Abstract
Philadelphia (Ph) chromosome is most commonly associated with chronic myelogenous leukemia (CML), a subset of precursor B-cell acute lymphoblastic leukemia and acute biphenotypic leukemia. In contrast only 1 % of acute myeloid leukemia (AML) show a consistent association with the Ph Chromosome. Before making a diagnosis of Ph + AML stringent criteria need to be applied in order to differentiate it from blast crisis stage of CML. It is important to identify this rare entity as patients who otherwise carry a poor prognosis with standard chemotherapy regimen, would benefit from therapy with imatinib mesylate. This article discusses the morphological, immunophenotype and clinical characteristics of a rare case of Ph + AML.
Keywords: BCR/ABL, PCR, Acute, Myeloid, Leukemia
Introduction
The exchange of genetic material between chromosome 9 and 22 results in formation of t(9;22)(q34;q11) or Philadelphia (Ph) chromosome which results in synthesis of the chimeric BCR/ABL non-receptor tyrosine kinase. This chromosomal anomaly is most commonly associated with chronic myelogenous leukemia (CML), a subset of precursor B cell acute lymphoblastic leukemia (B-ALL) and acute biphenotypic leukemia. However <1 % of de-novo acute myeloid leukemia (AML) show a consistent association with the Ph Chromosome [1]. Stringent clinical criteria have to be applied before making a diagnosis of Ph + AML as such an occurrence can also be seen in a blast crisis stage of a previously undiagnosed case of CML. The criteria include—an absence of a clinical history of a hematologic disorder, lack of evidence of chronic phase or accelerated phase of CML and lack of clinical and laboratory features of CML such as splenomegaly and basophilia [2]. Studies have shown that CML patients tend to have concurrent chromosomal anomalies such as extra copies of Ph + chromosome and trisomy 8 which are not seen in Ph + AML [3]. It is also seen that PH + AML more commonly return to a normal karyotype following induction chemotherapy in comparison to CML patients [3]. The importance in identifying this chimera in AML is that these patients have a poor prognosis with the standard chemotherapy regimen and if identified would benefit from the added therapy with imatinib mesylate [4]. We discuss the morphological, immunophenotype and clinical characteristics of a rare case of Ph + AML.
Case Report
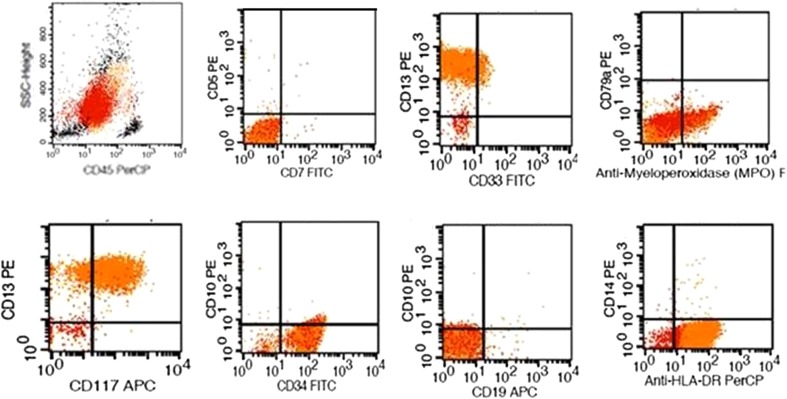
A 62 years elderly male presented to the Medical Out-patient department with history of fever, generalised weakness since 10 days and oral mucosal bleeding of 3 days duration. The patient had no known co-morbidities. Examination showed presence of fresh oral mucosal bleeding. However there was no splenomegaly, which was confirmed on ultrasonography. Investigations revealed hemoglobin of 7 gm/dl, leukocytosis (42,400/cumm) and thrombocytopenia (60,000/cumm). His peripheral blood examination showed leukocytosis with a differential count of predominant blasts (72 %). These blasts had scant amphophilic cytoplasm, large nuclei with open chromatin pattern and conspicuous 1-2 nucleoli. No cytoplasmic granularity or Auer rods were seen. Accompanying the blasts were mature lymphocytes and occasional myelocytes and metamyelocytes. However no mature neutrophils, eosinophils or basophils were seen. These blasts showed strong cytoplasmic positivity for myeloperoxidase (MPO) stain, establishing their myeloid lineage. Bone marrow aspirate and biopsy studies confirmed near total replacement of marrow by MPO positive blasts. Immunophenotyping was carried out on the peripheral blood sample using the BD FACS caliber machine. The gated blasts showed positivity for CD34, CD117, CD13, CD33 and cMPO (Fig. 1). No lymphoid markers were positive in the gated blast population, ruling out the possibility of precursor lymphoblastic leukemia and biphenotypic leukemia. Immunophenotyping and morphology favored the diagnosis of AML M1 subtype as per FAB classification. The patient was started on induction chemotherapy with cytarabine and daunorubicin. Meanwhile, a multiplex reverse transcriptase PCR was carried out on mRNA extracted from the peripheral blood to detect 28 possible mutations using primers from Hemavision kit. Initial step involved extraction of RNA from the blasts using Qiagen RNA extraction kit. The extracted RNA was subjected to an initial set of 8 primers through a nested PCR protocol to identify the lane of interest. This was followed by the split-out reaction which also involved a nested PCR protocol which identified the chimera t(9;22)(q34;q11) on Agarose gel electrophoresis (Fig. 2). A final diagnosis of ‘AML—not otherwise specified, without maturation’ was made as per the WHO 2008 classification. The patient did not go into hematological remission and succumbed to his illness on day 5 of treatment. As the presence of the BCR/ABL chimera was detected on the 5th day, the patient was not supplemented with imatinib mesylate.
Fig. 1.
Immunophenotyping shows positivity for CD34, CD117, CD13, CD33 and cMPO
Fig. 2.
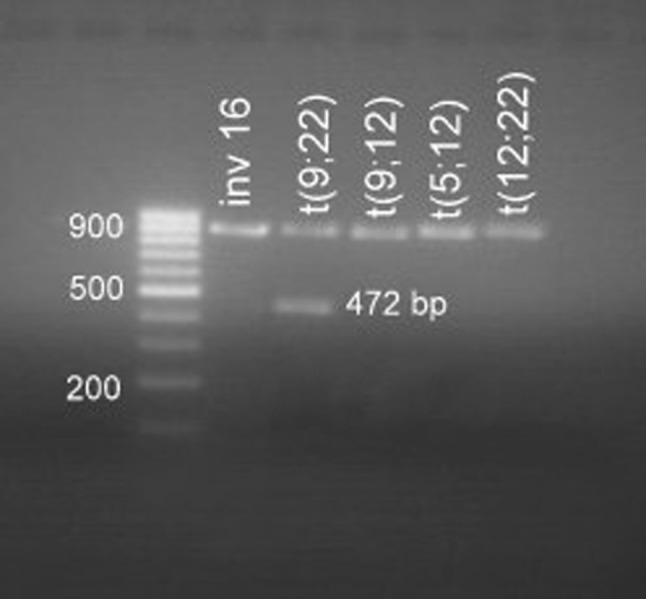
Split out reaction shows a positive band of 472 bp corresponding to t(9;22) on Agarose gel electrophoresis
Discussion
Incidence of de novo Ph + AML ranges from 0.45 to 1 % of all AML [5]. This subtype of AML has a male predominance with occurrence at a mean age of 52 years [5]. In principle there is no single clinical or hematologic feature which distinguishes Ph + AML from CML blast crisis. However, it is unlikely that our patient suffered from an undetected chronic phase CML, as there was the absence of clinical history suggestive of an antecedent hematologic disorder. Clinically, our patient did not show splenomegaly which is expected in cases having antecedent CML [2]. In addition, a classical hematologic picture of chronic or accelerated phase CML, with accompanying basophilia or dwarf megakaryocytes were not observed in our patient during his response to induction chemotherapy [3]. Morphologically, Ph + AML usually takes up a M1 or M2 subtype, though any FAB subtype other than M3 and M7 can be expected [5]. The immunophenotype showed the blasts to be of myeloid lineage with absence of positivity for lymphoid markers, ruling out a possibility of biphenotypic leukemia. Cytogenetic analysis of Ph + AML may show presence of cytogenetic abnormalities such as trisomy 8 and co-existence of normal metaphases [3]. However, cytogenetic analysis was not carried out in the index case. A recent study has shown molecular signature of Ph + AML to be distinct by showing a frequent association with NPM1 mutation and CML-BP in contrast showing a frequent association with ABL1 mutation [6]. Molecular analysis using Multiplex RT-PCR identified the presence of the BCR/ABL chimera, which is a rapid, sensitive and specific method for detection of known molecular aberrations in acute leukemia [7]. The protocol includes a control primer in each run to validate the reaction process. As an additional benefit, the high sensitivity of the protocol can be utilized in detection of minimal residual disease. Patients with Ph + AML have an aggressive clinical course and are at high risk for treatment failure or early relapse after induction therapy. However, studies have shown that patients have higher rates of remission and prolonged survival if imatinib is instituted early in therapy and is followed by allogenic stem cell transplantation. Though Ph + AML has been placed in the group of cytogenetic changes with poor prognosis, we recommend the use of Multiplex RT-PCR for early and rapid detection of the translocation in cases of AML which will help guiding therapy in the form of early institution of imatinib mesylate.
Conflict of interest
None identified.
References
- 1.Berger R, Chen SJ, Shen Z. Ph-positive acute leukemia. Cytogenetic and molecular aspects. Cancer Genet Cytogenet. 1990;44:143–152. doi: 10.1016/0165-4608(90)90041-8. [DOI] [PubMed] [Google Scholar]
- 2.Cuneo A, Ferrant A, Michaux JL, et al. Philadelphia chromosome-positive acute myeloid leukemia: cytoimmunologic and cytogenetic features. Haematologica. 1996;81:423–427. [PubMed] [Google Scholar]
- 3.Frankfurt O, Platanias LC. Philadelphia chromosome positive acute myeloid leukemia or de novo chronic myeloid leukemia-blast phase? Leuk Lymphoma. 2013;54(1):1–2. doi: 10.3109/10428194.2012.713105. [DOI] [PubMed] [Google Scholar]
- 4.Kondo T, Tasaka T, Sano F, et al. Philadelphia chromosome-positive acute myeloid leukemia (Ph + AML) treated with imatinib mesylate (IM): a report with IM plasma concentration and bsr-abl transcripts. Leuk Res. 2009;33(9):e137–e138. doi: 10.1016/j.leukres.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Soupir CP, Vergilio JA, Dal Cin P, et al. Philadelphia chromosome-positive acute myeloid leukemia: a rare aggressive leukemia with clinicopathologic features distinct from chronic myeloid leukemia in myeloid blast crisis. Am J Clin Pathol. 2007;127(4):642–650. doi: 10.1309/B4NVER1AJJ84CTUU. [DOI] [PubMed] [Google Scholar]
- 6.Konoplev S, Yin CC, Kornblau SM, et al. Molecular characterization of de novo Philadelphia chromosome-positive acute myeloid leukemia. Leuk Lymphoma. 2013;54(1):138–144. doi: 10.3109/10428194.2012.701739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salto-Tellez M, Shelat SG, Benoit B, et al. Multiplex RT-PCR For detection of leukemia-associated translocations; validation and application to routine molecular diagnostic practice. J Mol Diagn. 2003;5(4):231–236. doi: 10.1016/S1525-1578(10)60479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]