Abstract
Wiskott–Aldrich syndrome (WAS) is a rare inherited X-linked recessive immunodeficiency disease characterized by eczema, thrombocytopenia, immune deficiency, and bloody diarrhea and is caused by WASP gene mutations. This study reports a case of WAS with a novel mutation. A newborn Chinese infant was admitted to the hospital because of intermittent bloody stools, recurrent infections, and persistent thrombocytopenia. Genetic analysis of the coding sequences and flanking splice sites of the WASP gene showed a novel WASP gene deletion mutation (1144delA) at exon 10. Family history showed that both his mother and aunt had a heterozygous genotype of the WASP gene. The infant died at the age of 4 months due to persistent thrombocytopenia and severe pneumonia. A novel WASP gene deletion (1144delA) at exon 10 was identified in a Chinese infant with WAS. This base deletion results in a frame-shift mutation of the gene for an early stop codon at amino acid 444.
Keywords: Wiskott–Aldrich syndrome, Gene mutation, WASP gene
Introduction
Wiskott–Aldrich syndrome (WAS) is a rare inherited X-linked recessive immunodeficiency disease that is characterized by thrombocytopenia, recurrent infection, a high incidence of autoimmunity, and an increased risk of malignancies [1–3]. Clinically, the overwhelming majority of patients are male with the initial symptom of petechiae, due to thrombocytopenia. Spontaneous nose bleeding and bloody diarrhea are also common, and patients then quickly develop eczema within the first month of life. Recurrent bacterial infections develop by 3 months. Thereafter, the majority of WAS children suffer from autoimmune disorder or malignancies. Currently, WAS treatment is to correct the symptoms or try to with gene therapy. Genetically, WAS is linked to mutations of the Wiskott–Aldrich syndrome protein (WASP) gene on the short arm of the X chromosome, which spans approximately 9 kilobases of genomic DNA with 12 exons and encodes a protein with 502 amino acids that is mainly expressed in hematopoietic cells [1]. WASP contains several functional domains through which it interacts with proteins involved in intracellular signaling and regulation of the actin cytoskeleton [4]. To date, over 300 deletions, insertions, and splice site mutations in the WASP gene have been reported to cover all 12 exons [1–4]. The aim of this case report was to show a novel WASP gene mutation in a Chinese boy with WAS.
Case Presentation
A 4-month-old Chinese male infant was admitted to our department with intermittent bloody stools, recurrent infections, and persistent thrombocytopenia. On the second day after birth, he began showing repetitive bloody stools, fever, abdominal distension, and thus clinically diagnosed with WAS. Laboratory tests revealed normal coagulation levels after intermittent platelet transfusions, and complete blood count showed a low platelet count of 6.0 × 109/L. T and B lymphocytes and NK cell counts were 6.71 % CD3+ CD4+, 0.08 % CD4/CD8, 80.49 % CD3+ CD8+, and 23.38 % NK cells. The levels of IgG were 30.90 g/L, IgA 0.51 g/L, and IgM 1.21 g/L. Liver and renal function tests and cardiac enzymes were in normal ranges. Epstein–Barr virus, cytomegalovirus, toxoplasmosis, rubella virus, herpes simplex virus, mycoplasma pneumonia, and Chlamydia were all negative. According to a scoring system developed to describe the severity of WAS, the severity of this patient was about 4 [5]. Although parent and aunt of the patient had no symptoms of the disease, blood samples from his mother and aunt were collected and analyzed. Platelet counts were 180 × 109 and 212 × 109, respectively. All of the subjects provided informed, written consent to take part in this study. The patient was then treated with intravenous immunoglobulin infusion and thrombocyte transfusion on the 14th day of age. After the infection was controlled, the body temperature was stabilized and he started to gain weight, but symptoms (such as petechia, blood stools, abdominal distention, and hepatosplenomegaly) disappeared. The repeat blood culture also became negative, although the platelet count was still lower than 30 × 109/L. At 2 months of age, the patient was admitted to our hospital for follow-up after he was treated with dexamethasone and prednisone for approximately a month with intermittent platelet transfusions. He presented with recurrent infections and decreased platelet counts. Eczema appeared in a discontinuous fashion on his face and anterior chest at the 3rd month of age, and the thrush was persistent and died at the age of 4 months, due to persistent thrombocytopenia and severe pneumonia.
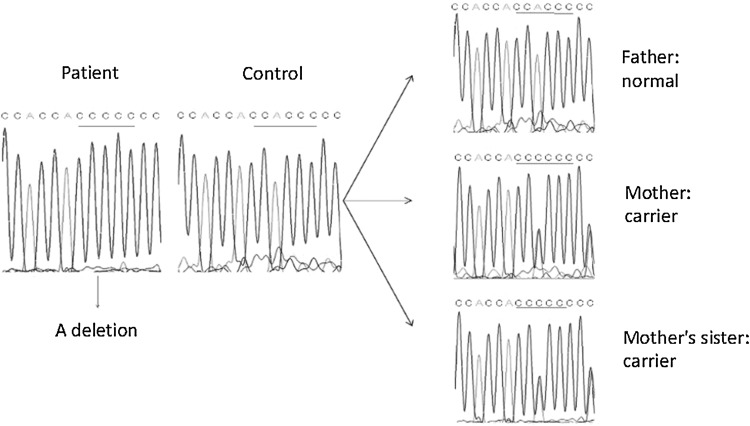
Furthermore, we performed molecular analysis of the WASP gene mutations in blood samples of the patient, parents, and aunt. Specifically, genomic DNA was extracted from whole blood using an SE Blood DNA Kit (Omega Bio-tec, Inc., Lilburn, Georgia), according to the manufacture’s instructions, and subjected to PCR amplification and DNA sequence of WASP coding sequences and flanking splice sites of all 12 exons, as described previously [6]. The results showed that WASP in this patient had a single base deletion (1144delA) in exon 10, putatively causing a frame-shift mutation with an early stop codon at amino acid 444 (Fig. 1). However, the patient’s mother and aunt have one mutated and one normal allele in WASP, indicating that they are the carriers (Fig. 1).
Fig. 1.
DNA sequence analysis of the WASP gene from blood samples. The data showed a deletion of WASP at exon 10 identified in the patient’s sample, and the mother and aunt’s data showed overlap of normal and mutated alleles, indicating carrier status of the mutated WASP allele
Discussion
To date, it has shown approximately 300 unique mutations in the WASP gene, spanning all 12 exons with missense, nonsense, and splice site mutations and insertions, deletions, and rearrangements. However, missense mutations are the predominant form of WASP mutations and typically localized in exons 1, 2, 3, and 4 [7, 8], while deletions and insertions are distributed throughout the WASP gene. In contrast, splice site mutations are predominantly in introns 6, 8, 9, and 10 [7, 8]. Clinically, the missense mutations or splice site mutations affecting non-invariant sites are often associated with the mild WAS symptoms, whereas the nonsense or frame-shift mutations are associated with severe WAS symptoms [5, 7, 9]. In this case report, we showed a novel WASP frame-shift mutation in a Chinese WAS patient whose WASP had a single base deletion (1144delA) in exon 10, putatively resulting in a frame-shift mutation with an early stop codon at amino acid 444. Based on clinical symptoms (such as persistent thrombocytopenia, difficult-to-control eczema, and frequent life-threatening infections), the severity of his patient was scored as 4, which was consistent with previous studies, where frame shift mutations are generally associated with a severe phenotype of WAS and contributed to the eventual death of the patients.
Molecularly, WASP is predominantly expressed in hematopoietic cells and responsible for regulating the dynamic actin cytoskeleton for a diversity of activities in hematopoietic cells. Moreover, WASP also plays an important role in the activation of T-lymphocytes via WASP regulation of T cell receptor signaling and cortical actin cytoskeleton rearrangements. WASP mutations impact T-lymphocyte functions, resulting in the immune deficiency and decreased antibody production, and in turn leading to the increase in infection susceptibility. In addition, the type of mutations in the WASP gene is significantly associated with the severity of the symptoms, because a truncated protein caused more significant symptoms than those with a missense mutation with a normal-length WASP [8]. However, to date, it remains unknown what causes WASP mutations and how WASP is mutated. Epidemiological studies showed that the combined incidence of WAS and X-linked thrombocytopenia is about 4–10 in 1 million live births, but there is no geographical preference [10]. In our current case report, DNA sequence data showed that both mother and aunt are carriers of mutated WASP allele, thus, WASP mutation in this patient could be inherited from the mother, and the aunt is the carrier of mutated WASP allele, although she had no symptoms, including no bleeding tendency, infections, or thrombocytopenia. WASP mutation in this patient showed a single base deletion (1144delA) in exon 10, resulting in a frame shift mutation and an early stop codon at amino acid 444; thus, this leads to the absence of WASP expression in the patient. In this case report, we did not detect expression of WASP protein, which is the limitation of this study.
We reported a Chinese infant with classic WAS with intermittent bloody stools, recurrent infections, and persistent thrombocytopenia. DAN sequence analysis revealed a novel mutation (1144delA) of the WASP gene in exon 10, resulting in a frame shift mutation and a stop codon at amino acid 444 in the patient. Family history analysis showed that the mutation of WASP gene was inherited from the mother. This observation further expands the genotype-phenotype correlations of WAS.
Acknowledgments
We thank Medjaden Bioscience Limited, Hong Kong, China, for assisting in the preparation of this manuscript. This study was supported by National Natural Science Foundation of China (No. 31071105).
References
- 1.Derry JM, Ochs HD, Francke U (1994) Isolation of a novel gene mutated in Wiskott–Aldrich syndrome. Cell 79(5):following 922 [PubMed]
- 2.Aldrich RA, Steinberg AG, Campbell DC. Pedigree demonstrating a sex-linked recessive condition characterized by draining ears, eczematoid dermatitis and bloody diarrhea. Pediatrics. 1954;13(2):133–139. [PubMed] [Google Scholar]
- 3.Remold-O’Donnell E, Rosen FS, Kenney DM. Defects in Wiskott–Aldrich syndrome blood cells. Blood. 1996;87(7):2621–2631. [PubMed] [Google Scholar]
- 4.Snapper SB, Rosen FS. The Wiskott–Aldrich syndrome protein (WASP): roles in signaling and cytoskeletal organization. Annu Rev Immunol. 1999;17:905–929. doi: 10.1146/annurev.immunol.17.1.905. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Q, Zhang M, Blaese RM, Derry JM, Junker A, Francke U, Chen SH, Ochs HD. The Wiskott–Aldrich syndrome and X-linked congenital thrombocytopenia are caused by mutations of the same gene. Blood. 1995;86(10):3797–3804. [PubMed] [Google Scholar]
- 6.Derry JM, Kerns JA, Weinberg KI, Ochs HD, Volpini V, Estivill X, Walker AP, Francke U. WASP gene mutations in Wiskott–Aldrich syndrome and X-linked thrombocytopenia. Hum Mol Genet. 1995;4(7):1127–1135. doi: 10.1093/hmg/4.7.1127. [DOI] [PubMed] [Google Scholar]
- 7.Imai K, Morio T, Zhu Y, Jin Y, Itoh S, Kajiwara M, Yata J, Mizutani S, Ochs HD, Nonoyama S. Clinical course of patients with WASP gene mutations. Blood. 2004;103(2):456–464. doi: 10.1182/blood-2003-05-1480. [DOI] [PubMed] [Google Scholar]
- 8.Jin Y, Mazza C, Christie JR, Giliani S, Fiorini M, Mella P, Gandellini F, Stewart DM, Zhu Q, Nelson DL, Notarangelo LD, Ochs HD. Mutations of the Wiskott–Aldrich Syndrome Protein (WASP): hotspots, effect on transcription, and translation and phenotype/genotype correlation. Blood. 2004;104(13):4010–4019. doi: 10.1182/blood-2003-05-1592. [DOI] [PubMed] [Google Scholar]
- 9.Albert MH, Bittner TC, Nonoyama S, Notarangelo LD, Burns S, Imai K, Espanol T, Fasth A, Pellier I, Strauss G, Morio T, Gathmann B, Noordzij JG, Fillat C, Hoenig M, Nathrath M, Meindl A, Pagel P, Wintergerst U, Fischer A, Thrasher AJ, Belohradsky BH, Ochs HD. X-linked thrombocytopenia (XLT) due to WAS mutations: clinical characteristics, long-term outcome, and treatment options. Blood. 2010;115(16):3231–3238. doi: 10.1182/blood-2009-09-239087. [DOI] [PubMed] [Google Scholar]
- 10.Suri D, Singh S, Rawat A, Gupta A, Kamae C, Honma K, Nakagawa N, Imai K, Nonoyama S, Oshima K, Mitsuiki N, Ohara O, Bilhou-Nabera C, Proust A, Ahluwalia J, Dogra S, Saikia B, Minz RW, Sehgal S. Clinical profile and genetic basis of Wiskott–Aldrich syndrome at Chandigarh, North India. Asian Pac J Allergy Immunol. 2012;30(1):71–78. [PubMed] [Google Scholar]