Abstract
Invasive pulmonary aspergillosis (IPA) is rarely reported in patients who have normal immune function. Recently, IPA risk was reported in nonimmunocompromised hosts, such as patients with chronic obstructive pulmonary disease and critically ill patients in intensive care units. Moreover, influenza infection is also believed to be associated with IPA among immunocompetent patients. However, most reports on IPA with influenza A infection, including pandemic influenza H1N1, and IPA associated with influenza B infection were scarcely reported. Here, we report probable IPA with a fatal clinical course in an immunocompetent patient with influenza B infection. We demonstrate IPA as a possible complication in immunocompetent patients with influenza B infection. Early clinical suspicion of IPA and timely antifungal therapy are required for better outcomes in such cases.
Keywords: Invasive Pulmonary Aspergillosis, Influenza B Virus, Immunocompetence
Introduction
Invasive pulmonary aspergillosis (IPA) has become an important life-threatening infection in severely immunocompromised patients with certain risk factors, including prolonged neutropenia and hematopoietic stem cell transplantation1. Recent evidence indicates that IPA can occur in patients without these factors; other associated conditions include chronic obstructive pulmonary disease, liver failure, alcoholism, and severe sepsis2,3,4. In patients with these conditions, characteristic radiologic signs of IPA are usually absent, and diagnostic examination is often delayed due to low clinical suspicion, resulting in high mortality rates5.
Several recent reports indicated that IPA could be a potential complication in immunocompetent patients with influenza infection6,7,8. However, previous reports of IPA complicating influenza infection mostly involved influenza A infections, particularly pandemic influenza H1N1. IPA associated with influenza B was scarcely reported9,10. We report probable IPA with a fatal clinical course in an immunocompetent patient with influenza B infection and reviewed literature.
Case Report
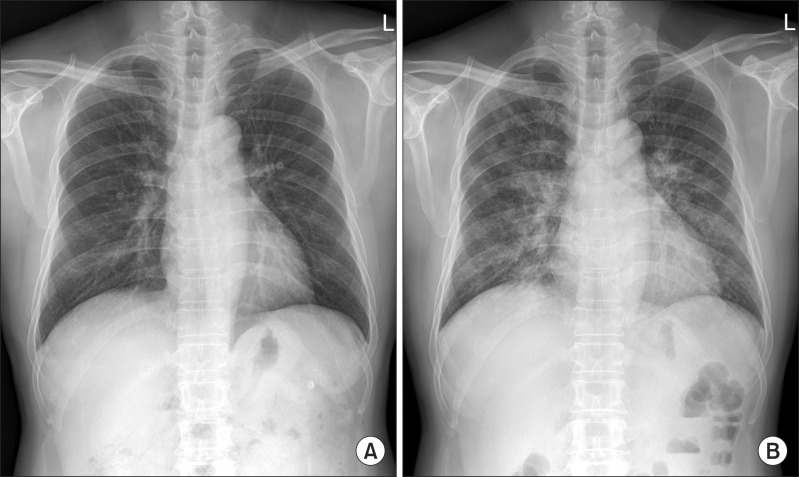
A 55-year-old previously healthy man was admitted to emergency department with 1-day history of dyspnea. He had fever, cough, and whitish sputum 7 days before admission. He was a former smoker with a history of 5-pack-year, and did not have alcohol abuse history. He had no known history of pulmonary diseases and underlying diseases such as diabetes. He took no medications or herbal medicines. Three days before present admission, he was admitted to a coronary care unit for atypical chest pain, and had normal coronary angiography. Simultaneously, chest radiography was normal (Figure 1A) and chest computed tomography (CT) revealed diffuse bronchial wall thickening, with no evidence of emphysema. He had flu-like symptoms, including fever, cough, sore throat, and myalgia, but had only received conservative medication without antiviral agents.
Figure 1.
(A) Normal chest radiograph on 3 days before admission. (B) Chest radiograph on admission showing bilateral pulmonary infiltrates and consolidations.
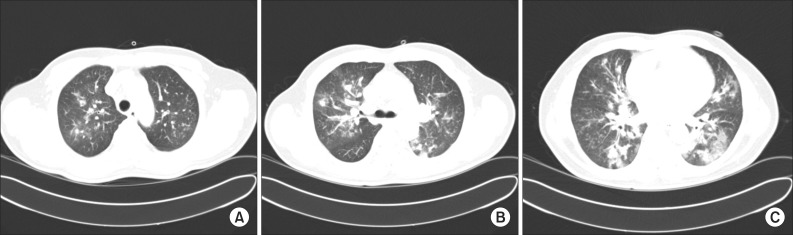
On current admission, blood pressure was 130/90 mm Hg, heart rate 113 beats/min, respiratory rate 26 breaths/min, and body temperature 37.8℃. He was conscious but showed acute ill-looking appearance. Physical examination showed normal conjunctiva with no palpable lymph nodes around his neck. Auscultation revealed bilateral expiratory wheezes. The initial laboratory evaluation revealed a white blood cells were 15,200/mm3 (neutrophils 84.9%, lymphocytes 8.6%, and eosinophils 0.5%), hemoglobin 16.0 g/dL, platelets 144,000/mm3, C-reactive protein 46.5 mg/L (reference range, 0-0.3 mg/L). Laboratory findings including liver, and renal function tests were within normal limits. Serologic tests for specific autoantibodies to nuclear antigens, and rheumatoid factor were all negative. Human immunodeficiency virus and venereal disease research laboratory test were negative, and CD4 count was within normal. Arterial blood gas analysis on supplemental oxygen at 3 L/min via nasal cannula revealed a pH of 7.45, PaO2 of 81.8 mm Hg, and PaCO2 of 38.5 mm Hg. Rapid influenza diagnostic test via nasopharyngeal swab specimen was negative. Anti-mycoplasma antibody, and urinary antigen tests for Legionella and pneumococcus were negative. The results of the initial sputum and sputum and blood cultures were all negative and a special stain for acid-fast bacilli was negative. A serum galactomannan (GM) assay on day 2 was positive, but we did repeat GM assay to exclude false positives due to low clinical suspicion based on no apparent immunodeficiency or underlying diseases. Chest radiograph revealed bilateral pulmonary infiltrates and consolidations (Figure 1B). Chest CT revealed newly developed patchy consolidations, ground-glass opacities, and centrilobular nodules in both lungs (Figure 2).
Figure 2.
(A-C) A chest computed tomography scan on admission revealed multifocal patchy consolidation, ground-glass opacities, and centrilobular nodules in both lungs.
He was initially prescribed ceftriaxone and clarithromycin for suspected community-acquired pneumonia. Acute exacerbation of previously undiscovered asthma was suspected due to bilateral wheezes without heart failure. On day 2, methylprednisolone of 62.5 mg/day was initiated, though he had no history of asthma or other pulmonary diseases. However, we switched to piperacillin/tazobactam plus levofloxacin on day 4 due to worsening leukocytosis and hypoxemia. He rapidly developed respiratory failure, and was transferred to the intensive care unit (ICU) and mechanically ventilated. After switching antibiotics, fever subsided and C-reactive protein level declined considerably (6.6 mg/L). Chest radiographic findings improved until day 8. Gram stain and culture of tracheal aspiration were all negative.
However, on day 9, his clinical condition rapidly deteriorated, with high fever and worsening chest radiographic findings. Bronchoscopy showed extensive yellow-whitish exudative pseudomembranes throughout his trachea and both main bronchi, with significant airway narrowing. Characteristic acute-angle branching hyphal elements were observed in bronchoalveolar lavage (BAL) fluid specimens (Figure 3); BAL fluid culture yielded numerous Aspergillus species colonies. A repeat serum GM assays was positive. Polymerase chain reaction of BAL fluid was positive for influenza B, although results of a rapid influenza diagnostic test on admission were negative. Because of low sensitivity of rapid test for influenza, we assumed that this patient's initial symptoms were closely related to influenza B infection. Based on European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) revised criteria1, we diagnosed this immunocompetent patient with probable IPA complicating influenza B infection. Therapy with voriconazole was initiated on day 9. However, he progressively worsened without documented bacterial infection, and died on day 14.
Figure 3.

Gomori's methenamine silver stain of bronchoalveolar lavage fluid specimen revealing characteristic septate hyphae branching at less than 45° (×400).
Discussion
IPA infections occur almost exclusively in immunocompromised patients1. Our patient was immunocompetent and was diagnosed with probable IPA associated with influenza B infection. He died due to rapidly progressive respiratory failure despite therapy with voriconazole. Previous reports indicate that influenza A infection may predispose otherwise healthy patients to invasive aspergillosis worldwide4, including Korea8. Based on a literature review from 1966 to 1998, Clancy and Nguyen5 found three cases of aspergillosis-caused acute community acquired pneumonia in immunocompetent hosts complicating influenza A infection. After the 2009 pandemic of influenza A/H1N1 virus, IPA was a much more frequent complication among critically ill H1N1 patients6,7. Interestingly, IPA associated with influenza B infection has not been reported in otherwise healthy hosts until a report by Hasejima et al. in 20059; cases with IPA complicating influenza B infection were extremely rare9,10. This might be related to findings that influenza B outbreaks are less extensive and less severe than influenza A outbreaks. Generally, influenza B virus does not undergo the same antigenic changes that result in pandemics caused by newly arising influenza A viruses9,11. To our knowledge, our case is the first study in Korea reporting IPA complicating influenza B infection with a fatal clinical course in an immunocompetent patient.
Diagnosis of proven IPA usually requires histopathological confirmation with evidence of associated tissue damage, and a positive culture result for a specimen from a normally sterile site1. Our case was a probable case because we did not obtain tissue biopsies or perform an autopsy. However, tissue diagnostic specimens are often difficult to obtain from critically ill patients, particularly for those on mechanical ventilation, as with our case. Thus, the limitations of these definitions of IPA should be considered when using them in clinical practice. Criteria of proven IPA could be applied to any patient, regardless of immune status, whereas those for probable IPA were proposed only for immunocompromised patients. Failure to meet criteria for proven IPA does not necessarily imply the absence of IPA; rather, it only implies that evidence is insufficient to support diagnosis of IPA. Based on results of bronchoscopic findings and positive BAL fluid culture, repeatedly positive serum GM assays provide strong evidence suggestive of IPA. Serum GM assays are an indirect diagnostic test with good sensitivity for detecting invasive aspergillosis only in patients with hematologic malignancies. Although the diagnostic utility of a single GM assay in immunocompetent patients is low and remains to be determined12, two consecutive positive results of serum GM assays suggest IPA13. Thus, we believe that our patient may have IPA and died of IPA complicating influenza B infection.
Influenza infection may have caused our case to be susceptible to fungal infections6. Influenza viruses are known to cause cell-mediated destruction of airway epithelium and mucociliary clearance, and making patients prone to secondary invasions by Aspergillus species. Moreover, our patient briefly received corticosteroids, possibly facilitating rapid IPA progression, because serum GM assay was positive before initiating corticosteroids. Therapy with corticosteroids decreases antifungal activities of neutrophils and macrophages against Aspergillus species. Corticosteroids before ICU admission appear to be a major risk factor for developing IPA among ICU patients with H1N1 virus infections7.
Until recently, voriconazole was considered as the primary treatment for invasive aspergillosis12. However, up to 50% of high-risk patients were unresponsive to this treatment, and nearly 30% of these patients died within 12 weeks after treatment14. Thus, the outcome with IPA is directly associated with early detection and appropriate timely antifungal therapy. Our case was admitted within a clinical setting of community acquired pneumonia, resulted in the delayed recognition of Aspergillus species as a potential pathogen.
In summary, we have reported on an immunocompetent adult with fatal probable IPA accompanied by influenza B infection. Early suspicion of IPA and timely antifungal therapy are important to provide better outcomes in such cases.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meersseman W, Lagrou K, Maertens J, Van Wijngaerden E. Invasive aspergillosis in the intensive care unit. Clin Infect Dis. 2007;45:205–216. doi: 10.1086/518852. [DOI] [PubMed] [Google Scholar]
- 3.Trof RJ, Beishuizen A, Debets-Ossenkopp YJ, Girbes AR, Groeneveld AB. Management of invasive pulmonary aspergillosis in non-neutropenic critically ill patients. Intensive Care Med. 2007;33:1694–1703. doi: 10.1007/s00134-007-0791-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens DA, Melikian GL. Aspergillosis in the 'nonimmunocompromised' host. Immunol Invest. 2011;40:751–766. doi: 10.3109/08820139.2011.614307. [DOI] [PubMed] [Google Scholar]
- 5.Clancy CJ, Nguyen MH. Acute community-acquired pneumonia due to Aspergillus in presumably immunocompetent hosts: clues for recognition of a rare but fatal disease. Chest. 1998;114:629–634. doi: 10.1378/chest.114.2.629. [DOI] [PubMed] [Google Scholar]
- 6.Lat A, Bhadelia N, Miko B, Furuya EY, Thompson GR., 3rd Invasive aspergillosis after pandemic (H1N1) 2009. Emerg Infect Dis. 2010;16:971–973. doi: 10.3201/eid1606.100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wauters J, Baar I, Meersseman P, Meersseman W, Dams K, De Paep R, et al. Invasive pulmonary aspergillosis is a frequent complication of critically ill H1N1 patients: a retrospective study. Intensive Care Med. 2012;38:1761–1768. doi: 10.1007/s00134-012-2673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon OK, Lee MG, Kim HS, Park MS, Kwak KM, Park SY. Invasive pulmonary aspergillosis after influenza a infection in an immunocompetent patient. Tuberc Respir Dis. 2013;75:260–263. doi: 10.4046/trd.2013.75.6.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasejima N, Yamato K, Takezawa S, Kobayashi H, Kadoyama C. Invasive pulmonary aspergillosis associated with influenza B. Respirology. 2005;10:116–119. doi: 10.1111/j.1440-1843.2005.00593.x. [DOI] [PubMed] [Google Scholar]
- 10.Ohnishi T, Andou K, Kusumoto S, Sugiyama H, Hosaka T, Ishida H, et al. Two cases of successfully treated invasive pulmonary aspergillosis following influenza virus infection. Nihon Kokyuki Gakkai Zasshi. 2007;45:349–355. [PubMed] [Google Scholar]
- 11.Labella AM, Merel SE. Influenza. Med Clin North Am. 2013;97:621–645. doi: 10.1016/j.mcna.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 13.Hope WW, Walsh TJ, Denning DW. Laboratory diagnosis of invasive aspergillosis. Lancet Infect Dis. 2005;5:609–622. doi: 10.1016/S1473-3099(05)70238-3. [DOI] [PubMed] [Google Scholar]
- 14.Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]