opted for CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) chemotherapy without rituximab. Soon after first CHOP chemotherapy, her symptoms improved with evidence of moderate tumour lysis. At that stage the liver function parameters started to improve and her liver size reduced clinically. HCV viral load remained stable during chemotherapy despite not receiving antiviral therapy. She received a total of 6 cycles of CHOP chemotherapy at 21 days intervals, which were tolerated well. A repeat CT scan after completion of chemotherapy showed complete resolution of all the liver masses and the hepatic architecture appeared normal (Fig. 3a, b). Later she was commenced on antiviral therapy with ribavirin and peg-interferon which led to the reduction of hepatitis C viral load. Currently more than 30 months after completion of chemotherapy, she remains in complete remission of lymphoma and her liver function tests were consistently stable.
Fig. 3.
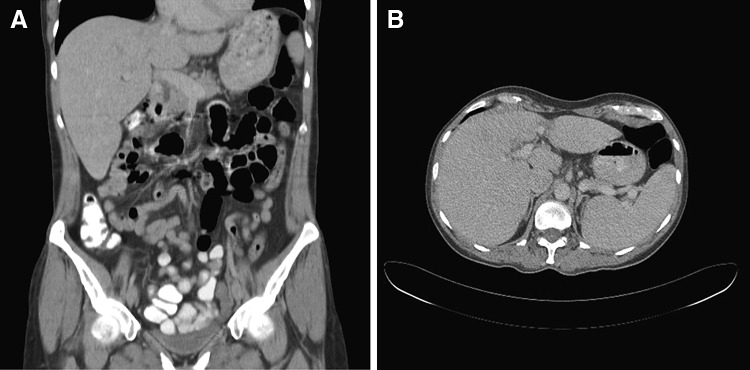
a, b CT scan coronal and transverse view findings after completion of 6 cycles of CHOP chemotherapy in November 2011
Discussion
HCV infection is associated with an increase incidence of B-cell non-Hodgkin lymphoma (NHL) of approximately 20 to 30 % [1]. DLBCL and marginal zone B-cell lymphoma are the common histologic sub-types of NHL in HCV infected patients [2]. The underlying mechanism of lymphoma in HCV infected patients remains unclear. Since HCV is a lymphotropic virus, a direct oncogenic role of HCV has been postulated but not proved [3]. More likely mechanism is HCV induced chronic antigenic stimulation leading to proliferation of specific B-cell clones [4].
Even though secondary hepatic involvement can occur in approximately 20 % of NHL patients, PHL is very rare occurring in 0.016 % of all NHL [5]. PHL is known to be associated with primary biliary cirrhosis, immunosuppressive therapy, autoimmune disease and viruses like hepatitis B, hepatitis C, HIV and EBV. HCV infection was found to be prevalent in approximately 20 % of patients with PHL. In HCV infected patients DLBCL is the commonest histological sub-type of PHL followed by MALT lymphoma [6]. Approximately one-third of patients with PHL present with a solitary liver nodule at presentation, one-third can have multiple liver nodules, and the remaining present with diffuse liver infiltration. Since PHL can mimic HCC or liver metastases clinically and radiologically, surgical biopsy is often required for diagnosis [7]. In a Japanese study comprising of twenty PHL patients, 8 out of 12 DLBCL and 2 out of 8 MALT lymphoma cases had positive serum HCV antibodies. These patients received surgery and/or chemotherapy with or without rituximab. In the patients with primary hepatic MALT lymphoma, median survival was more than 84 months, which was significantly longer than that of primary hepatic DLBCL cases (27 months, p < 0.05) [6].
Immunosuppression associated with chemotherapy, steroids or rituximab can alter the balance between viral proliferation and host immune response. Hepatitis can occur due to viral reactivation or during immune reconstitution after completion of chemotherapy. Severe reactivation of hepatitis B virus (HBV) and flare of hepatitis is common in NHL patients undergoing immunosuppressive therapy and is rare with HCV [8]. Liver function impairment in HCV infected patients who underwent chemotherapy is generally mild [grade I/II elevation of aspartate aminotransferase (AST) and alanine aminotransferase (ALT)] [9]. In a study it was observed that addition of rituximab to chemotherapy posed a tenfold risk of severe hepatotoxicity (grade III/IV elevation of AST and ALT) when compared with chemotherapy alone. Despite that there was no significant increase in mortality rates in patients with HCV infection treated with rituximab [10]. Early antiviral therapy prevents worsening of hepatitis in B-cell NHL with co-existent HBV receiving rituximab and chemotherapy [11]. In contrast, for patients with concomitant B-cell NHL and Hepatitis-C infection, role of antiviral therapy in conjunction with chemotherapy is unclear [2]. However, commencement of antiviral therapy after completion of chemotherapy may be associated with improved outcomes. In a retrospective study, 25 HCV-positive patients received antiviral treatment with interferon and ribavirin following completion of chemotherapy for non-Hodgkin’s lymphoma. Among them none of the patients who achieved virological response experienced relapse of lymphoma, while 29 % of the non-responders relapsed [12].
Our patient presented with multifocal liver lesions which resembled HCC clinically and radiologically. After the diagnosis of PHL was confirmed, there were difficulties in decision-making regarding management of her lymphoma. Even though rituximab was omitted in the chemotherapy regimen, there was an excellent initial response and prolonged remission of PHL. Her hepatitis C viral load and liver function tests remained stable throughout treatment with chemotherapy + steroids and did not require antiviral therapy. Even though some studies have reported high incidence of hepatotoxicity after rituximab treatment in DLBCL and co-existent HCV infection, there is dearth of evidence regarding safety of rituximab in concomitant HCV infection and PHL. In such patients the role of anti-viral therapy in combination with chemotherapy is also unclear. The response rates and prognosis after chemotherapy in patients with PHL and coexistent HCV infection is also uncertain. These therapeutic dilemmas may be difficult to solve since clinical trials in PHL patients with co-existent HCV infection will be hard to perform due to rarity of the disease.
Acknowledgments
Conflict of interest
None.
References
- 1.Giordano TP, Henderson L, Landgren O, Chiao EY, Kramer JR, El-Serag H, et al. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA. 2007;297(18):2010–2017. doi: 10.1001/jama.297.18.2010. [DOI] [PubMed] [Google Scholar]
- 2.Foran JM. Hepatitis C in the rituximab era. Blood. 2010;116(24):5081–5082. doi: 10.1182/blood-2010-09-307827. [DOI] [PubMed] [Google Scholar]
- 3.Gasparotto D, De Re V, Boiocchi M. Hepatitis C virus, B-cell proliferation and lymphomas. Leuk Lymphoma. 2002;43(4):747–751. doi: 10.1080/10428190290016845. [DOI] [PubMed] [Google Scholar]
- 4.Negri E, Little D, Boiocchi M, La Vecchia C, Franceschi S. B-cell non-Hodgkin’s lymphoma and hepatitis C virus infection: a systematic review. Int J Cancer. 2004;111(1):1–8. doi: 10.1002/ijc.20205. [DOI] [PubMed] [Google Scholar]
- 5.Bronowicki JP, Bineau C, Feugier P, Hermine O, Brousse N, Oberti F, et al. Primary lymphoma of the liver: clinical-pathological features and relationship with HCV infection in French patients. Hepatology. 2003;37(4):781–787. doi: 10.1053/jhep.2003.50121. [DOI] [PubMed] [Google Scholar]
- 6.Kikuma K, Watanabe J, Oshiro Y, Shimogama T, Honda Y, Okamura S, et al. Etiological factors in primary hepatic B-cell lymphoma. Int J Pathol. 2012;460(4):379–387. doi: 10.1007/s00428-012-1199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steller EJ, van Leeuwen MS, van Hillegersberg R, Schipper ME, Rinkes IH, Molenaar IQ. Primary lymphoma of the liver—a complex diagnosis. World J Radiol. 2012;4(2):53–57. doi: 10.4329/wjr.v4.i2.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner NC, Dusheiko G, Jones A. Hepatitis C and B-cell lymphoma. Ann Oncol. 2003;14(9):1341–1345. doi: 10.1093/annonc/mdg363. [DOI] [PubMed] [Google Scholar]
- 9.Visco C, Arcaini L, Brusamolino E, Burcheri S, Ambrosetti A, Merli M, et al. Distinctive natural history in hepatitis C virus positive diffuse large B-cell lymphoma: analysis of 156 patients from northern Italy. Ann Oncol. 2006;17(9):1434–1440. doi: 10.1093/annonc/mdl131. [DOI] [PubMed] [Google Scholar]
- 10.Ennishi D, Maeda Y, Niitsu N, Kojima M, Izutsu K, Takizawa J, et al. Hepatic toxicity and prognosis in hepatitis C virus-infected patients with diffuse large B-cell lymphoma treated with rituximab-containing chemotherapy regimens: a Japanese multicenter analysis. Blood. 2010;116(24):5119–5125. doi: 10.1182/blood-2010-06-289231. [DOI] [PubMed] [Google Scholar]
- 11.Niitsu N, Hagiwara Y, Tanae K, Kohri M, Takahashi N. Prospective analysis of hepatitis B virus reactivation in patients with diffuse large B-cell lymphoma after rituximab combination chemotherapy. J Clin Oncol. 2010;28(34):5097–5100. doi: 10.1200/JCO.2010.29.7531. [DOI] [PubMed] [Google Scholar]
- 12.La Mura V, De Renzo A, Perna F, D’Agostino D, Masarone M, Romano M, et al. Antiviral therapy after complete response to chemotherapy could be efficacious in HCV-positive non-Hodgkin’s lymphoma. J Hepatol. 2008;49(4):557–563. doi: 10.1016/j.jhep.2008.06.025. [DOI] [PubMed] [Google Scholar]