Abstract
We describe a sixteen year old with Wilson’s disease on copper chelation and subsequent high dose oral zinc who developed severe anemia and neutropenia. Bone marrow aspirate done to evaluate the cause of bicytopenia revealed trilineage dysplasia. Correlating the clinical context with bone marrow and biochemical parameters, copper deficiency was suspected and he was given a trial of therapy, following which the hematological parameters improved. This case highlights hypocupremia as a reversible cause of bone marrow dysplasia in patients with Wilson’s disease on chelation, where serum copper levels are not useful in the diagnosis. We also believe that monitoring of the blood counts in patients on copper chelation may provide a clue to impending copper deficiency.
Keywords: Wilson’s disease, Cytopenia, Bone marrow dysplasia, Hypocupremia
Introduction
Wilson’s disease is associated with retention of copper which is treated with chelation to prevent deposition of copper in the liver and basal ganglion. However, excessive treatment and subsequent copper deficiency may cause cytopenias and dysplasia in the bone marrow. Recognition of this reversible cause of dysplasia and monitoring of the blood counts in patients of Wilson’s on chelation may provide a clue to impending copper deficiency in such patients.
Case Report
A sixteen year old male, a known case of Wilson’s disease (WD) presented with features of persistent anemia and leukopenia of a year’s duration. He had been diagnosed as suffering from WD two years previously and had been on regular medication with penicillamine and anticonvulsants and trihexyphenidine for his CNS symptoms. As the anemia and neutropenia did not respond to treatment with hematinics, he was advised bone marrow aspiration, which he refused. He went elsewhere where penicillamine was stopped and high dose zinc sulphate started, after which his anemia and neutropenia worsened. He came back to us after 9 months.
On examination he had severe pallor and mild splenomegaly. He did not have petechiae, purpura or overt or focal neurological signs. A partial Kayser Fleischer ring was identified on slit lamp examination.
On investigation his hemoglobin was 5.8 g/dL, red blood cell count 1.9 million/cumm, red cell distribution width (RDW) 17.3 % and mean corpuscular width (MCV) 94 fL. The peripheral smear showed dimorphic red cell morphology and neutropenia. There were no features of hemolysis. Serum levels of folic acid, B12 and ferritin were normal. Liver function tests were mildly deranged with mildly increased enzymes, prothrombin time and activated partial thromboplastin time. Renal function tests were normal. Serum ceruloplasmin measured <2.0 mg/dL (normal 25–43 mg/dL), serum copper was decreased to 31 µg/dL (normal 70–140.0 µg/dL) and urine copper excretion in 24 h was 32.3 µg (normal 15–60 µg/day). Serum zinc measured 438.4 µg/dL (normal range 72.6–127 µg/dL).
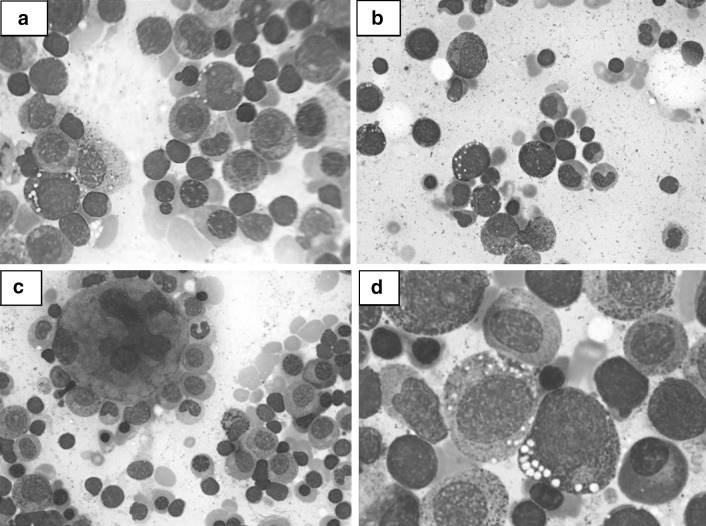
Bone marrow aspirate showed a hypercellular marrow with features of dyserythropoiesis, dysmyelopoiesis and dysmegakaryopoiesis (Fig. 1a, b, c). Dyserythropoiesis was present in 60 % of the erythoid elements in the form of megaloblastoid change and budding nuclei; dysmyelopoiesis was identified in 30 % of myeloid precursors and was seen as hypogranular forms, hypolobated forms and occasional ring nuclei. Dysmegakaryopoiesis was noted in 80 % with abnormal nuclear lobulation. Early erythroid and granulocytic precursors showed vacuolization of the cytoplasm (Fig. 1d). Occasional ring sideroblasts were noted and a few plasma cells showed large iron positive inclusions. A final morphological diagnosis of dysplastic marrow was offered. Based on the history of treatment with the chelating agent, penicillamine, decreased serum copper levels and bone marrow findings of dysplasia and vacuolated erythroid and granulocyte precursors, a diagnosis of bone marrow dysplasia and cytopenia due to copper deficiency was entertained. The patients’ zinc supplementation was stopped and he was reviewed after 2 weeks, when his anemia and neutropenia had improved. He was then started on copper supplements at a dose of 2.5 mg/day after which there was further improvement in his hematological parameters, confirming the diagnosis of cytopenia due to copper deficiency. Eight weeks after stopping zinc and later starting low dose copper supplementation the patient’s hemoglobin, complete blood count and MCV returned to normal. He refused a repeat bone marrow examination. At present copper has been stopped, he has been restarted on low dose penicillamine and alternate day zinc therapy, with maintenance of his hematological parameters. He has resumed normal activities.
Fig. 1.
a Bone marrow aspirate with dyserythropoiesis and vacuolated erythroblast. Giemsa ×400. b Dysmyelopoiesis with dys-synchronous nuclei and vacuolated myeloid cells. Giemsa ×400. c Megakaryocyte with markedly abnormal nucleus. Giemsa ×400. d Vacuolization of immature granulocytes. Giemsa ×1,000
Table 1 summarizes the hematological and biochemical parameters at various times during the course of the disease.
Table 1.
Hematology profile of the patient at the time of presentation, after cessation of zinc and after copper supplementation
| Blood parameter (reference range) | At presentation | After zinc withdrawal (2 weeks after diagnosis) | After copper supplementation (4 weeks after diagnosis) | After copper supplementation (8 weeks after diagnosis) |
|---|---|---|---|---|
| Hemoglobin (13–16 g/L) | 5.8 g/L | 8.9 g/L | 10.5 g/L | 13.6 g/L |
| RBC count (4.5–5.3 × 106/cumm) | 1.9 × 106/cumm | 3.1 × 106/cumm | 4.0 × 106/cumm | 5.3 × 106/cumm |
| Mean corpuscular volume (78–98 fL) | 94 fL | 88.7 fL | 83.5 fL | 76.5 fL |
| Leucocyte count (4.5–10.5 × 109/L) | 1.4 × 109/L | 2.7 × 109/L | 5.1 × 109/L | 6.3 × 109/L |
| Platelet count (150–400 × 109/L) | 243 × 109/L | 322 × 109/L | 428 × 109/L | 296 × 109/L |
| Neutrophil count (1.9–3.3 × 109/L) | 0.46 × 109/L | 0.83 × 109/L | 1.84 × 109/L | 3.23 × 109/L |
| Serum zinc level (72.6–127 µg/dL) | 438.4 µg/dL | NA | 246.2 µg/dL | 257.4 µg/dL |
| Serum copper (70–140.0 µg/dL) | 31.0 µg/dL | NA | 63 µg/dL | 19.0 µg/dL |
NA Not available
Discussion
WD is an autosomal recessive disease associated with defect in the WD gene (ATP7B) located on chromosome 13. Mutation in the WD gene results in retention of copper in the liver as well as in impaired export of copper into bile [1]. In WD, serum copper and serum ceruloplasmin are less than normal, while 24 h urinary excretion of copper and liver copper are increased. In response to penicillamine treatment, urinary copper excretion is initially markedly increased and, following continued administration, urinary copper levels may become normal, as in our patient.
The hematological side effects of the chelating agent penicillamine include leukopenia, thrombocytopenia, agranulocytosis and rarely aplastic anaemia. As our patient had anemia and neutropenia, not responding to routine treatment, a bone marrow examination was done to establish the cause of bicytopenia. It showed trilineage dysplasia and vacuolation of some of the erythroid and myeloid elements which when correlated with clinical and copper levels suggested a diagnosis of hypocupremia. Copper deficiency can have protean hematological manifestations with or without neurological manifestations and has been reported to result in anemia, neutropenia and less commonly thrombocytopenia [2, 3]. The RBC’s may be macrocytic, microcytic or normocytic; neutropenia is consistently reported and the absolute neutrophil count is usually <1,000/cumm [3, 4].
In copper deficiency, the bone marrow has been reported as variably cellular with features of dyserythropoiesis, dysmyelopoiesis variable number of ringed sideroblasts and at times trilineage dysplasia, which may be erroneously diagnosed as myelodysplastic syndrome (MDS) [3, 5]. However, vacuolization of both erythroid and myeloid precursors has been consistently reported [2, 3, 6, 7] and iron incorporation in plasma cells is occasionally mentioned in copper deficiency [2, 3]. Though the BMA findings are not specific, when correlated with clinical and copper levels they point to the correct diagnosis of dysplasia due to copper deficiency and may help to differentiate the dysplasia of hypocupremia from that of MDS [2–5]. In otherwise normal individuals, a diagnosis of copper deficiency can be established by demonstration of low levels of serum copper. However, in WD, low serum copper and serum ceruloplasmin are usual and a diagnosis of copper deficiency requires either analysis of liver copper content or clinical response after therapeutic trial with copper supplementation [4] as was done in this case. Hematological response to treatment is good and the bone marrow features of dysplasia are reversible, in contrast to myelodysplastic syndrome, which it mimics morphologically [8]. In a study of 22 patients with copper deficiency, 93 % of the hematological parameters normalized completely within 8–12 weeks after initiating copper treatment and removing zinc exposure [2]. In some case reports, recovery follows discontinuation of zinc alone [7].
The mechanism by which copper deficiency induces anemia and other cytopenias is unknown. However, copper is an essential cofactor for a number of redox enzymes essential for optimal erythropoiesis, including cytochrome oxidase and ceruloplasmin ferroxidase and it is hypothesized that decreased activity of these enzymes may lead to anemia [3, 4]. The cause of neutropenia remains obscure, though it is observed consistently [3]. Peled and coworkers suggest that copper deficiency results in the inhibition of differentiation and self-renewal of CD34 (+) hematopoietic progenitor cells [9]. Zinc toxicity may lead to secondary copper deficiency [6, 7] and oral zinc is also known to decrease copper levels by induction of the intestinal protein methallothionein, which preferentially binds copper, prevents its absorption and enhances its excretion [8]. Our patients symptoms worsened following zinc supplementation which may have contributed to the copper deficiency and severity of symptoms.
Acquired copper deficiency leading to anemia and neutropenia has been reported following total parental nutrition [3, 6], following copper chelation with triphenylene triamine dihydrochloride [10], following high doses of zinc [7] and in patients treated for WD [2].
In conclusion, in a patient with WD on treatment who develops cytopenia, copper deficiency may be considered in the presence of bone marrow dysplasia. In addition, the hematologic parameters of patients on chelation and zinc therapy should be monitored regularly, as copper deficiency can cause anemia not responding to routine therapy.
References
- 1.Frydman M, Bonne-Tamir B, Farrer LA, Conneally PM, Magazanik A, Ashbel S, Goldwitch Z. Assignment of the gene for Wilson disease to chromosome 13: linkage to the esterase D locus. Proc Natl Acad Sci USA. 1985;82:1819–1821. doi: 10.1073/pnas.82.6.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabreyes AA, Abbasi HN, Forbes KP, McQuaker G, Duncan A, Morrison I. Hypocupremia associated cytopenia and myelopathy:a national retrospective review. Eur J Haematol. 2013;90:1–9. doi: 10.1111/ejh.12020. [DOI] [PubMed] [Google Scholar]
- 3.Gregg XT, Reddy V, Prchal JT. Copper deficiency masquerading as myelodysplastic syndrome. Blood. 2002;100:1493–1495. doi: 10.1182/blood-2002-01-0256. [DOI] [PubMed] [Google Scholar]
- 4.Green R. Anemia resulting from other nutritional deficiencies. Trace metal deficiencies. In: Kaushansky K, Lichtman MA, Beutler E, Kipps TJ, Seligsohn U, Prchal JT, editors. Williams hematology. 8. New York: Mc Graw Hill; 2010. p. 609. [Google Scholar]
- 5.Lazarchick J. Update on anemia and neutropenia in copper deficiency. Curr Opin Hematol. 2012;19:58–60. doi: 10.1097/MOH.0b013e32834da9d2. [DOI] [PubMed] [Google Scholar]
- 6.Irving JA, Mattman A, Lockitch G, Farerell K, Wadsworth LD. Element of caution : a case of reversible cytopenias associated with excessive zinc supplementation. CMAJ. 2003;169:129–131. [PMC free article] [PubMed] [Google Scholar]
- 7.Willis MS, Monaghan SA, Miller ML, Mc Kenna RW, Perkins WD, Levinson BS, Bhushan V, Kroft SH. Zinc induced copper deficiency. A report of three cases initially recognized on bone marrow examination. Am J Clin Pathol: 2005;123:125–131. doi: 10.1309/V6GVYW2QTYD5C5PJ. [DOI] [PubMed] [Google Scholar]
- 8.Broun ER, Greist A, Tricot G, Hoffman R. Excessive zinc ingestion. A reversible cause of sideroblastic anemia and bone marrow depression. JAMA. 1990;264:1441–1443. doi: 10.1001/jama.1990.03450110087033. [DOI] [PubMed] [Google Scholar]
- 9.Peled T, Landau E, Prus E, Treves AJ, Fibach E. Cellular copper content modulates differentiation and self renewal in cultures of cord blood–derived CD34 + cells. Br J Hematol. 2002;116:661–665. doi: 10.1046/j.0007-1048.2001.03316.x. [DOI] [PubMed] [Google Scholar]
- 10.Condamine L, Hermine O, Alvin P, Levine M, Rey C, Courtecuisse V. Acquired sideroblastic anaemia during treatment of Wilson’s disease with triethylene tetramine dihydrochloride. Br J Hematol. 1993;83:166–168. doi: 10.1111/j.1365-2141.1993.tb04648.x. [DOI] [PubMed] [Google Scholar]