Abstract
Primary ciliary dyskinesia (PCD) is characterized by the congenital impairment of mucociliary clearance. When accompanied by situs inversus, chronic sinusitis and bronchiectasis, PCD is known as Kartagener syndrome. The main consequence of impaired ciliary function is a reduced mucus clearance from the lungs, and susceptibility to chronic respiratory infections due to opportunistic pathogens, including nontuberculous mycobacteria (NTM). There has been no report of NTM lung disease combined with Kartagener syndrome in Korea. Here, we report an adult patient with Kartagener syndrome complicated with Mycobacterium abscessus lung disease. A 37-year-old female presented to our hospital with chronic cough and sputum. She was ultimately diagnosed with M. abscessus lung disease and Kartagener syndrome. M. abscessus was repeatedly isolated from sputum specimens collected from the patient, despite prolonged antibiotic treatment. The patient's condition improved and negative sputum culture conversion was achieved after sequential bilateral pulmonary resection.
Keywords: Kartagener Syndrome; Primary Ciliary Dyskinesia; Bronchiectasis; Nontuberculous Mycobacteria; Mycobacterium Infections, Nontuberculous
Introduction
Primary ciliary dyskinesia (PCD) is associated with abnormal ciliary structure and function, which results in the retention of mucus and bacteria in the respiratory tract, leading to chronic oto-sino-pulmonary disease, situs abnormalities and abnormal sperm motility1. The initial recognition of this syndrome in 1933 was based on the triad of chronic sinusitis, bronchiectasis, and situs inversus, known as Kartagener syndrome2. About 55% of PCD patients present with situs inversus2.
PCD causes abnormal and permanent dilatation of the bronchi, known as bronchiectasis, which is caused by inflammation and destruction of the structural components of the bronchial walls. Most experts accept that a "vicious cycle" of infection and inflammation is created by this basic defect in host airway defenses. This generates airway damage and further impairs airway clearance; eventually chronic microbial colonization/infection develops, leading to further infection and inflammation with subsequent destruction of the conducting airways1,2. Airway infections with Haemophilus influenzae, Staphylococcus aureus, and Streptococcus pneumoniae are common, infections with Pseudomonas aeruginosa have also been reported, usually in adults1. Approximately 15% of adult patients with PCD have positive sputum cultures of non-tuberculous mycobacteria (NTM); however, the prevalence in children with PCD is lower3.
To the best of our knowledge, there has been no report of NTM lung disease combined with Kartagener syndrome in Korea, although the number of patients with NTM lung disease has been increasing in recent years4,5. Here, we report an adult case of Kartagener syndrome complicated with Mycobacterium abscessus lung disease who was successfully treated with bilateral pulmonary resection combined with antibiotic therapy.
Case Report
A 37-year-old female was referred to our hospital for further management of NTM lung disease. During the 4 months before the patient arrived at our hospital, she had a productive cough and hemoptysis. She was initially diagnosed as having "smear-positive pulmonary tuberculosis," and received anti-tuberculosis drugs. During treatment, her symptoms did not improve and NTM were repeatedly isolated from her sputum specimens. She was then referred to our hospital.
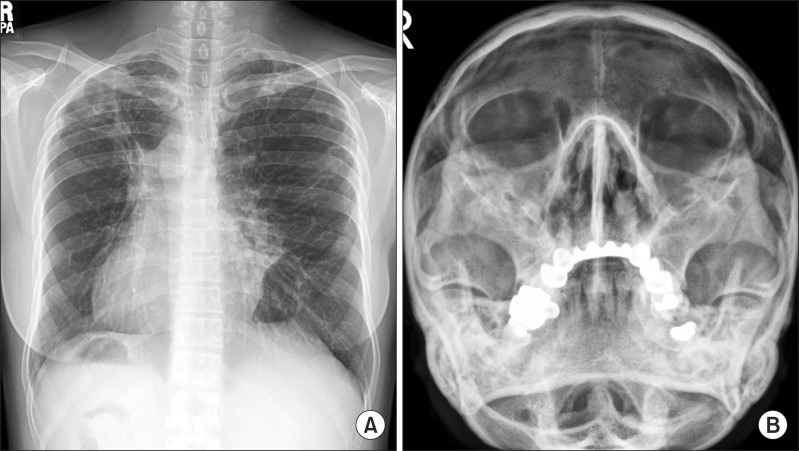
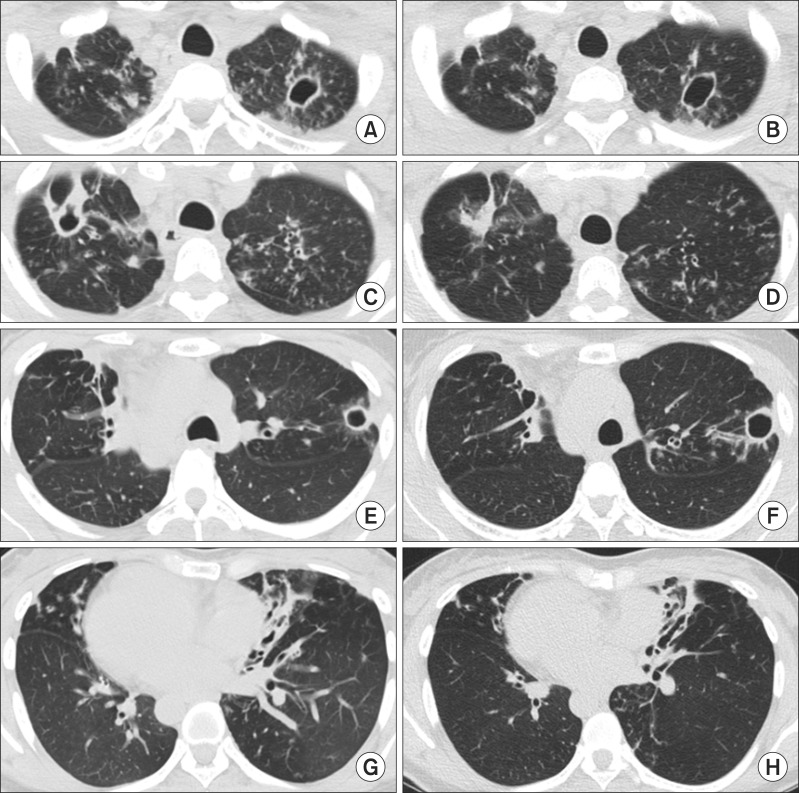
The patient was a nonsmoker. Twenty years earlier, the patient had undergone surgery for chronic rhinosinusitis. Chest radiography showed dextrocardia and situs inversus and paranasal sinus radiography showed total opacification involving the bilateral ethmoid sinus and bilateral maxillary sinus (Figure 1A, B). Chest high-resolution computed tomography (HRCT) scans showed multiple cavitary lesions in both upper lobes, bronchiectasis, and multiple nodules in the left middle lobe and the lingular segment of the right upper lobe (Figure 2A, C, E, G). The patient's sputum was positive for acid-fast bacilli (AFB) stain, and M. abscessus (sensu stricto) was repeatedly isolated from sputum specimens collected from the patient. The identification of M. abscessus (sensu stricto) was performed using a polymerase chain reaction-restriction fragment length polymorphism analysis of rpoB and sequence analysis of rpoB and hsp656. Drug susceptibility test showed the isolate was susceptible amikacin (minimum inhibitory concentration [MIC], 16 µg/mL) and imipenem (MIC, 4 µg/mL), intermediate to cefoxitin (MIC, 32 µg/mL), and resistant to ciprofloxacin (MIC, >16 µg/mL), moxifloxacin (MIC, 8 µg/mL) and doxycycline (MIC, >32 µg/mL). The isolate had inducible resistance to clarithromycin, as it was susceptible (MIC, ≤0.5 µg/mL) to clarithromycin at day 3 but resistant to clarithromycin (MIC, >64 µg/mL) at day 14.
Figure 1.
(A) Chest radiography showed dextrocardia and situs inversus. Note the cavitary lesions in the right upper lobe and nodulostreaky opacity, suggesting bronchiectasis in the left middle lung zones. (B) Paranasal sinus radiography showed total opacification involving the bilateral ethmoid sinus and bilateral maxillary sinus.
Figure 2.
(A, C, E, G) Chest high-resolution computed tomography showed multiple cavities in both upper lobes. Note the severe bronchiectasis in the left middle lobe and the lingular segment of the right upper lobe. (B, D, F, H) Although the cavitary lesion in the right upper lobe improved after 20 months of antibiotic treatment, the size of the multiple cavities in the left upper lobe increased.
The patient received oral clarithromycin and fluoroquinolone antibiotics (initially ciprofloxacin, later moxifloxacin), along with an initial 4-week course of intravenous amikacin and cefoxitin7. Although the cavitary lesion in the right upper lobe improved after 20 months of antibiotic therapy, the size of the multiple cavities in the left upper lobe increased (Figure 2B, D, F, H). The patient's symptoms worsened and follow-up AFB staining of sputum and sputum cultures were persistently positive.
Considering the patient's age, persistent symptoms, and aggravation of cavitary lesions in the left lung after antibiotic therapy, we decided to perform surgical resection. Preoperative pulmonary function test revealed that forced vital capacity was 2.82 L (95% predicted) and forced expiratory volume in one second was 1.46 L (63% predicted). The patient underwent a left upper bilobectomy (left upper lobectomy plus left middle lobectomy). She was discharged 8 days after surgery without postoperative complications. After surgery, her symptoms improved. However, cultures of the patient's sputum were persistently positive for M. abscessus, although AFB staining of sputum showed negative conversion.
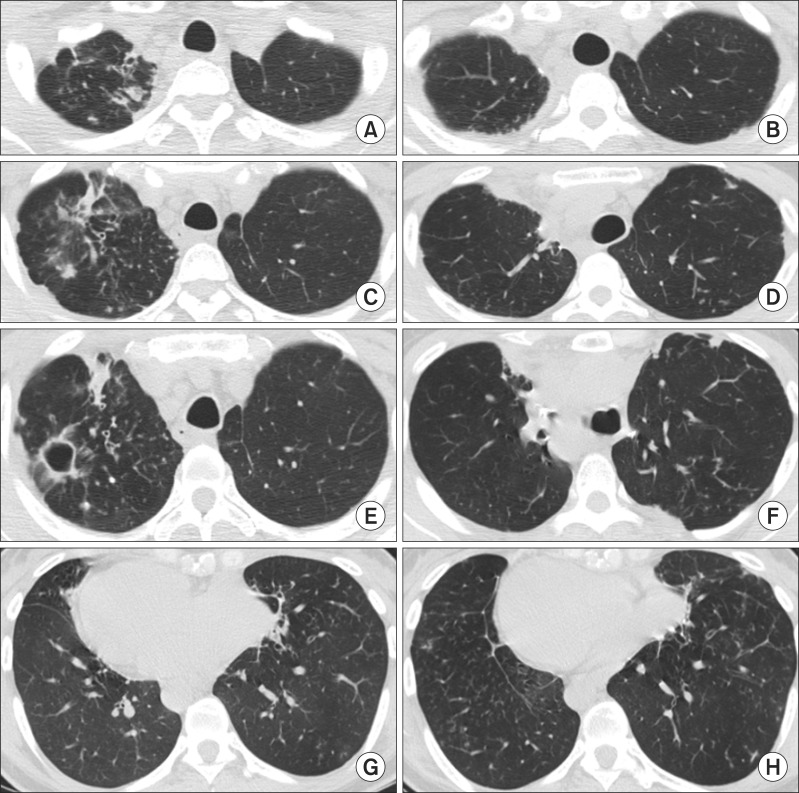
At 9 months after surgery, an AFB-positive sputum smear was obtained, and the cavitary lesion in the right upper lobe was aggravated (Figure 3A, C, E, G). Thus, a right upper lobectomy was performed and the patient was discharged 8 days after surgery without postoperative complications. Soon after the second operation, the patient's symptoms improved and negative sputum smears and culture conversion were achieved. The patient was maintained on oral antibiotic treatment with clarithromycin and moxifloxacin. Antibiotic therapy was successfully discontinued 24 months after the second operation. At 2 years since the second surgery, the patient is doing well: sputum AFB smears and cultures are consistently negative and follow-up chest HRCT scans indicate no new lesions (Figure 3B, D, F, H).
Figure 3.
(A, C, E, G) At 9 months after the left-sided surgery, chest high-resolution computed tomography showed an aggravation of the cavitary lesion in the right upper lobe. (B, D, F, H) At 24 months of the right-sided surgery, follow-up chest high-resolution computed tomography scans indicate no new lesions.
Discussion
PCD is a genetically heterogeneous recessive disorder of motile cilia that leads to oto-sino-pulmonary disease1,2. Kartagener syndrome, which consists of situs inversus totalis, bronchiectasis and sinusitis, is a well-known but less common presentation of PCD. In patients with PCD, recurrent and chronic bacterial infections in the lower airways inevitably occur, and there is age-dependent development of bronchiectasis, which is a nearly universal complication of PCD in adults3.
Initially, sputum cultures are positive for H. influenzae, S. pneumoniae, and S. aureus. Once bronchiectasis is evident on chest HRCT, P. aeruginosa and other opportunistic pathogens may be present. NTM may also be isolated; a previous study from the United States reported that NTM infections are not uncommon, especially in older patients, and that NTM were present in 8 of 59 patients (14%) with PCD in whom sputum for microbiologic testing was obtained (Mycobacterium avium complex [n=3], M. abscessus [n=2], M. gordonae [n=2], and mixed M. avium complex/M. gordonae [n=1])3.
The prevalence of lung diseases caused by NTM is increasing worldwide, affecting both immunocompetent and immunocompromised individuals8. In Korea, M. abscessus complex is the second most common pathogen responsible for lung diseases caused by NTM, after M. avium complex6,7,9. M. abscessus complex (M. abscessus sensu lato) are divided into three closely related subspecies: M. abscessus (sensu stricto), Mycobacterium massiliense, and Mycobacterium bolletii6,10,11. Within the M. abscessus complex, M. abscessus (44-53%) and M. massiliense (45-55%) are equally distributed, while M. bolletii is a relatively rare pathogen (1-2%) in Korea6,10,11.
M. abscessus (sensu stricto) is resistant in vitro to many antibiotics and thus is very difficult to treat6,7. Isolates are usually susceptible in vitro to some parenteral agents (e.g., amikacin, cefoxitin, and imipenem) and to macrolides (e.g., clarithromycin and azithromycin)8. Combination therapy with intravenous amikacin and cefoxitin or imipenem and an oral macrolide has been recommended by the American Thoracic Society/Infectious Diseases Society of America and many other experts8. However, treatment response rates are not satisfactory and optimal therapeutic regimens and treatment durations are not well established6,7.
Curative therapy for M. abscessus lung disease is more likely to be obtained with limited disease and a combination of surgical resection of the involved lung and chemotherapy8. Patients with focal lung disease and symptoms that significantly impair their quality of life who can withstand lung resection should be treated surgically after an initial period on antimicrobials to lessen the microbial burden8,12. Some patients who have bilateral disease could undergo staged sequential surgery13,14,15. The fact that sputum conversion was obtained only after the second pulmonary resection underscores the necessity of bilateral resection in this patient.
In summary, this is the first reported case of NTM lung disease in a patient with Kartagener syndrome in Korea. AFB smear cannot differentiate between Mycobacterium tuberculosis and NTM. The recovery rate of NTM from respiratory specimens and the number of patients with NTM lung disease has been rapidly increasing in Korea. An early differential diagnosis of NTM lung disease from pulmonary tuberculosis is important, as the therapeutic regimen differs from that of pulmonary tuberculosis. This case also suggests that if medical treatment fails for NTM lung disease, resection of the diseased lobes should be considered, even in patients with bilateral lesions.
Acknowledgements
This study was supported by a grant of the Korean Health technology R&D Project, Ministry for Health & Welfare, Republic of Korea (A120647).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Barbato A, Frischer T, Kuehni CE, Snijders D, Azevedo I, Baktai G, et al. Primary ciliary dyskinesia: a consensus statement on diagnostic and treatment approaches in children. Eur Respir J. 2009;34:1264–1276. doi: 10.1183/09031936.00176608. [DOI] [PubMed] [Google Scholar]
- 2.Knowles MR, Daniels LA, Davis SD, Zariwala MA, Leigh MW. Primary ciliary dyskinesia. Recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med. 2013;188:913–922. doi: 10.1164/rccm.201301-0059CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noone PG, Leigh MW, Sannuti A, Minnix SL, Carson JL, Hazucha M, et al. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med. 2004;169:459–467. doi: 10.1164/rccm.200303-365OC. [DOI] [PubMed] [Google Scholar]
- 4.Yoo JW, Jo KW, Kim MN, Lee SD, Kim WS, Kim DS, et al. Increasing trend of isolation of non-tuberculous mycobacteria in a tertiary university hospital in South Korea. Tuberc Respir Dis. 2012;72:409–415. doi: 10.4046/trd.2012.72.5.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koh WJ, Chang B, Jeong BH, Jeon K, Kim SY, Lee NY, et al. Increasing recovery of nontuberculous mycobacteria from respiratory specimens over a 10-year period in a tertiary referral hospital in South Korea. Tuberc Respir Dis. 2013;75:199–204. doi: 10.4046/trd.2013.75.5.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, et al. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med. 2011;183:405–410. doi: 10.1164/rccm.201003-0395OC. [DOI] [PubMed] [Google Scholar]
- 7.Jeon K, Kwon OJ, Lee NY, Kim BJ, Kook YH, Lee SH, et al. Antibiotic treatment of Mycobacterium abscessus lung disease: a retrospective analysis of 65 patients. Am J Respir Crit Care Med. 2009;180:896–902. doi: 10.1164/rccm.200905-0704OC. [DOI] [PubMed] [Google Scholar]
- 8.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 9.Koh WJ, Jeong BH, Jeon K, Lee NY, Lee KS, Woo SY, et al. Clinical significance of the differentiation between Mycobacterium avium and Mycobacterium intracellulare in M avium complex lung disease. Chest. 2012;142:1482–1488. doi: 10.1378/chest.12-0494. [DOI] [PubMed] [Google Scholar]
- 10.Koh WJ, Stout JE, Yew WW. Advances in the management of pulmonary disease due to Mycobacterium abscessus complex. Int J Tuberc Lung Dis. 2014;18:1141–1148. doi: 10.5588/ijtld.14.0134. [DOI] [PubMed] [Google Scholar]
- 11.Lee SH, Yoo HK, Kim SH, Koh WJ, Kim CK, Park YK, et al. The drug resistance profile of Mycobacterium abscessus group strains from Korea. Ann Lab Med. 2014;34:31–37. doi: 10.3343/alm.2014.34.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koh WJ, Kim YH, Kwon OJ, Choi YS, Kim K, Shim YM, et al. Surgical treatment of pulmonary diseases due to nontuberculous mycobacteria. J Korean Med Sci. 2008;23:397–401. doi: 10.3346/jkms.2008.23.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell JD, Bishop A, Cafaro A, Weyant MJ, Pomerantz M. Anatomic lung resection for nontuberculous mycobacterial disease. Ann Thorac Surg. 2008;85:1887–1892. doi: 10.1016/j.athoracsur.2008.02.041. [DOI] [PubMed] [Google Scholar]
- 14.Yu JA, Pomerantz M, Bishop A, Weyant MJ, Mitchell JD. Lady Windermere revisited: treatment with thoracoscopic lobectomy/segmentectomy for right middle lobe and lingular bronchiectasis associated with non-tuberculous mycobacterial disease. Eur J Cardiothorac Surg. 2011;40:671–675. doi: 10.1016/j.ejcts.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 15.Lee SH, Min JW, Um SW, Han SS, Han SK, Shim YS, et al. Sequential bilateral lung resection in a patient with Mycobacterium abscessus lung disease refractory to medical treatment. Yonsei Med J. 2010;51:141–144. doi: 10.3349/ymj.2010.51.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]