Abstract
In 2012, a 25-years-old pregnant woman presented with thromocytosis for 4 months, blood counts showed platelets 701 × 109/L. Bone marrow examination disclosed a feature of hypercellular marrow in erythrocytic,granulocytic and megakaryocytic series. Cytogenetic analysis showed t(9;22)(q34;q11) in 100 % of metaphase. The percentage of BCR–ABL-positive FISH signals was 37 % in the peripheral blood. Molecular analysis showed the presence of the JAK2V617F mutation and BCR–ABL mRNA b3a2 transcript. A diagnosis of concomitant presence of essential thrombocythemia and chronic myelocytic leukemia was made. Based on this case and literatures reported before, it might be necessary to detect JAK2-V617F mutation and BCR–ABL fusion gene concomitantly in myeloproliferative neoplasms patients.
Keywords: Myeloproliferative neoplasms, Essential thrombocythemia, Chronic myelocytic leukemia, JAK2V617F mutation, BCR–ABL
The frequency of dual BCR–ABL transcript and JAK2V617F might be low in myeloproliferative neoplasms (MPN) but probably higher than expected. Computerized literature searches of the PUBMED database were performed. The searches of the English language literatures from 2007 to 2013 combined the terms “CML”, “BCR–ABL” and “JAK2V617F” showed 41 patients had a concomitant BCR–ABL translocation and JAK2V617F mutation. Almost all of the cases were expressing the P210 oncoprotein (b3a2 or b2a2 junctions) except two cases who expressed P190 protein (e1a2 junction) [1] and P230 protein (e19a2 junction) [2] respectively. Reviewing these cases, three type of scenarios were observed: (1) Initially diagnosed with chronic myelocytic leukemia (CML) and subsequently proceed to manifest a JAK2V617F(+) myeloproliferative phenotype after treatment with imatinib; (2) Initially diagnosed with CML and found to be co-existing with JAK2V617F(+) polycythernia vera (PV), primary myelofibrosis (PMF) or essential thrombocythemia (ET); (3) Initially diagnosed with JAK2V617F(+)PV, PMF or ET, and proceeded to CML several years later. Overall, PV and PMF were the common Ph (-) MPN coexistance with CML, ET was unlikely involved. Men were affected more frequently than women (24 vs 12), 28 of 36 (77.7 %) patients were above 50 years old, only one case was reported below 30 years old [3]. We present here a pregnant woman with the initial diagnosis of ET in the presence of JAK2V617F mutation and BCR–ABL translocation.
Patients and Methods
In July 2012, a 25-years-old pregnant woman (20 weeks) presented with an increased thrombocytosis (701 × 109/L) for 4 months and physical examination did not show splenomegaly. Her white blood cell counts (WBC) and hemoglobin concentration were within reference range (WBC 9.1 × 109/L, Hb 127 g/L). Regarding the pregnant status, further examination such as bone marrow (BM) was not done at that time. Her whole blood count showed an increasing WBC (14.5 × 109/L) while platelet remained at the previous level (716 × 109/L) on 2 days before delivery. Concomitant JAK2V617F mutation and BCR–ABL fusion gene (b3a2) of the peripheral blood were detected by allele-specific polymerase chain reaction [4] and 2-step reverse transcription PCR (RT-PCR), respectively (Fig. 1). Direct sequencing assay revealed heterozygous mixed-type 1894 G > T mutation in JAK2 (Fig. 2). Real-time quantitative PCR (RQ-PCR) of RNA showed the BCR–ABL transcript at a high level (ratio BCR–ABL/ABL: 161 %). The presence of the t (9;22) was confirmed by fluorescence in situ hybridization (FISH) using extra-signal probe (VYSIS) on peripheral blood, 37 % analyzed nuclei showed 1 yellow, 2 red and 1 green signal (Fig. 3). After she gave birth to a healthy girl on December 5th, BM sample was collected. BM smears revealed a hypercellular marrow with unusually increased number of platelet-producing megakaryocytes and abundant platelets, a normal myeloid: erythroid ratio (1.6:1) and normal percentage of myelocytes in differential maturation (Table 1), the neutrophil alkaline phosphatase score was 20. Cytogenetic analysis revealed a translocation (9;22)(q34;q11) in the 100 % metaphases examined and FISH showed 67 % cells with BCR–ABL fusion signal in BM sample. According to clinical manifestation and morphology, a diagnosis of ET with concomitant JAK2V617F and BCR–ABL was made.
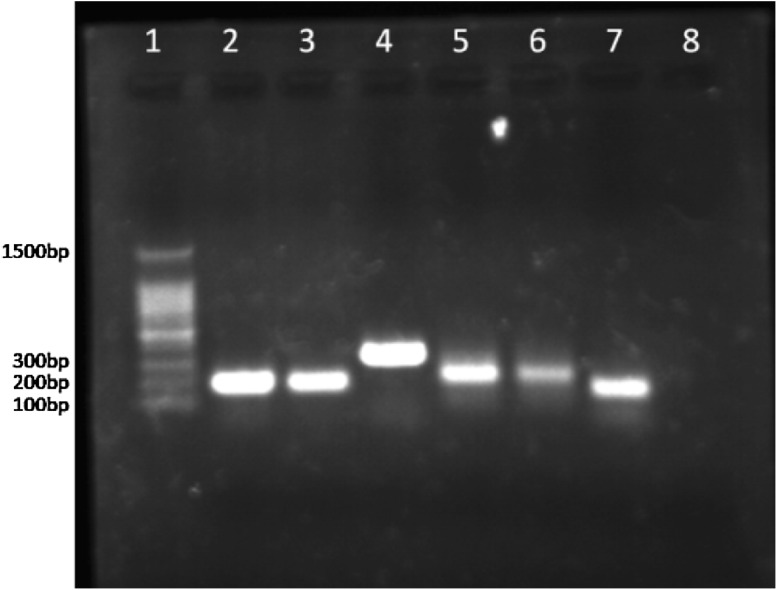
Fig. 1.
BCR–ABL gene rearrangement analysis using RT-PCR, JAK2V617F detected by PCR. The No. 1 lane shows molecular weight markers (from 100 bp to 1500 bp). No. 2 lane JAK2V617F positive control (203 bp), No. 3 lane JAK2V617F mutation allele (from patient’s PB), No. 4 lane wild allele for JAK2V617F (364 bp), No. 5 lane: BCR–ABL positive control (314 bp), No. 6 lane: BCR–ABL fragment from patient’s PB, No. 7 lane ABL internal control (184 bp), No. 8 lane negative control
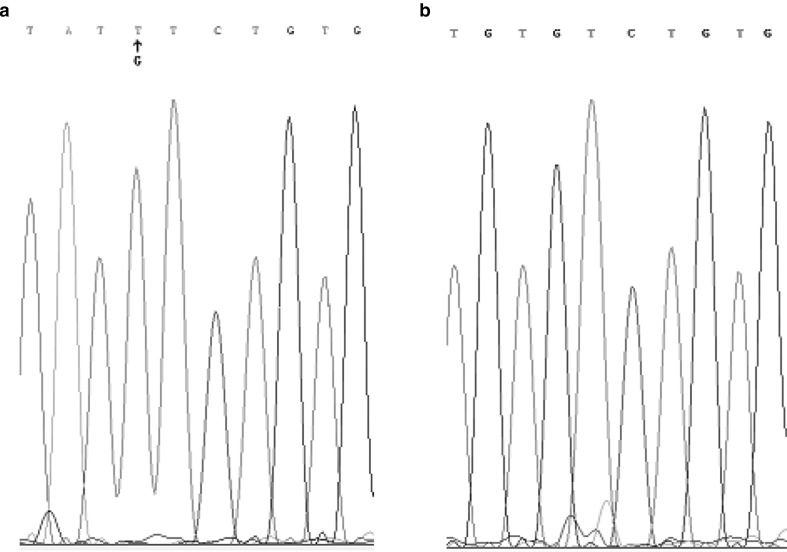
Fig. 2.
JAK2 V617F mutation by DNA sequence analysis. a mutation type allele, b Wild type allele
Fig. 3.
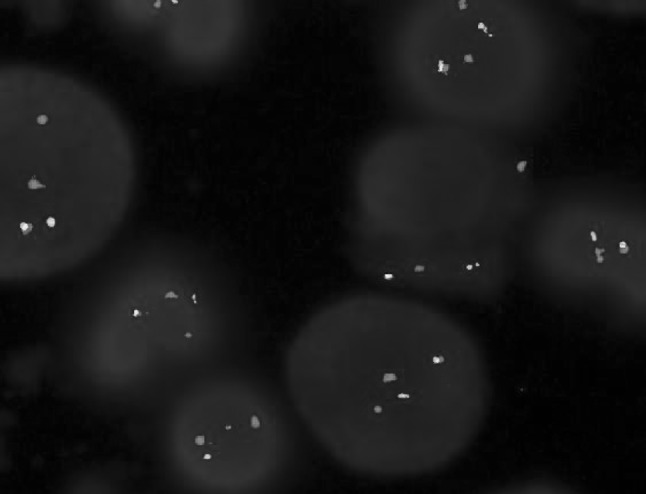
FISH using the LSI BCR–ABL ES probe, positive cells showed 1Y, 2R, 1G fusion signals
Table 1 .
BM features at the time of CML and ET diagnosis
| Cell name | Percentage (%) | Reference value |
|---|---|---|
| Blasts | 3 | 0.3–2 |
| Promyelocyte | 1 | 1–8 |
| Neutrophilic myelocyte | 6 | 5–20 |
| Neutrophilic metamyelocyte | 8 | 9–18 |
| Band form neutrophilic granulocyte | 9 | 4–14 |
| Neutrophilic segmented granulocyte | 20 | 7–30 |
| Eosinophilic metamyelocyte | 2 | 0.5–4 |
| Eosinophilic granulocyte band form | 1 | |
| Eosinophilic granulocyte segmented form | 2 | |
| Basophilic granulocyte segmented form | 1 | 0–1 |
| Small lymphocyte | 11 | 3–20 |
| Monocyte | 3 | 0.5–5 |
| Pronormoblast | 1 | 0.5–4 |
| Early erythroblast | 4 | 1–5 |
| Polychromatic normoblast | 10 | 12–20 |
| Acidophilic normoblast | 18 | 6–10 |
Discussion
MPN are classified into two major categories, CML and ph (−) MPN, such as PV, ET and PMF. MPN are often found in elderly patients and men are affected more frequently than women. Since the post-JAK2V617F era, totally 41 MPN patients have been reported with concurrent BCR–ABL junction and JAK2V617F mutation. The male and female ratio was 2:1 (24 vs 12), except for five cases without description [5]. The median age of male and female cases was 62.5 years (male: 32–71) and 68 years (female: 27–82). 11 of 12 female cases were above 52, only one case was a 27 years-old pregnant woman who was diagnosed PV and CML [3]. Few cases of concomitant ET and CML have been reported in women under the age of 30. Up to January 2013, the patient here is the second pregnant case and the youngest woman with ET and CML.
Before the JAK2V617F era, PV, PMF or ET transition to CML had been reported in the literature. This pattern of disease-process was further proved by JAK2V617F mutation. The other two patterns involving JAK2V617F (+) PV, PMF or ET concurrent with CML and CML developed to JAK2V617F (+) PV, PMF or ET were reported. Although the dual genetic abnormalities were detected simultaneously in the patient here presented with ET, could we make a diagnosis of Ph (+) ET? It led the researchers to postulate the relationship between the BCR–ABL and JAK2V617F anomalies in a single patient. Which aberration happened earlier? One possibility is that the BCR–ABL is a second leukemogenic event [6] in JAK2V617F positive clone. Several reports [7–11] observed PV, PMF or ET with only JAK2V617F mutant clone could evolve to CML. Even in those patients who successfully treated by IM, the retrospective assessment of stored DNA samples showed that JAK2V617F mutation was already existed [12–14]. They hypothesized previously existing JAK2V617F (+) clones were masked by dominant BCR–ABL clones. Cultured colonies from a patient with both positive JAK2V617F and BCR–ABL showed the presence of JAK2V617F mutation alone or together with BCR–ABL transcripts, but in contrast, BCR–ABL transcripts were never detected alone [11]. These results also speculated JAK2V617F mutations occurs in hemopoietic stem cells as an initial step with a sub-clone acquiring an additional BCR–ABL translocation. However, other group did not found JAK2V617F clones in the previously stored samples in JAK2V617F (+) MPN and CML patients [7, 15, 16].
JAK2V617F mutation and BCR–ABL transcription were proved to exist in stem cells, respectively. Bornhäuser [8] found that the two aberrations can present in one stem cell clone (CD34+cell). Another question is in the progress of cell differentiation, whether the aberrations exist in two separated clones or in one cell clone [17]. One hypothesis is there are two independent clones each having BCR–ABL and JAK2 V617F mutation [12, 18–20]. Evidence favoring this theory is JAK2V617F diminished when BCR–ABL mRNA burden increased and reappeared once BCR–ABL mRNA levels were reduced by IM therapy [7, 19]. Another theory is JAK2V617F exist in all the myeloid cells, BCR–ABL limited to a small compartment of myeloid progenitor cells. IM induced suppression of the BCR–ABL clone but not the JAK2V617F clone. The latter remained in previous level or even higher than before. Cambier [21] and Bornhauser [8] proved that JAK2V617F mutation could be detected in granulocytic colonies and erythroid colonies, while BCR–ABL fusion existed only in granulocytic colonies but not in erythroid colonies. The case reported here should be classed into this situation because our FISH results showed t(9;22) existed in 37 % nucleiod cells. The third hypothesis is that a single stem cell clone concurrently possesses both BCR–ABL and JAK2V617F mutation. As for this mechanism, Campiotti et al. [22] observed IM therapy caused the regression of CML cells, also including those with JAK2V617F mutation. Bocchia [10] discovered evidence for the concurrent presence of BCR–ABL transcript and JAK2V617F in most of the erythroid and myeloid colonies at the time of CML diagnosis.
Together with previous reports, we would propose that clinicians need to be aware of the possibility that CML might occur on the background of JAK2V617F(+) MPN, and treatment of CML might unmask a concomitant Ph (-) MPN. It is necessary to test the concomitant presence of JAK2-V617F mutation and BCR–ABL status in MPN patients, especially in those CML patients who failed to obtain an optimal response to IM therapy or abnormal elevated blood values (Hb and/or platelets). Most recently, Inokuchi [23] reported dasatinib successfully repressed both BCR–ABL and JAK2V617F clone in the patient [24] who resisted to IM. On the contrary, Hyeoung-Joon Kim [25] reported dasatinib and nilotinib suppressed the BCR–ABL clone not the JAK2V617F clone in the past. It seems that tyrosine kinase inhibitor associated with JAK2 inhibitor could be the best choice for these dual anomalous patients.
Footnotes
You-wen Qin and Yi-ning Yang are co-first authors.
Contributor Information
You-wen Qin, Email: youwenqin@hotmail.com.
Chun Wang, Phone: +86-21-63240090, FAX: +86-21-63097008, Email: wangchun2@medmail.com.cn.
References
- 1.Caocci G, Atzeni S, Orrù N, et al. Response to imatinib in a patient with chronic myeloid leukemia simultaneously expressing p190BCR–ABL oncoprotein and JAK2V617F mutation. Leuk Res. 2010;34(1):e27–e29. doi: 10.1016/j.leukres.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Hussein K, Bock O, Seegers A, et al. Myelofibrosis evolving during imatinib treatment of a chronic myeloproliferative disease with coexisting BCR–ABL translocation and JAK2V617F mutation. Blood. 2007;109(9):4106–4107. doi: 10.1182/blood-2006-12-061135. [DOI] [PubMed] [Google Scholar]
- 3.Toogeh G, Ferdowsi S, Naadali F, et al. Concomitant presence of JAK2 V617F mutation and BCR–ABL translocation in a pregnant woman with polycythemia vera. Med Oncol. 2011;28(4):1555–1558. doi: 10.1007/s12032-010-9570-8. [DOI] [PubMed] [Google Scholar]
- 4.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 5.Cappetta M, Pérez V, Zubillaga MN, et al. Concomitant detection of BCR–ABL translocation and JAK2 V617F mutation in five patients with myeloproliferative neoplasm at diagnosis. Int J Lab Hematol. 2013;35(1):e4–e5. doi: 10.1111/ijlh.12010. [DOI] [PubMed] [Google Scholar]
- 6.Conchon MR, Costa JL, Novaes MM, et al. Simultaneous detection of JAK2 V617F mutation and Bcr–Abl translocation in a patient with chronic myelogenous leukemia. Int J Hematol. 2008;88(2):243–245. doi: 10.1007/s12185-008-0131-2. [DOI] [PubMed] [Google Scholar]
- 7.Hussein K, Bock O, Theophile K, et al. Chronic myeloproliferative diseases with concurrent BCR–ABL junction and JAK2V617F mutation. Leukemia. 2008;22(5):1059–1062. doi: 10.1038/sj.leu.2404993. [DOI] [PubMed] [Google Scholar]
- 8.Bornhäuser M, Mohr B, Oelschlaegel U, et al. Concurrent JAK2(V617F) mutation and BCR–ABL translocation within committed myeloid progenitors in myelofibrosis. Leukemia. 2007;21(8):1824–1826. doi: 10.1038/sj.leu.2404730. [DOI] [PubMed] [Google Scholar]
- 9.Mirza I, Frantz C, Clarke G, et al. Transformation of polycythemia vera to chronic myelogenous leukemia. Arch Pathol Lab Med. 2007;131(11):1719–1724. doi: 10.5858/2007-131-1719-TOPVTC. [DOI] [PubMed] [Google Scholar]
- 10.Bocchia M, Vannucchi AM, Gozzetti A, et al. Insights into JAK2-V617F mutation in CML. Lancet Oncol. 2007;8(10):864–866. doi: 10.1016/S1470-2045(07)70295-4. [DOI] [PubMed] [Google Scholar]
- 11.Hummel JM, Kletecka MC, Sanks JK, et al. Concomitant BCR–ABL1 translocation and JAK2(V617F) mutation in three patients with myeloproliferative neoplasms. Diagn Mol Pathol. 2012;21(3):176–183. doi: 10.1097/PDM.0b013e318246975e. [DOI] [PubMed] [Google Scholar]
- 12.Krämer A, Reiter A, Kruth J, et al. JAK2-V617F mutation in a patient with Philadelphia-chromosome-positive chronic myeloid leukaemia. Lancet Oncol. 2007;8(7):658–660. doi: 10.1016/S1470-2045(07)70206-1. [DOI] [PubMed] [Google Scholar]
- 13.Pardini S, Fozza C, Contini S, et al. A case of coexistence between JAK2V617F and BCR/ABL. Eur J Haematol. 2008;81(1):75–76. doi: 10.1111/j.1600-0609.2008.01063.x. [DOI] [PubMed] [Google Scholar]
- 14.Pieri L, Spolverini A, Scappini B, et al. Concomitant occurrence of BCR–ABL and JAK2V617F mutation. Blood. 2011;118(12):3445–3446. doi: 10.1182/blood-2011-07-365007. [DOI] [PubMed] [Google Scholar]
- 15.Pingali SR, Mathiason MA, Lovrich SD, et al. Emergence of chronic myelogenous leukemia from a background of myeloproliferative disorder: jAK2V617F as a potential risk factor for BCR–ABL translocation. Clin Lymphoma Myeloma. 2009;9(5):E25–E29. doi: 10.3816/CLM.2009.n.080. [DOI] [PubMed] [Google Scholar]
- 16.Tefferi A, Levitt R, Lasho TL, et al. Postimatinib therapy emergence of a new JAK2V617F clone and subsequent development of overt polycythemia vera in a patient with chronic myelogenous leukaemia. Eur J Haematol. 2010;85(1):86–87. doi: 10.1111/j.1600-0609.2010.01458.x. [DOI] [PubMed] [Google Scholar]
- 17.Krämer A. JAK2-V617F and BCR–ABL–double jeopardy? Leuk Res. 2008;32(10):1489–1490. doi: 10.1016/j.leukres.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Gattenlohner S, Völker HU, Etschmann B, et al. BCR–ABL positive chronic myeloid leukemia with concurrent JAK2(V617F) positive myelodysplastic syndrome/myeloproliferative neoplasm (RARS-T) Am J Hematol. 2009;84(5):306–307. doi: 10.1002/ajh.21296. [DOI] [PubMed] [Google Scholar]
- 19.Bee PC, Gan GG, Nadarajan VS, et al. A man with concomitant polycythaemia vera and chronic myeloid leukemia: the dynamics of the two disorders. Int J Hematol. 2010;91(1):136–139. doi: 10.1007/s12185-009-0471-6. [DOI] [PubMed] [Google Scholar]
- 20.Véronèse L, Tchirkov A, Richard-Pebrel C, et al. A thrombocytosis occurring in Philadelphia positive CML in molecular response to imatinib can reveal an underlying JAK2(V617F) myeloproliferative neoplasm. Leuk Res. 2010;34(4):e94–e96. doi: 10.1016/j.leukres.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 21.Cambier N, Renneville A, Cazaentre T, et al. JAK2V617F-positive polycythemia vera and Philadelphia chromosome-positive chronic myeloid leukemia: one patient with two distinct myeloproliferative disorders. Leukemia. 2008;22(7):1454–1455. doi: 10.1038/sj.leu.2405088. [DOI] [PubMed] [Google Scholar]
- 22.Campiotti L, Appio L, Solbiati F, et al. JAK2-V617F mutation and Philadelphia positive chronic myeloid leukemia. Leuk Res. 2009;33(11):e212–e213. doi: 10.1016/j.leukres.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Inokuchi K, Yamaguchi H, Tamai H, et al. Disappearance of both the BCR/ABL1 fusion gene and the JAK2V617F mutation with dasatinib therapy in a patient with imatinib-resistant chronic myelogenous leukemia. J Clin Exp Hematop. 2012;52(2):145–147. doi: 10.3960/jslrt.52.145. [DOI] [PubMed] [Google Scholar]
- 24.Inami M, Inokuchi K, Okabe M, et al. Polycythemia associated with the JAK2V617F mutation emerged during treatment of chronic myelogenous leukemia. Leukemia. 2007;21(5):1103–1104. doi: 10.1038/sj.leu.2404591. [DOI] [PubMed] [Google Scholar]
- 25.Kim YK, Shin MG, Kim HR, et al. Simultaneous occurrence of the JAK2V617F mutation and BCR–ABL gene rearrangement in patients with chronic myeloproliferative disorders. Leuk Res. 2008;32(6):993–995. doi: 10.1016/j.leukres.2007.10.018. [DOI] [PubMed] [Google Scholar]