Abstract
Background and Purpose
The A2DS2 score was recently developed from the Berlin Stroke Registry for predicting in-hospital pneumonia after acute ischemic stroke and performed well in an external validation in the North-west Germany Stroke Registry. It could be a useful tool for risk stratification in clinical practice or stroke trials. We aimed to prospectively validate the predictive value of A2DS2 score in a Chinese stroke population.
Methods
The prognostic model was used to predict stroke-associated pneumonia (SAP) from Henan Province Stroke Registry (HNSR) in which data were prospectively collected. The receiver-operating characteristic curves were plotted, and the C statistics were calculated to assess the discrimination ability. The Hosmer–Lemeshow goodness-of-fit test and the plot of observed versus predicted SAP risk were used to assess model calibration.
Results
Among 1142 eligible patients, the overall in-hospital SAP was 18.8%, which ranged from 9.0% in patients with lower A2DS2 scores (0–4) to 65.0% in those with higher scores of 5 to 10 (P for trend <0.001). The C statistic was 0.836 (95% confidence interval, 0.803–0.868) through the A2DS2 score, suggesting excellent discrimination in the HNSR. The A2DS2 score also showed excellent calibration (Cox and Snell R 2 = 0.243) in the external validation sample from the HNSR.
Conclusions
The A2DS2 score could reliably predict in-hospital SAP in Chinese stroke patients. It might be helpful for the assessment of increased risk monitoring and prophylactic treatment in identified high-risk patients for SAP in clinical routine.
Introduction
Pneumonia is a major cause of in-hospital morbidity and mortality in acute stroke population [1]–[11], and attributes to increase the length of hospital stay and hospitalization cost [12]–[16].
Previous studies have identified several factors being independently associated with stroke-associated pneumonia (SAP) in acute stroke, such as older age, stroke severity, dysphagia, impaired level of consciousness, diabetes mellitus, location of stroke infarction and so on [11], [12], [16]–[27]. Some of these findings have been translated into scoring systems aiming at risk stratification for SAP [19]–[24]. However, no reliable scoring system is currently available in routine clinical practice or stroke trials.
The A2DS2 score (Table S1) was recently developed from the Berlin Stroke registry (BSR) cohort to assess the risk of in-hospital SAP based on routinely collected data and externally validated its predictive properties in the independent North-west Germany Stroke Registry(NGSR) [22]. It may be a useful tool for clinical practice and stroke trials. We aimed to prospectively validate the A2DS2 score in a Chinese stroke population.
Methods
Patients selection
Patients included in this study were from the database of the Henan Province Stroke Registry (HNSR), which is a prospective single-center hospital–based cohort study of consecutive patients who had transient ischemic attack (TIA) and acute ischemic stroke (stroke onset to hospital ≤7 days) [28].
Detailed baseline data were registered prospectively using standardized case report forms. Acute ischemic stroke registry forms were completed by neurologists with similar levels of training and experience. To be eligible for this study, subjects had to meet the following criteria: (1) age ≥18 years; (2) hospitalized with a primary diagnosis of acute ischemic stroke according to World Health Organization criteria [29]; (3) stroke confirmed by CT or MRI. Patients were excluded if any of the components of the A2DS2 score were not available.
Ethics Statement
The study was approved by the central Institutional Review Board at the first affiliated hospital of Zhengzhou university. All patients or their designated relatives were informed about study participation, and informed written consent was obtained.
Data definitions
The following variables were analyzed for the present study: (1)demographics (age and sex); (2)stroke risk factors: hypertension (history of hypertension or antihypertensive medication use), diabetes mellitus (history of diabetes mellitus or antidiabetic medication use), dyslipidemia (history of dyslipidemia or lipid-lowering medication use), atrial fibrillation (history of atrial fibrillation or documentation of atrial fibrillation at admission), coronary heart disease, history of stroke and TIA, current smoking, and excess alcohol consumption (≥2 standard alcohol beverages per day); (3)admission stroke severity based on National Institutes of Health Stroke Scale score (NIHSS); (4)clinical feature: dysphagia (symptom of dysphagia, or abnormal of swallowing water test, or decline of consciousness level, or cough asthenia); (5)stroke subtype: according to the Oxfordshire Community Stroke Project criteria (OCSP) [30]; (6)complication: pneumonia; (7)length of hospital stay.
In this study, SAP was diagnosed by treating physician according to the Centers for Disease Control and Prevention criteria for hospital-acquired pneumonia [31], on a basis of clinical and laboratory indices of respiratory tract infection (fever, cough, auscultatory respiratory crackles, new purulent sputum, or positive sputum culture), and supported by typical chest X-ray or CT findings. Only hospital-acquired pneumonia was documented and pneumonia before stroke was not considered. Data on in-hospital SAP was prospectively recorded.
Outcomes
The main outcome of interest was in-hospital SAP.
Statistical analysis
The Shapiro-Wilk test was used to check the normality of continuous variables. Student t test was used in the case of normality, and Mann-whitney test was used in the case of abnormality. The differences between categorical variables were analyzed by χ2 test. Discrimination was assessed by calculating the area under the receiver operating characteristic curve (AUROC). Calibration was assessed by performing the Hosmer–Lemeshow goodness of fit test and was graphically depicted in the plot of observed versus predicted SAP risk according to 7 deciles of predicted risk. Univariate and multivariable logistic regression was performed to determine the independent predictors of SAP after acute ischemic stroke. Variables associated with SAP at a significance level of 0.1 in univariate analysis were included in a multivariable logistic regression model using a stepwise backward elimination procedure.
All tests were 2-tailed and statistical significance was determined at an α level of 0.05. Statistical analyses were performed with the SPSS 16.0.
Results
Patients flow
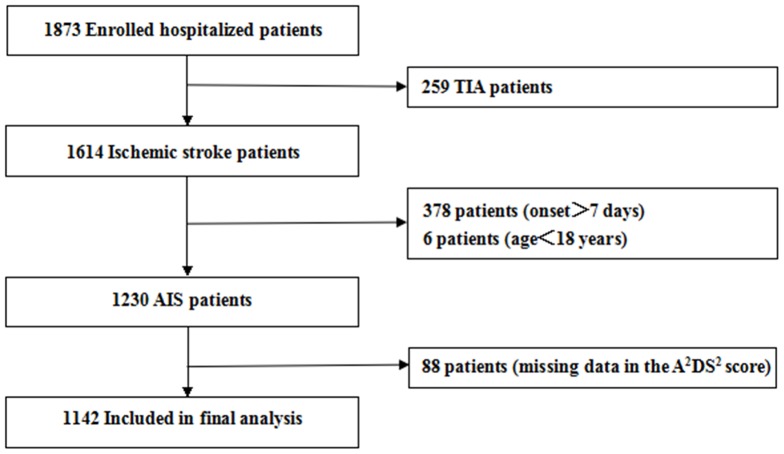
From Jan 2009 to Dec 2012, a total of 1873 patients of acute ischemic stroke were registered in the HNSR cohort. Of these, we excluded 259 patients with TIA, 378 patients with onset>7 days, 6 patients with age <18 years, and an additional 88 patients who had missing data of ≥1 components in the A2DS2 score. Finally, 1142 eligible patients were included in our analysis (Figure 1).
Figure 1. Flowchart of study population.
The flowchart was used to illustrate how the study population was selected.
Baseline characteristics
The baseline characteristics of the HNSR and BSR cohort were summarized in the Table 1. The patients in HNSR were quite different from those in BSR in demographic characteristics and risk factors. The Chinese patients were younger, had a higher proportion of men, presented somewhat milder severity, and lower incidence of atrial fibrillation. Among the 1142 eligible patients, the overall in-hospital SAP was 18.8%, which increased from 9.0% in patients with lower A2DS2 scores (0–4) to 65.0% in those with higher scores of 5 to 10 (P for trend <0.001).
Table 1. Comparison of Patient Baseline Characteristics Between the HNSR and BSR Cohort Which Was Initially Used to Introduce the A2DS2 Score.
| Characteristic | HNSR Cohort | BSR Cohort |
| (n = 1142) | (n = 15335) | |
| Age, y | ||
| Mean(SD) | 60.3±13.1 | 71.2±13.1 |
| Median(IQR) | 61(51–70) | 72(64–81) |
| Age group, no.(%), y | ||
| ≤64 | 692(60.6) | 4006(26.1) |
| 65–74 | 285(25.0) | 4659(30.6) |
| 75–84 | 140(12.3) | 4311(28.1) |
| ≥85 | 25(2.2) | 2323(15.1) |
| Male sex, no.(%) | 723(63.3) | 7759(50.6) |
| Stroke severity, NIHSS | ||
| Median(IQR) | 4(1–7) | 4(2–10) |
| NIHSS categories, no.(%) | ||
| 0–4 | 654(57.3) | 6290(50.4) |
| 5–15 | 420(36.8) | 5098(37.1) |
| 16+ | 68(6.0) | 1707(12.4) |
| Dysphagia, no.(%) | 217(19.0) | 3505(22.9) |
| Comorbidities, no.(%) | ||
| Hypertension | 673(58.9) | 11358(85.9) |
| Diabetes mellitus | 267(23.4) | 4783(31.2) |
| Atrial fibrillation | 51(4.5) | 4139(27.0) |
| Previous stroke | 299(26.2) | 4320(28.2) |
| Length of stay, d | ||
| Mean(SD) | 15.7(9.3) | 9.2(7.3) |
| Median(IQR) | 14.0(10–18) | 8.0(5–11) |
| Pneumonia, no.(%) | 215(18.8%) | 7.2% |
HNSR, Henan Province Stroke Registry; BSR, Berlin Stroke Registry; SD, standard deviation; IQR, interquartile range; NIHSS, National Institutes of Health Stroke Scale.
Discrimination and calibration
The A2DS2 score showed excellent discrimination (C statistic: 0.836, 95% confidence interval: 0.803–0.868, P<0.001) (Figure 2).
Figure 2. Receiver-operator curve of the A2DS2 score in the HNSR cohort.
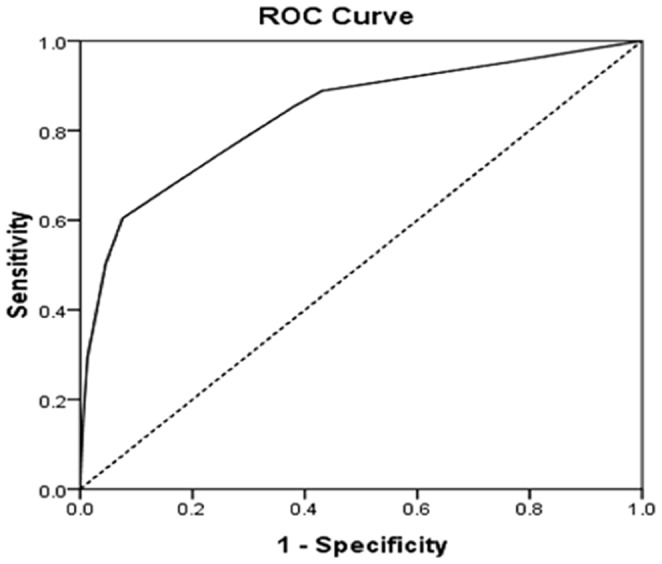
The A2DS2 score showed excellent discrimination with an area under the receiver operating characteristic curve of 0.836 (95% confidence interval, 0.803–0.868).
The sensitivity, specificity, positive predictive value, negative predictive value and Youden index for each individual point were listed in Table 2. Acute ischemic stroke patients were divided into low A2DS2 group (0–4) and high A2DS2 group (5–10) based on optimal cut-off point which represents maximum Youden index.
Table 2. Discrimination abilities of each point about the A2DS2 score.
| A2DS2 score | sensitivity | specificity | PPV | NPV | Youden index |
| 0 | 0.1000 | 0.0000 | 0.188 | NA | 0 |
| 1 | 0.9628 | 0.1855 | 0.215 | 0.956 | 0.1483 |
| 2 | 0.8884 | 0.5696 | 0.324 | 0.957 | 0.4580 |
| 3 | 0.8558 | 0.6170 | 0.341 | 0.949 | 0.4728 |
| 4 | 0.7442 | 0.7562 | 0.415 | 0.927 | 0.5004 |
| 5 | 0.6047 | 0.9245 | 0.650 | 0.910 | 0.5292* |
| 6 | 0.5023 | 0.9547 | 0.720 | 0.892 | 0.4570 |
| 7 | 0.2930 | 0.9871 | 0.840 | 0.858 | 0.2801 |
| 8 | 0.1581 | 0.9946 | 0.872 | 0.836 | 0.1527 |
| 9 | 0.0419 | 0.9989 | 0.900 | 0.818 | 0.0408 |
PPV, positive predictive value; NPV, negative predictive value; NA, not applicable.
*maximum Youden index.
The Hosmer–Lemeshow goodness of fit test showed that the A2DS2 score also had an excellent calibration (Cox and Snell R 2 = 0.243) (Table 3). The plot of observed versus predicted risk of in-hospital SAP after acute ischemic stroke showed high correlation (Pearson correlation coefficient: 0.987) between observed and predicted risk in the external validation cohort (Figure 3).
Table 3. Discrimination and calibration of the A2DS2 score in different cohort.
| BSR Cohort | NGSR Cohort | HNSR Cohort | |
| Discrimination | |||
| AUROC(95%CI) | 0.837(0.826–0.849) | 0.835(0.828–0.842) | 0.836(0.803–0.868) |
| Calibration, goodness of fit | |||
| Cox and Snell R 2 | 0.106 | 0.112 | 0.243 |
| Nagelkerke R 2 | 0.259 | 0.264 | 0.392 |
BSR, Berlin Stroke Registry; NGSR, North-west Germany Stroke Registry; HNSR, Henan Province Stroke Registry; AUROC, area under the receiver operating characteristic curve.
Figure 3. The plot of observed versus predicted risk of SAP in the HNSR cohort.
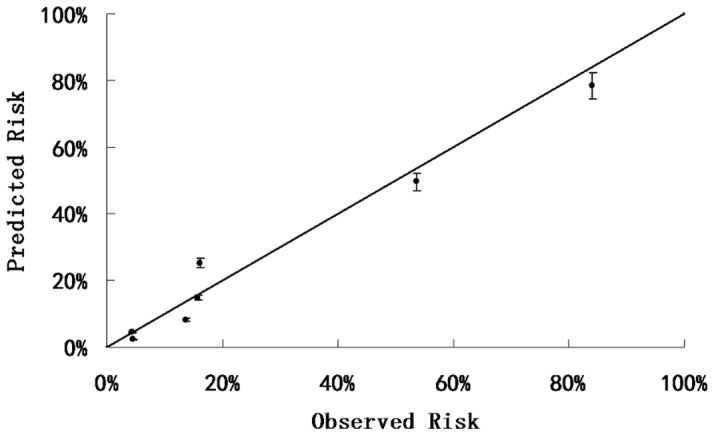
There was a high correlation between observed and expected probability of SAP (Pearson correlation coefficient, 0.987).
Univariate and multivariable logistic regression analysis
The univariate logistic regression analysis results of the risk factors were shown in the Table 4. Age, atrial fibrillation, dysphagia, admission NIHSS score, history of stroke or TIA, diabetes mellitus, OCSP subtype and length of stay were associated with SAP at the significance level of 0.1. The A2DS2 score together with other significant variables were included in the multivariable logistic regression analysis using a stepwise backward elimination procedure. The multivariate logistic regression analysis demonstrated that length of stay, history of stroke and the A2DS2 score were associated with a higher risk of SAP (Table 5).
Table 4. Risk factors for SAP by univariate logistic regression.
| Characteristics | N | Pneumonia | OR(95%CI) | P Value |
| Age, y | ||||
| <75 | 977 | 16.3% | 1.0(reference) | |
| 75+ | 165 | 33.9% | 2.64(1.84–3.81) | 0.001* |
| Male sex | 723 | 18.7% | 0.97(0.72–1.32) | 0.861 |
| Dysphagia | 217 | 67.3% | 25.51(17.53–37.12) | 0.001* |
| Comorbidities | ||||
| Atrial fibrillation | 51 | 49.0% | 4.56(2.58–8.07) | 0.001* |
| Hypertesion | 673 | 18.9% | 1.01(0.75–1.36) | 0.964 |
| Diabetes mellitus | 343 | 23.9% | 1.57(1.15–2.15) | 0.004* |
| Previous stroke | 299 | 24.7% | 1.64(1.19–2.25) | 0.002* |
| Previous TIA | 53 | 28.3% | 1.76(0.95–3.25) | 0.071* |
| Dyslipidemia | 94 | 14.9% | 0.74(0.41–1.33) | 0.309 |
| Coronary heart disease | 115 | 19.1% | 1.02(0.63–1.67) | 0.930 |
| Excess alcohol consumption | 270 | 16.7% | 0.83(0.58–1.19) | 0.299 |
| Current smoking | 336 | 17.3 | 0.86(0.62–1.20) | 0.382 |
| NIHSS on admission | ||||
| 0–4 | 654 | 7.8% | 1.0(reference) | |
| 5–15 | 420 | 27.4% | 4.46(3.12–6.37) | 0.001* |
| 16+ | 68 | 72.1% | 30.49(16.70–55.67) | 0.001* |
| OCSP subtype | ||||
| LACI | 222 | 12.2% | 1.0(reference) | |
| PACI | 403 | 19.4% | 1.73(1.08–2.78) | 0.021* |
| TACI | 40 | 37.5% | 4.33(2.03–9.23) | 0.001* |
| POCI | 256 | 23.0% | 2.16(1.32–3.55) | 0.002* |
| Length of stay | 921 | 20.4% | 1.10(1.08–1.12) | 0.001* |
OR, Odds Ratio; CI, Confidence Interval; TIA, Transient Ischemic Attack; NIHSS, National Institutes of Health Stroke Scale score; OCSP, Oxfordshire Community Stroke Project; LACI, Lacunar infarction; PACI, Partial anterior circulation infarct; TACI, Total anterior circulation infarct; POCI, Posterior circulation infarct.
Table 5. Risk factors for SAP by multivariate logistic regression.
| Risk factor | OR | 95%CI | P Value |
| Length of stay | 1.06 | 1.04–1.09 | 0.001 |
| History of stroke | 1.64 | 1.03–2.60 | 0.037 |
| The A2DS2 score | 1.76 | 1.58–1.95 | 0.001 |
OR, Odds Ratio; CI, Confidence Interval.
Discussion
Our results showed that the A2DS2 score has an excellent discrimination (C statistic: 0.836) and calibration (Cox and Snell R 2 = 0.243) of predicting the risk of SAP in the HNSR cohort, even if the Chinese patients were significantly different in clinical baseline from those in the Germany (mainly with younger median age, milder stroke severity and lower incidence of atrial fibrillation). This is a prospective external validation study for the A2DS2 score through a different independent population from the BSR cohort.
The A2DS2 score was developed based on routinely collected data available directly after hospital admission within a large stroke register in Berlin, and showed good discrimination and calibration abilities both in different population (Germany and Chinese). No reliable scoring system was available in routine clinical practice or stroke research before the A2DS2 score. The A2DS2 score could be used to identify patients who are at high risk of developing SAP after stroke, which is important for clinical practice and stroke trials, especially in the study of early prophylactic antibiotics [32], [33].
In the present study, the incidence of in-hospital SAP (18.8%) was significantly higher than the BSR cohort (7.2%). Length of stay may play an important role. The HNSR cohort had longer hospital stay than the BSR cohort (15.7 VS 9.2 days). After adjusting for potential confounders, length of stay was significantly associated with development of SAP (adjusted OR, 1.06; 95% CI, 1.04–1.09; P<0.001). Previous studies also reported that length of stay was highly associated with SAP [13], [16], along with the increasing risk of opportunistic infection. Furthermore, the conventional ward in our department (not stroke unit) may increase the risk of cross-infection with the frequent visit of family.
There were also some limitations in this study. Firstly, this is a single center study that could have selected bias. Secondly, the exact date of new-onset SAP during hospitalization wasn't documented, the present study just showed that the length of stay was highly associated with SAP, but could not come to a conclusion as to whether patients with a longer length of stay was more likely to develop pneumonia or whether diagnosis of pneumonia led to a longer hospitalization. Therefore, whether the A2DS2 risk model can apply to any stroke care institution needs further investigation.
Conclusions
In conclusion, our study prospectively validates the predictive accuracy of the A2DS2 score in Chinese patients by identifying the in-hospital SAP risk of acute ischemic stroke. It might be helpful for the assessment of increased risk monitoring and prophylactic treatment in identified high-risk patients for SAP in clinical routine and stroke trials.
Supporting Information
Components of the A2DS2 score.
(DOC)
Raw data of the present study.
(XLS)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by the Health Department of Henan Province (201202005) and the Education Department of Henan Province (12A320046). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Koennecke HC, Belz W, Berfelde D, Endres M, Fitzek S, et al. (2011) Factors influencing in-hospital mortality and morbidity in patients treated on a stroke unit. Neurology 77: 965–972. [DOI] [PubMed] [Google Scholar]
- 2. Emsley HC, Hopkins SJ (2008) Acute ischaemic stroke and infection: recent and emerging concepts. Lancet neurology 7: 341–353. [DOI] [PubMed] [Google Scholar]
- 3. Heuschmann PU, Kolominsky-Rabas PL, Misselwitz B, Hermanek P, Leffmann C, et al. (2004) Predictors of in-hospital mortality and attributable risks of death after ischemic stroke: the German Stroke Registers Study Group. Archives of internal medicine 164: 1761–1768. [DOI] [PubMed] [Google Scholar]
- 4. Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D (2011) Post-stroke infection: a systematic review and meta-analysis. BMC neurology 11: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pandian JD, Kaur A, Jyotsna R, Sylaja PN, Vijaya P, et al. (2012) Complications in acute stroke in India (CAST-I): a multicenter study. Journal of stroke and cerebrovascular diseases: the official journal of National Stroke Association 21: 695–703. [DOI] [PubMed] [Google Scholar]
- 6. Forti P, Maioli F, Procaccianti G, Nativio V, Lega MV, et al. (2013) Independent predictors of ischemic stroke in the elderly: prospective data from a stroke unit. Neurology 80: 29–38. [DOI] [PubMed] [Google Scholar]
- 7. Grube MM, Koennecke HC, Walter G, Meisel A, Sobesky J, et al. (2013) Influence of acute complications on outcome 3 months after ischemic stroke. PloS one 8: e75719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hong KS, Kang DW, Koo JS, Yu KH, Han MK, et al. (2008) Impact of neurological and medical complications on 3-month outcomes in acute ischaemic stroke. European journal of neurology: the official journal of the European Federation of Neurological Societies 15: 1324–1331. [DOI] [PubMed] [Google Scholar]
- 9. Tong X, Kuklina EV, Gillespie C, George MG (2010) Medical complications among hospitalizations for ischemic stroke in the United States from 1998 to 2007. Stroke; a journal of cerebral circulation 41: 980–986. [DOI] [PubMed] [Google Scholar]
- 10. Kumar S, Selim MH, Caplan LR (2010) Medical complications after stroke. Lancet neurology 9: 105–118. [DOI] [PubMed] [Google Scholar]
- 11. Hilker R, Poetter C, Findeisen N, Sobesky J, Jacobs A, et al. (2003) Nosocomial pneumonia after acute stroke: implications for neurological intensive care medicine. Stroke; a journal of cerebral circulation 34: 975–981. [DOI] [PubMed] [Google Scholar]
- 12. Kammersgaard LP, Jorgensen HS, Reith J, Nakayama H, Houth JG, et al. (2001) Early infection and prognosis after acute stroke: the Copenhagen Stroke Study. Journal of stroke and cerebrovascular diseases: the official journal of National Stroke Association 10: 217–221. [DOI] [PubMed] [Google Scholar]
- 13. Ingeman A, Andersen G, Hundborg HH, Svendsen ML, Johnsen SP (2011) In-hospital medical complications, length of stay, and mortality among stroke unit patients. Stroke; a journal of cerebral circulation 42: 3214–3218. [DOI] [PubMed] [Google Scholar]
- 14. Katzan IL, Dawson NV, Thomas CL, Votruba ME, Cebul RD (2007) The cost of pneumonia after acute stroke. Neurology 68: 1938–1943. [DOI] [PubMed] [Google Scholar]
- 15. Wilson RD (2012) Mortality and cost of pneumonia after stroke for different risk groups. Journal of stroke and cerebrovascular diseases: the official journal of National Stroke Association 21: 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Finlayson O, Kapral M, Hall R, Asllani E, Selchen D, et al. (2011) Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology 77: 1338–1345. [DOI] [PubMed] [Google Scholar]
- 17. Chumbler NR, Williams LS, Wells CK, Lo AC, Nadeau S, et al. (2010) Derivation and validation of a clinical system for predicting pneumonia in acute stroke. Neuroepidemiology 34: 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sellars C, Bowie L, Bagg J, Sweeney MP, Miller H, et al. (2007) Risk factors for chest infection in acute stroke: a prospective cohort study. Stroke; a journal of cerebral circulation 38: 2284–2291. [DOI] [PubMed] [Google Scholar]
- 19. Harms H, Grittner U, Droge H, Meisel A (2013) Predicting post-stroke pneumonia: the PANTHERIS score. Acta neurologica Scandinavica 128: 178–184. [DOI] [PubMed] [Google Scholar]
- 20. Katzan IL, Cebul RD, Husak SH, Dawson NV, Baker DW (2003) The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology 60: 620–625. [DOI] [PubMed] [Google Scholar]
- 21. Aslanyan S, Weir CJ, Diener HC, Kaste M, Lees KR (2004) Pneumonia and urinary tract infection after acute ischaemic stroke: a tertiary analysis of the GAIN International trial. European journal of neurology: the official journal of the European Federation of Neurological Societies 11: 49–53. [DOI] [PubMed] [Google Scholar]
- 22. Hoffmann S, Malzahn U, Harms H, Koennecke HC, Berger K, et al. (2012) Development of a clinical score (A2DS2) to predict pneumonia in acute ischemic stroke. Stroke; a journal of cerebral circulation 43: 2617–2623. [DOI] [PubMed] [Google Scholar]
- 23. Ji R, Shen H, Pan Y, Wang P, Liu G, et al. (2013) Novel risk score to predict pneumonia after acute ischemic stroke. Stroke; a journal of cerebral circulation 44: 1303–1309. [DOI] [PubMed] [Google Scholar]
- 24. Kwon HM, Jeong SW, Lee SH, Yoon BW (2006) The pneumonia score: a simple grading scale for prediction of pneumonia after acute stroke. American journal of infection control 34: 64–68. [DOI] [PubMed] [Google Scholar]
- 25. Galovic M, Leisi N, Muller M, Weber J, Abela E, et al. (2013) Lesion location predicts transient and extended risk of aspiration after supratentorial ischemic stroke. Stroke; a journal of cerebral circulation 44: 2760–2767. [DOI] [PubMed] [Google Scholar]
- 26. Walter U, Knoblich R, Steinhagen V, Donat M, Benecke R, et al. (2007) Predictors of pneumonia in acute stroke patients admitted to a neurological intensive care unit. Journal of neurology 254: 1323–1329. [DOI] [PubMed] [Google Scholar]
- 27. Kemmling A, Lev MH, Payabvash S, Betensky RA, Qian J, et al. (2013) Hospital acquired pneumonia is linked to right hemispheric peri-insular stroke. PloS one 8: e71141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Song B, Fang H, Zhao L, Gao Y, Tan S, et al. (2013) Validation of the ABCD3-I score to predict stroke risk after transient ischemic attack. Stroke; a journal of cerebral circulation 44: 1244–1248. [DOI] [PubMed] [Google Scholar]
- 29. Stroke—1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke; a journal of cerebral circulation 20: 1407–1431. [DOI] [PubMed] [Google Scholar]
- 30. Bamford J, Sandercock P, Dennis M, Burn J, Warlow C (1991) Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 337: 1521–1526. [DOI] [PubMed] [Google Scholar]
- 31. Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM (1988) CDC definitions for nosocomial infections, 1988. American journal of infection control 16: 128–140. [DOI] [PubMed] [Google Scholar]
- 32. Westendorp WF, Vermeij JD, Vermeij F, Den Hertog HM, Dippel DW, et al. (2012) Antibiotic therapy for preventing infections in patients with acute stroke. The Cochrane database of systematic reviews 1: CD008530. [DOI] [PubMed] [Google Scholar]
- 33. Harms H, Prass K, Meisel C, Klehmet J, Rogge W, et al. (2008) Preventive antibacterial therapy in acute ischemic stroke: a randomized controlled trial. PloS one 3: e2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Components of the A2DS2 score.
(DOC)
Raw data of the present study.
(XLS)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.