Introduction
Methemoglobin (MetHb) has an oxidized ferric iron (Fe+3) instead of regular reduced ferrous form (Fe+2) of normal hemoglobin [1]. This structural change of MetHb is responsible for its inability to bind oxygen [1, 2].
A serum level of 1 % is physiological for MetHb and has no significant negative effect on oxygen transporting. However, under conditions causing oxidative stress, MetHb levels rise and cyanosis occurs with levels above 10–15 %. Systemic symptoms such as tachycardia and vomiting besides lethargy and coma may be observed with increasing levels. A MetHb level above 70 % is considered lethal [2].
We report the noninvasive monitoring of a newborn with methemoglobinemia presented with cyanosis due to local anesthetic (combined lidocaine and prilocaine) administration for circumcision.
Case Report
An 18-day-old male infant was admitted to our emergency room with sudden severe cyanosis. His parents reported a local anesthetic application (combination of lidocaine and prilocaine) for circumcision 4 h ago. The infant’s physical examination was normal except cyanosis (Fig. 1). He had no respiratory problem. His heart rate was 122/min and rhythmic, respiratory rate was 40/min. The patient was monitored noninvasively with Massimo Rainbow SET® Radical 7 pulse oximeter, USA in the neonatal intensive care unit which showed a MetHb value of 29 % (Fig. 2). The first blood gas analysis taken at the same time was as follows: pH 7.39, pO2 82.9, pCO2 26.5, MetHb 29.9 %, HCO3 15.8, SaO2 100 %. Hematologic and biochemical parameters were normal. Echocardiography performed for the differential diagnosis of cyanosis was normal. Glucose-6-phosphate dehydrogenase (G6PD) level was also normal.
Fig. 1.
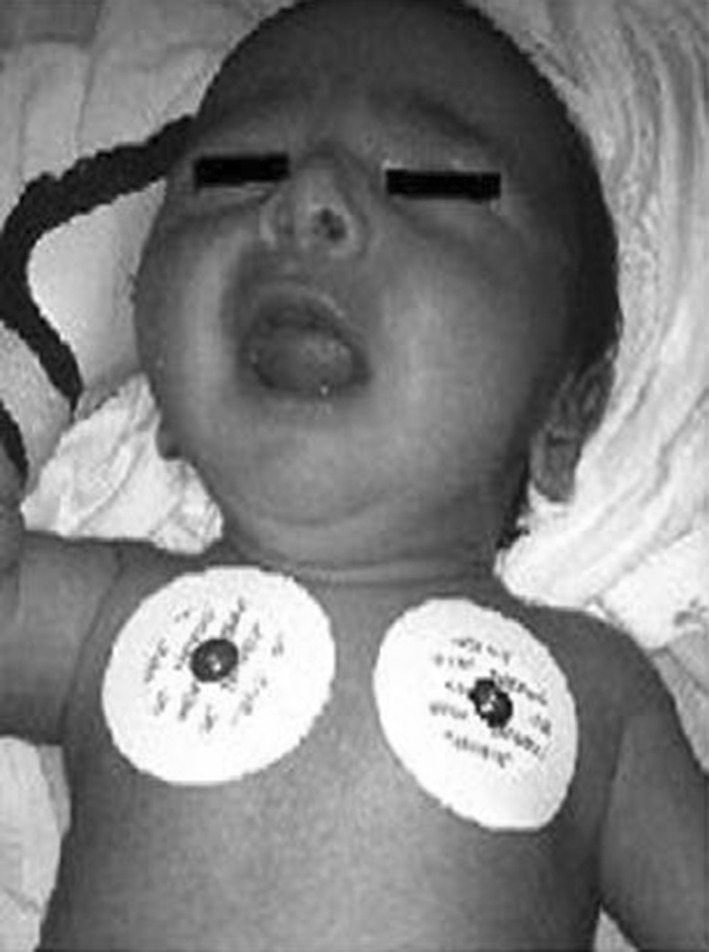
Picture of the patient with cyanosis
Fig. 2.
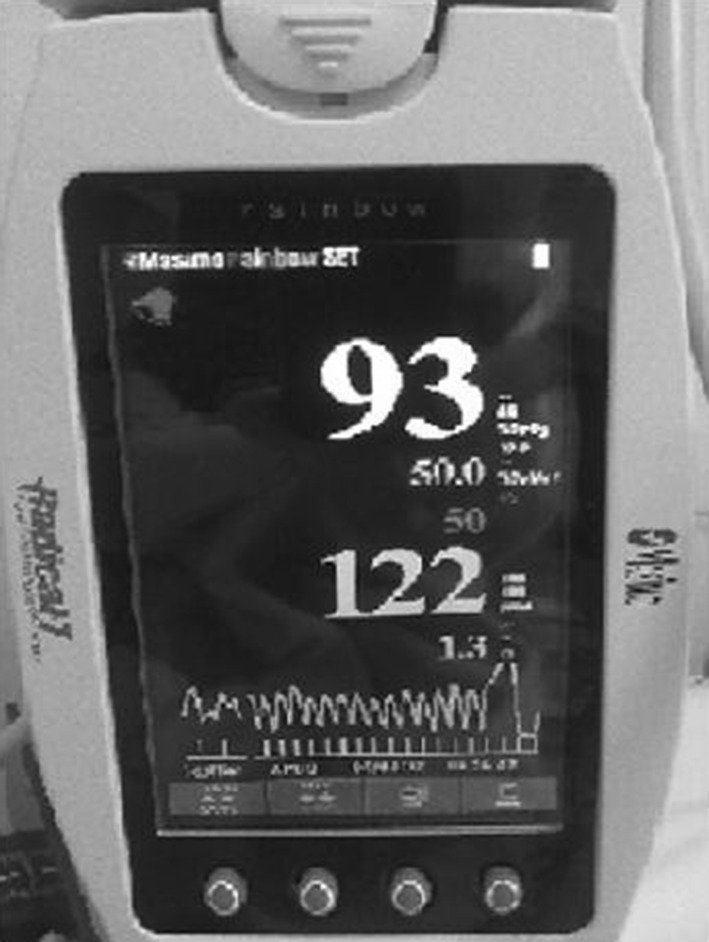
Screen of the pulse oximeter with a methemoglobin level of 50 %
MetHb levels gradually increased to a maximum level of 50 % in Massimo monitor within 10 min when 2 mg/kg dosage of intravenous methylene blue infusion was started. Methemoglobinemia was successfully treated with this infusion of 10 min and MetHb level decreased to 3.8 % at the end of the infusion and to 1.1 % 1 h later. The cyanosis resolved and no further increase in MetHb levels was observed. The patient was discharged from the hospital with no complication. Methemoglobin levels are serially measured during the follow-ups and no evidence is detected to support congenital methemoglobinemia.
Discussion
Methemoglobinemia may develop due to congenital or more commonly to acquired reasons [3]. Acquired form of methemoglobinemia is more frequent among infants in the first 4 months of life. The reduction capacity of hemoglobin and therefore oxygen carrying capacity of the erythrocytes decrease together with exposure to oxidant drugs and toxins. This may be due to the easier oxidation of the fetal hemoglobin and also to the lower NADH reductase enzyme levels at birth increasing in the first 4 months of life reaching to the normal limits at the end of the fourth month [2].
The main chemicals causing toxic (acquired) methemoglobinemia are benzocaine, chloroquine, dapsone, flutamide, lidocaine, metoclopramide, nitrites, nitric oxide, prilocaine, primaquin, riluzole, sodium nitrate and sulfonamides. Mostly, complete recovery may be observed with removal of the etiologic agent in patients with MetHb levels below 30 %. Above this level, treatment with methylene blue is required [4].
Circumcision in newborn period rather than in later childhood is becoming more popular in Muslim countries. Our 18 days old patient was circumcised after the local anesthetic administration which contains 25 mg lidocaine and 25 mg prilocaine in 1 g. O-toluidine, a prilocaine metabolite, may cause methemoglobinemia which is usually reversible [5, 6]. This topical agent’s efficacy and reliability is limited in the first 3 months of life and the clinical findings of methemoglobinemia may be observed even within the therapeutic dosage of 1–2 mg/kg in this age group [7].
Noninvasive monitoring of the hemoglobin levels is necessary for rapid diagnosis and may be life saving. Recently, new devices measuring MetHb levels noninvasively have been developed especially for the neonatal intensive care units in order to detect “methemoglobinemia” occurring as a side effect of nitric oxide treatment in infants with persistent pulmonary hypertension. These devices are also designed to be used as a pulse oximeter. The Massimo Rainbow SET® Radical 7 pulse oximeter can noninvasively measure MetHb and carboxyhemoglobin, as well as conventional blood oxygen saturation (SpO2). As Goldstein et al. [8] stated, the measurements of MetHb and SpO2 correlates with blood gas analysis in neonates. We used this device to monitor MetHb levels continuously at the diagnosis and during the treatment of our patient.
Methylene blue is the first treatment choice; but can be performed only after the exclusion of G6PD deficiency. It may act as an oxidant agent and may result with hemolysis in G6PD deficiency [9].
In normal conditions, MetHb is rapidly degraded by NADH-methemoglobin reductase (cytocrom b5 reductase) in erythrocytes. There is another system of MetHb reductase in erythrocytes using NADPH as a cofactor. The physiologically inactive methemoglobin reductase enzyme is activated with methylene blue by translating the electron from NADPH to MetHb [9]. Methylene blue with a dosage of 2 mg/kg was used in our patient after exclusion of G6PD deficiency and MetHb levels gradually decreased to 1.1 from 52 %.
Vitamin C (ascorbic acid) reduces the MetHb through a non-enzymatic pathway and may be used as an alternative treatment and it is the primary option in patients with G6PD deficiency [2, 10].
In conclusion, the clinical findings of methemoglobinemia may be observed even with the therapeutic dosage of local anesthetic agents due to the rapid passage to the circulation from the skin in the first 3 months of life. Therefore, a careful consideration about circumcision indication in the newborn period is required. Noninvasive diagnosis and treatment follow-up is possible with the new pulse oximeter devices that follow not only oxyhemoglobin saturation but also pathologic MetHb levels as in our patient who were successfully treated with methylene blue infusion.
References
- 1.Hopkins U. Methemoglobinemia. Univ Md Sch Pharm. 2000;17:1–4. [Google Scholar]
- 2.Quirolo K, Vichinsky E. Hemoglobin disorders. In: Behrman RE, Kleigman RM, Jenson HB, editors. Nelson textbook of pediatrics. Philadelphia: Saunders; 2004. pp. 1623–1634. [Google Scholar]
- 3.Denshaw-Burke M, Curran AL et al (2013) Methemoglobinemia. http://emedicine.medscape.com/article/204178-overview
- 4.Osterhoudt KC. Methemoglobinemia. In: Ford M, Delaney K, Ling L, Erickson T, editors. Ford: clinical toxicology. 1. Philadelphia: W.B. Saunders Company; 2001. pp. 211–217. [Google Scholar]
- 5.Tetzlaff JE. The pharmacology of local anesthetics. Anesthesiol Clin N Am. 2000;18:213–233. doi: 10.1016/S0889-8537(05)70161-9. [DOI] [PubMed] [Google Scholar]
- 6.Duncan PG, Kobrinsky N. Prilocaine-induced methemoglobinemia in a newborn infant. Anesthesiology. 1983;59:75–76. doi: 10.1097/00000542-198307000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Kumar AR, Dunn N, Naqvi M. Methemoglobinemia associated with a prilocaine-lidocaine cream. Clin Pediatr. 1997;36:239–240. doi: 10.1177/000992289703600410. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein MR, Saesim D, Macknet M et al (2008) Do pulse CO-oximeter measures of Spmet and SpO2 correlate with blood gas CO-oximetry in neonates? Anestesiology 109: A1218 poster
- 9.Mansouri A. Methemoglobinemia. Am J Med Sci. 1985;289:200–209. doi: 10.1097/00000441-198505000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med. 1999;34:646–656. doi: 10.1016/S0196-0644(99)70167-8. [DOI] [PubMed] [Google Scholar]


