Abstract
Aberrant expression of CD5 has rarely been reported in B cell lineage acute lymphoblastic leukemia (B-ALL). We report the rare immunophenotypic expression of CD5 in a 20-year-old male who was diagnosed to have B-ALL on bone marrow examination. Cytogenetic analysis revealed a mosaic supernumerary marker chromosome. The patient died due to acute pancreatitis after admission. CD5 positive B-ALL may represent a distinct clinicopathologic subcategory of B-ALL with an aggressive clinical course.
Introduction
B lymphoblastic leukaemia (B-ALL) is a neoplasm of haematopoietic precursor cells characterized by the expression of various B-cell lineage associated antigens. CD5 expression in B-cell lineage chronic lymphoproliferative disorders (B-CLPD) is typically seen in chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma, or blastoid variant of mantle cell lymphoma (MCL) and occasionally some other B cell lymphomas [1]. CD5 expression in B-ALL is very uncommon with only few case reports and small series reported in literature [2–4]. Aberrant T-cell antigen expression is said to be associated with poor prognosis, and as a useful marker to identify patients at increased risk for relapse and for harbouring adverse cytogenetic abnormalities [5, 6]. In this report we present a case of 20 year old male with CD5+ B-ALL with karyotype showing small supernumerary marker chromosome (SMC). To our knowledge, this is the first report of CD5+ B-ALL from Indian literature.
Case Presentation
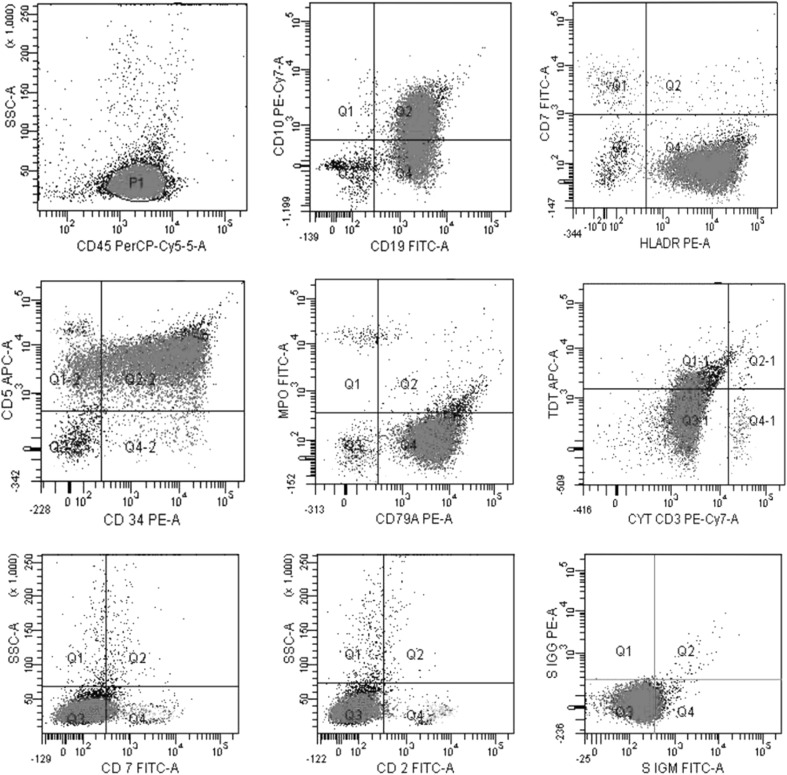
A 20-year-old male presented with history of blood in stools and abdominal pain of 5 days duration with associated fever, weakness and bony pains of 2 months duration. On examination, he was febrile with temperature of 100 °F with marked pallor and bilateral discrete enlarged inguinal lymph nodes, 1–2 cm in size. Systemic examination revealed hepatomegaly with liver enlarged 10 cm below costal margin. Computed tomographic scan of the abdomen showed a liver span of 26 cm with mild ascites. A complete blood count showed pancytopenia with haemoglobin of 3.1 g/dL, total leucocyte count 3.8 × 109/L and platelet count of 10 × 109/L. Peripheral blood smear showed 88 % blasts, 10 % lymphocytes and 2 % neutrophils with few nucleated red cells. Bone marrow aspirate smears showed 95 % blasts with French American British L2 morphology (Fig. 1). Blasts were negative for all cytochemical stains. Immunophenotyping on BM aspirate was carried out on FACS calibur flow cytometer after staining the cells with fluorescent conjugated antibodies (BD Biosciences, USA). Gating on CD45/side scatter showed blasts with moderate expression of CD45. A count of 10,000 events showed expression of CD34 (81.6 %), HLA DR (97 %), CD19 (99 %), CD10 (59.3 %), CD79a (98.5 %), and TDT (12.6 %). Bright expression of CD5 (96 %) was seen (Fig. 2). Other T cell antigens, namely, cytoplasmic CD3 (0.1 %), CD2 (1.3 %), CD7 (1.9 %) and myelomonocytic markers MPO, CD13, CD33, CD117 were negative. The other B-cell markers (CD23, CD22 and cytoplasmic and surface immunoglobins (Ig) light chains) were also negative. Immunohistochemistry for cyclin D1 on bone marrow biopsy was negative. Thus, a diagnosis of B-ALL with aberrant expression of CD5 was established. Conventional cytogenetics showed karyotype of 47,XY,+mar[6]/46,XY,[14]. Patient was treated with broad spectrum antibiotics, antifungals and transfusion support with partial improvement in his general condition. However, prior to being taken up for induction chemotherapy, he developed an acute abdomen, was diagnosed to have acute pancreatitis, and succumbed to his illness.
Fig. 1.
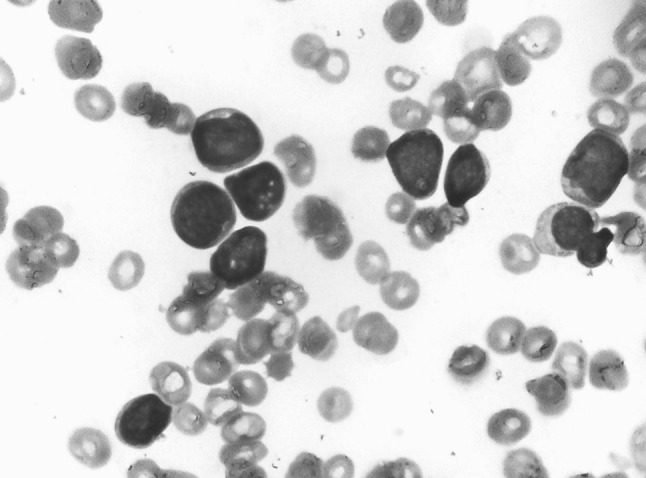
Bone marrow aspirate smear showing lymphoblasts with French American British L2 morphology (Jenner Giemsa, ×400)
Fig. 2.
Flow cytometric immunophenotyping of bone marrow aspirate showing leukemic lymphoblasts (red) expressing bright CD19, CD10, HLADR, CD34 and CD5 and no MPO, CD3, CD2, CD7 and surface Ig. Green events represent mature lymphocytes. (Color figure online)
Discussion
CD5 is a 67-kd T-cell membrane glycoprotein expressed on T-cells and on a subset of T-independent and memory B-cells. CD5 in humans appears to contribute to B-cell survival and interleukin 10 production through induction of anergic state in lymphocytes [7]. In clinical practice, CD5 is typically co-expressed by CLL and MCL. It is also reported in some other B-CLPDs, including diffuse large B-cell lymphoma (DLBCL), splenic marginal zone lymphoma (SMZL), lymphoplasmacytic lymphoma and even in some cases of follicular lymphoma [1]. Aberrant expression of CD5 in B-ALL is uncommon, seen in 2 % in a series of 200 cases of B-ALL cases [5] and 4.5 % cases in a series of 134 cases [6]. Blastoid variant of MCLs was considered in the differential diagnosis as both entities are CD19+, CD5+ and display an aggressive evolution. However, neither the t(11;14) nor bright surface Ig pattern seen on immunophenotyping in MCL were present in this patient.
Other B cell lymphoproliferative disorders showing CD5 expression namely CLL/PL, DLBCL, and SMZL were ruled out on the basis of morphology and expression of CD34. T-cell ALL were ruled out on the basis of negative expression for CD2, CD3 and CD7.
A mosaic SMC was reported in this patient’s karyotypic analysis. This is characteristically seen in patients with developmental disorders [8]. Although its association with hematologic malignancies is not well defined, centrosome amplification has been suggested as a marker of aggressiveness and invasive phenotype in some solid cancers [9]. Our patient died prior to administration of chemotherapy after an episode of acute pancreatitis. Aberrant expression of CD5 in B-ALL is very rare and may represent a distinct clinicopathologic subcategory of B-ALL with an aggressive clinical course.
Acknowledgments
Conflict of interest
The author(s) declare that they have no conflict of interest.
References
- 1.Jevremovic D, Dronca RS, Morice WG, McPhail ED, Kurtin PJ, Zent CS, et al. CD5+ B-cell lymphoproliferative disorders: beyond chronic lymphocytic leukemia and mantle cell lymphoma. Leuk Res. 2010;34(9):1235–1238. doi: 10.1016/j.leukres.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 2.Peterson MR, Noskoviak KJ, Newbury R. CD5-positive B-cell acute lymphoblastic leukemia. Pediatr Dev Pathol. 2007;10(1):41–45. doi: 10.2350/06-03-0057.1. [DOI] [PubMed] [Google Scholar]
- 3.Subirá D, Roman A, Jiménez-Garófano C, Prieto E, Martínez-Delgado B, Aceituno E, et al. Brief report. CD19/CD5 acute lymphoblastic leukemia. Med Pediatr Oncol. 1998;31(6):551–552. doi: 10.1002/(SICI)1096-911X(199812)31:6<551::AID-MPO23>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed D, Ahmed TA, Ahmed S, Tipu HN, Wiqar MA. CD5-positive acute lymphoblastic leukemia. J Coll Physicians Surg Pak. 2008;18(5):310–311. [PubMed] [Google Scholar]
- 5.Seegmiller AC, Kroft SH, Karandikar NJ, McKenna RW. Characterization of immunophenotypic aberrancies in 200 cases of B acute lymphoblastic leukemia. Am J Clin Pathol. 2009;132(6):940–949. doi: 10.1309/AJCP8G5RMTWUEMUU. [DOI] [PubMed] [Google Scholar]
- 6.Hussein S, Gill KZ, Sireci AN, Colovai AI, Small T, Emmons FN, et al. Aberrant T-cell antigen expression in B lymphoblastic leukaemia. Br J Haematol. 2011;155(4):449–456. doi: 10.1111/j.1365-2141.2011.08870.x. [DOI] [PubMed] [Google Scholar]
- 7.Gary-Gouy H, Harriague J, Bismuth G, Platzer C, Schmitt C, Dalloul AH. Human CD5 promotes B-cell survival through stimulation of autocrine IL-10 production. Blood. 2002;100(13):4537–4543. doi: 10.1182/blood-2002-05-1525. [DOI] [PubMed] [Google Scholar]
- 8.Reddy KS, Aradhya S, Meck J, Tiller G, Abboy S, Bass H. A systematic analysis of small supernumerary marker chromosomes using array CGH exposes unexpected complexity. Genet Med. 2013;15(1):3–13. doi: 10.1038/gim.2012.78. [DOI] [PubMed] [Google Scholar]
- 9.Ogden A, Rida PCG, Aneja R. Heading off with the herd: how cancer cells might maneuver supernumerary centrosomes for directional migration. Cancer Metastasis Rev. 2013;32(1–2):269–287. doi: 10.1007/s10555-012-9413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]