Abstract
Background
Breonadia salicina (Rubiaceae) is a critically endangered plant at the local scale native to southwestern Saudi Arabia. To understand the levels and partitioning of genetic variation across populations and geographical regions of this species, we assessed its genetic diversity using inter-simple sequence repeat (ISSR) markers.
Results
Fourteen ISSR primers selected from 43 primers gave rise to 211 amplified loci, of which 68 were polymorphic. The percentage of polymorphic loci (PPL) at the population level ranged from 17.1 to 23.7%, with an average of 21.3%. Nei’s gene diversity (h) and Shannon’s information index (I) were 0.086 and 0.125, respectively. At the species level, PPL was 32.2%, while h and I were 0.116 and 0.172, respectively. A hierarchical analysis of molecular variance revealed a high level of genetic differentiation among populations (17% of total variance, P = 0.001), consistent with the gene differentiation coefficient (GST = 0.256). Nevertheless, the evaluated genetic diversity was very low within populations; while relatively high among populations, levels were insufficient for long-term survival. Saudi Arabian accessions were also compared to accessions of a population from Yemen, where the species is more widespread. The Yemeni population also showed low genetic diversity but clustered separately.
Conclusions
Breonadia salicina in Saudi Arabia is characterized by low within-population genetic diversity and high among-population genetic differentiation. Based on our findings, this locally endangered species is on the verge of local extinction. The species’ survival depends on successful implementation of suggested strategies for its long-term conservation.
Keywords: Breonadia salicina, Genetic variation, Genetic structure, Molecular markers, ISSR, Conservation biology
Background
The conservation of threatened and endangered medicinal species is indispensable for the future of humankind. The flora of Saudi Arabia is rich in diversity, with numerous rare and endangered plant species. The number of threatened species is increasing annually as a result of adverse environmental conditions and anthropogenic activities [1]. Over-exploitation, including habitat destruction, expansion of urban activities, over-grazing, selective species removal, and mutilation, is leading to genetic erosion. Physical syndromes of declining existence, such as fragmented natural habitats, endangerment, thin populations, narrowed genetic diversity, rarity, paucity of regeneration, and reproductive inefficiencies, are prevalent in the kingdom of Saudi Arabia [2] and may lead to the eventual local extinction of B. salicina and many other endangered species.
Breonadia Ridsdale (Rubiaceae) is a monotypic genus distributed in tropical Africa and the southern Arabian Peninsula. Breonadia salicina (Vahl) Hepper & JRI Wood (Figure 1) is considered to be one of the most critically endangered plant species of southwestern Saudi Arabia [2–4]. Reaching up to 40 m in height and 2 m in diameter, trees of B. salicina usually grow along high escarpments from 500 to 2000 m above sea level, near the banks, or in the water of permanent streams and rivers [3,5]. B. salicina is monoecious with small, pale mauve and sweetly scented flowers. They are present in compact round axillary heads up to 40 mm in diameter on long slender stalks up to 60 mm, with 2 leaf-like bracts along their length. All floral parts are in fives, expanding into a funnel-shaped crag and 5-lobed cup-shaped disc. The stamens are inserted in the crag of the tube projecting from the spout. The ovary grows in the leaf from November to March with two chambers and sprightly yellow balls [6]. Breonadia salicina is a potentially medicinally and economically valuable plant that has been used for the treatment of many human diseases. Because of the presence of numerous secondary metabolites, the entire plant is widely used to treat arthritis, diabetes, diarrhea, cancer, headaches, gastrointestinal illness, fevers, wounds, ulcers, and bacterial and fungal infections [7–9]. In South Africa, B. salicina bark is used for stomach complaints and as an astringent [10], and root decoctions are used for the treatment of tachycardia [11]. The wood is yellowish, hard, heavy, very durable, termite resistant, and oily to the touch [6], making it an excellent, highly valued timber for furniture and house construction. Furthermore, B. salicina is a well-known firewood in Tanzania [12].
Figure 1.

Breonadia salicina . (A–B) Trees from Wadi Lejib, Jizan Province (A) and Rabuaa, Asir Province (B). (C–D) Leaf morphology.
Assessment of within- and between-population genetic diversity of rare and endangered plants not only enhances our understanding of population dynamics, adaptation, and evolution, but also provides useful information for the development of conservation strategies [13]. Such an assessment is important because a species’ long-term survival and evolution depends on the maintenance of sufficient genetic variability within and among populations to accommodate new selection pressures brought about by environmental changes [14]. Molecular analysis of genetic variation among individuals of a population can be used to monitor the genetic variability of a declining population and to assess genetic fragmentation outcomes of surviving populations [15,16].
In recent decades, a series of techniques involving genetic markers have been developed to analyze and estimate genetic diversity. Compared with other genetic markers, DNA-based markers have superior reproducibility for the evaluation of genetic diversity. Among various marker systems, the inter-simple sequence repeat (ISSR) marker system uses repeat-anchored primers to amplify DNA sequences between two inverted simple sequence repeats [17]. ISSR markers are reproducible under a variety of conditions at relatively low expense, and enable the use of higher primer annealing temperatures and the generation of longer sequences than other markers. Because ISSRs are dominant markers, allelic variation cannot be detected, as homozygous and heterozygous states cannot be distinguished at individual loci [18]. Nevertheless, these markers have been successfully used in hundreds of studies for the assessment of genetic diversity of endangered plant species [19–21].
Comparisons with common congeners may decrease the contradictory effects of phylogeny [22,23]. To determine whether a rare species indeed exhibits low genetic diversity, it is therefore advantageous to compare genetic diversity measures in the rare species with those of a more widespread, common congener [24]. As mentioned above, however, Breonadia is a monotypic genus; to solve this problem, comparison with widespread B. salicina in Yemen can be used as an alternative for better understanding of genetic diversity.
Literature on B. salicina is limited, and information regarding its genetic basis is lacking. As a consequence, the purpose of this study was to investigate patterns of genetic diversity within and among natural populations of B. salicina in Saudi Arabia using ISSR markers and to compare their levels of genetic diversity with those of a population from Yemen, where the species is more widespread. Informed by the results, we also aimed to address possible strategies for long-term conservation of B. salicina.
Methods
Endangered plant survey
Previous information regarding B. salicina population levels and localities was insufficient. Therefore, to understand the spatial distribution and current state of this endangered plant species, we initially conducted searches at sites where B. salicina had been most recently collected and for which usable locality information was available. Information was obtained from King Saud University Herbarium records, site data from vegetation mapping, and botanists. A qualified botanist subsequently conducted a thorough survey of similar habitats in other areas for B. salicina. In the case of B. salicina, the most suitable habitat—along 500- to 2000-m high escarpments and above permanent streams and rivers—is characteristic of many sites in southwestern Saudi Arabia.
Collection of plant material
Following the survey, leaf samples of fully-grown trees of B. salicina were collected from most individuals present in three fragmented natural populations located in southwestern Saudi Arabia and a single individual plant found in Al-Baha, Saudi Arabia. The remaining individuals (nine individuals) in the same populations were not sampled because of the difficulty of reaching their positions. For better comparison of genetic diversity, we also sampled a population from Yemen, as the tree is widely distributed in that country (Figure 2). After collection and labeling, all samples were preserved in silica gel until DNA isolation. Identification based on leaf and fruit morphological characters was performed at the Department of Botany and Microbiology, College of Science, King Saud University, Riyadh, and vouchers were deposited in the herbarium (Table 1).
Figure 2.
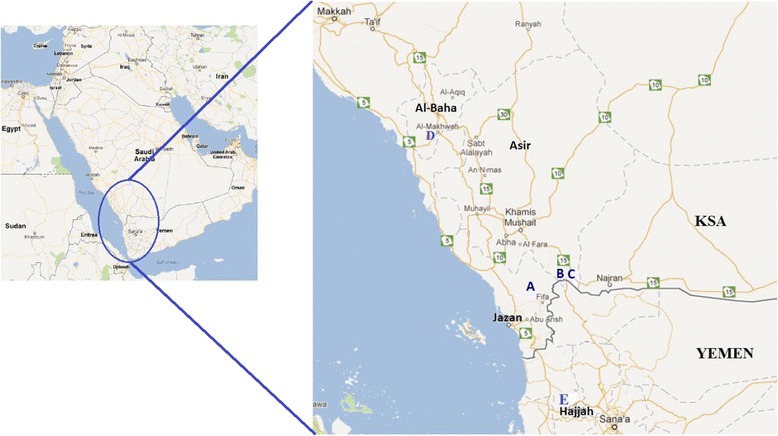
Map showing locations of the populations of Breonadia salicina in Saudi Arabia and Yemen sampled in this study. (A) Population 1: Wadi Lejib; (B) Population 2: Rabuaa, “Wadi Afkah”; (C) Population 3: Rabuaa, “Main Entrance”; (D) Al-Baha single individual; (E) Yemeni population.
Table 1.
Sampled populations of Breonadia salicina and their locations
| Populations | Location | Province (region) | Number of sampled individuals | Number of individuals within population | Coordinates | Elevation (m) |
|---|---|---|---|---|---|---|
| Population 1 | Wadi Lejib | Jizan (KSA) | 6 | 9 | 17° 36′ N | 1610 |
| 42° 56′ E | ||||||
| Population 2 | Rabuaa (Wadi Afkah) | Asir (KSA) | 7 | 11 | 17° 33′ N | 1680 |
| 43° 18′ E | ||||||
| Population 3 | Rabuaa (Main Entrance) | Asir (KSA) | 5 | 7 | 17° 34′ N | 1715 |
| 43° 19′ E | ||||||
| Al-Baha Individual | Jabal Shada Al-Aala | Asir (KSA) | 1 | 1 | 19° 51′ N | 1060 |
| 41° 18′ E | ||||||
| Yemeni population | Mapyan | Hajjah (Yemen) | 8 | 15 | 15° 43′ N | 1810 |
| 43° 34′ E |
Extraction and purification of genomic DNA
Genomic DNA was isolated and purified using a modification of the cetyltrimethylammonium bromide CTAB method [25].
PCR amplifications and ISSR profiling
ISSR primers were synthesized by Macrogen (Seoul, South Korea) and Sigma (UK). A set of 43 ISSR primers were screened for reproducibility and suitability for genetic diversity analysis after optimization of primer annealing temperatures according to their Tm values. DNA was diluted to a working concentration of 20 ng/μl. Amplifications were performed in 25-μl volumes using PCR beads (GE Healthcare, Spain) [26] on a Veriti 96-well thermocycler (Applied Biosystems), with the tubes vortexed and briefly spun (≈10 s) after addition of all components. PCR products were separated on a 1.3% agarose gel in 1× Tris/Borate/EDTA buffer TBE containing 0.5 μg/ml ethidium bromide and electrophoresed at 5 V/cm. Gels were photographed using a Syngene bio-imaging gel documentation system (Ingenius).
Analysis of ISSR banding patterns
For determination of genetic diversity, banding patterns generated by the ISSR primers were analyzed by comparing bands present in various lanes. We scored the most prominent and distinct bands, assigning for each band a ‘1’ if present and ‘0’ if absent. To assess genetic diversity, basic parameters including Nei’s gene diversity index (h), the Shannon index (I), percentage of polymorphic loci (PPL), population diversity (HS), total gene diversity (HT) within populations, and inter-population differentiation (GST) [27] were calculated from the data using the program POPGENE 1.32 [28]. A non-parametric analysis of molecular variance (AMOVA) was used to investigate the partitioning of genetic variation within and among regional populations using GenAlEx 6.5 software [29,30]. The generated data were analyzed with the NTSYS-pc 2.21 software package [31] to evaluate genetic similarity and phylogenetic relationships within and among populations using the Unweighted Pair Group Method with Arithmetic Mean (UPGMA) algorithm [32].
Results and discussion
Current status of B. salicina in Saudi Arabia
Our survey revealed that only one wild population remained in Wadi Lejib (Jizan Province) from previously reported locations, and that one had vanished in Wadi Fadah (Jizan Province). Similarly, our recent exploration discovered only one surviving individual of the formerly [33] thin (three- to four-tree) population of B. salicina in Al-Baha Province. On the other hand, two wild populations were newly identified and sampled from Asir Province in southwestern Saudi Arabia. The few known relict populations in the southwestern part of the country contained less than 30 individuals; no young seedlings were observed in these populations, except in Wadi Lejib (Jizan Province) (Table 1). Although some recreational activities take place in Wadi Lejib, many endangered plants—including this species—are thriving there [3]. Threats to B. salicina are due to several factors: environmental pressures, anthropogenic activities, including the current low rate of natural management, and increased habitat fragmentation and deterioration. Regardless of the reasons, an important consequence is that reductions in population size are leading to a situation of inbreeding, reducing within-population genetic diversity.
Genetic diversity
The application of ISSR markers in the present study revealed genetic diversity levels that were low within populations but relatively high among populations. Out of the 43 ISSR primers tested, 14 (Table 2) produced well-reproducible, scorable patterns. The 14 primers generated a total of 211 prominent, reproducible DNA bands ranging in length from 185 to 2,200 bp (Figure 3); 68 of these were polymorphic across all accessions, reflecting poor allelic diversity in the sampled populations. Bands per primer ranged from 10 (UBC 42) to 25 (UBC 34). The average number of loci per primer was 15.1, while the average number of polymorphic loci per primer was 4.9. The largest number of polymorphic loci was 13 (52%), generated from primer UBC 34, and the lowest, from primer UBC 26, was 1 (9.1%). At the population level, a low range of genetic diversity was observed. PPL per population ranged from 17.1 to 23.7%, h varied from 0.0714 to 0.0968, and I ranged from 0.1031 to 0.1405 (Table 3).
Table 2.
ISSR primers used in this study
| S.N | Name | Sequence |
|---|---|---|
| 1 | UBC-10 | GAG AGA GAG AGA GAG AT |
| 2 | UBC-11 | GAG AGA GAG AGA GAG AC |
| 3 | UBC-12 | GAG AGA GAG AGA GAG AA |
| 4 | UBC-18 | CAC ACA CAC ACA CAC AG |
| 5 | UBC-25 | ACA CAC ACA CAC ACA CT |
| 6 | UBC-26 | ACA CAC ACA CAC ACA CC |
| 7 | UBC-27 | ACA CAC ACA CAC ACA CG |
| 8 | UBC-34 | AGA GAG AGA GAG AGA GYT |
| 9 | UBC-35 | AGA GAG AGA GAG AGA GYC |
| 10 | UBC-41 | GAG AGA GAG AGA GAG AYC |
| 11 | UBC-42 | GAG AGA GAG AGA GAG AYG |
| 12 | UBC-48 | ACA CAC ACA CAC ACA CYT |
| 13 | UBC-49 | ACA CAC ACA CAC ACA CYA |
| 14 | UBC-50 | ACA CAC ACA CAC ACA CYG |
Figure 3.
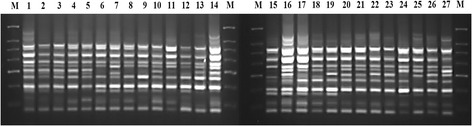
ISSR profile resulting from amplification of genomic DNA of Saudi Arabian and Yemeni Breonadia salicina accessions with primer UBC-10. M: 100-bp ladder; 1–27, accessions of B. salicina.
Table 3.
Genetic diversity within Saudi Arabian and Yemeni natural populations of Breonadia salicina based on ISSR markers
| Population | # of Polymorphic Loci | PPL | h | I |
|---|---|---|---|---|
| Pop. 1 | 50 | 23.7 | 0.097 (0.1817) | 0.141 (0.2597) |
| Pop. 2 | 49 | 23.2 | 0.090 (0.1741) | 0.132 (0.2502) |
| Pop. 3 | 36 | 17.1 | 0.071 (0.1629) | 0.103 (0.2325) |
| Average | 45 | 21.3 | 0.086 | 0.125 |
| Pop. Yemen | 51 | 24.2 | 0.094 (0.179) | 0.137 (0.255) |
Standard deviations are in parentheses. h, Nei’s gene diversity; I, Shannon’s information index; PPL, Percentage of polymorphic loci.
At the species level, PPL, h, and I were 32.2%, 0.116, and 0.172, respectively (Table 4). This level of genetic diversity is low compared with that uncovered in many other ISSR-based studies of endangered and threatened plants [34–36]. The average PPL in B. salicina across the three natural Saudi Arabian populations and the Yemeni population at the species level was considerably lower than that reported in Psychotria ipecacuanha, Galium cracoviense, and Palicourea coriacea, other species in the Rubiaceae [37–39]. Mean h within the three sampled Saudi Arabian populations of B. salicina showed considerably lower diversity (h = 0.086) than that obtained in many common species using RAPD markers (h = 0.22 or 0.23) [40]. The low genetic diversity of B. salicina may be due to its breeding system, restricted geographic attribution, gene flow, genetic drift, and/or natural selection. The type of breeding system is the main factor affecting genetic diversity both among and within populations [41,42]. At present, no comprehensive studies of the B. salicina mating system have been conducted. The genetic diversity found in B. salicina at the population level (h = 0.0861) is lower than the average for both outcrossing-plant species and mixed breeding (h = 0.27 and 0.18, respectively) and closely similar to that of self-pollinating plants (h = 0.091) using the same data source [40]. Based on this result, the genetic diversity of B. salicina is more compatible with selfing behavior, which often reduces within-population genetic variation and increases it among populations. In addition, habitat fragmentation leads to decreased genetic diversity and increased inter-population divergence, likely the result of restricted gene flow among fragmented populations, increased inbreeding, and random genetic drift within populations [43]. Small-sized populations have less genetic diversity than large-sized ones, as pollinators are more attracted to the latter [44–46].
Table 4.
Genetic diversity among Saudi Arabian populations of Breonadia salicina estimated using ISSR markers
| h | I | H T | H S | G ST | Number of polymorphic loci | PPL | |
|---|---|---|---|---|---|---|---|
| ISSR | 0.116 (0.183) | 0.172 (0.266) | 0.116 (0.034) | 0.086 (0.021) | 0.256 | 68 | 32.2 |
The individual sampled from Al-Baha is excluded. Standard deviations are in parentheses. h, Nei’s gene diversity; I, Shannon’s Information index; H T, total gene diversity; H S, population diversity; G ST, Coefficient of genetic differentiation; PPL, Percentage of polymorphic loci.
On the other hand, ISSR primers generated a total of 51 polymorphic bands, with an average of 3.6, from the Yemeni population. The largest number of polymorphic bands, 9, was generated twice—from primers UBC 34 (36%) and 50 (56.3%); the lowest number, 1 (8.3%), was obtained using primer UBC 25. Upon combination of ISSR data from Yemeni and Saudi Arabian populations, a high level of genetic diversity was expected within the Yemeni population. The opposite was true, however, with low genetic diversity observed within the Yemeni population with respect to PPL, h, and other evaluated parameters, similar to the Saudi Arabian populations. PPL, h, and I in the Yemeni population were 24.2%, 0.094, and 0.137, respectively (Table 3).
Genetic structure
In population genetics, gene differentiation based on GST values is classified as low (<0.05), medium (0.05–0.15), or high (>0.15) [47]. The GST value uncovered for B. salicina (0.256) would thus be considered high; by indirect comparison, however, it was much lower than values previously reported using ISSR markers (0.34) [40].
These findings raise the possibility that all present-day B. salicina populations were formerly part of a single population that became fragmented owing to anthropogenic activities and various ecological factors. An alternative, more likely hypothesis is that these populations were larger in the past and were probably bordered by intervening populations (which are now extinct, e.g., Wadi Fadah) that ensured low levels of gene diversity coupled with this level of genetic differentiation.
The calculated GST value for B. salicina was supported by an AMOVA, which indicated that 17% of the total variation was partitioned between populations among regions. When populations were analyzed without grouping, AMOVA indicated that 84.86% of the total variation in the studied populations was due to differences within populations, with the remaining 15.14% accounted for by among-population differences (Table 5).
Table 5.
Distribution of genetic variability within and between Saudi Arabian Breonadia salicina populations and regions measured by AMOVA of ISSR data
| Source of variance | Df | Sum of square | Variance component | % total of variance | Significance | |
|---|---|---|---|---|---|---|
| 1. Groups based on geographical origin | Variance among regions | 1 | 19.972 | 0.000 | 0% | |
| Variance among populations | 1 | 23.617 | 2.236 | 17% | P < 0.001 | |
| Variance within populations | 15 | 158.633 | 10.576 | 83% | ||
| Total | 17 | 202.222 | 12.811 | 100% | ||
| 2. Without grouping | Variance among populations | 2 | 43.589 | 1.887 | 15.14% | P < 0.001 |
| Variance within populations | 15 | 158.633 | 10.576 | 84.86% | ||
| Total | 17 | 202.222 | 12.463 | 100% |
df, degrees of freedom.
When groups were based on geographic origin (i.e., provinces of sampled populations; Table 1), among-region, among-population, and within-population contributions to the observed variance were 0%, 17%, and 83%, respectively. The null value of the variance among regions might be due to the proximity of different populations.
Genetic relationships among B. salicina populations
Based on ISSR allele frequencies, Nei’s genetic identity (IN) values of the Saudi Arabian populations had a very narrow range, varying from 0.9514 to 0.9705, and pairwise genetic distances were between 0.0299 and 0.0498 (Table 6). Genetic identity based on IN was highest between populations 1 and 2 and lowest between populations 1 and 3, although these values were nearly identical.
Table 6.
Nei’s unbiased measures of genetic identity and genetic distance between Saudi Arabian and Yemeni Breonadia salicina populations based on ISSR data*
| Pop ID | 1 | 2 | 3 | Yemen |
|---|---|---|---|---|
| 1 | **** | 0.9705 | 0.9514 | 0.9210 |
| 2 | 0.0299 | **** | 0.9572 | 0.9354 |
| 3 | 0.0498 | 0.0437 | **** | 0.9434 |
| Yemen | 0.0823 | 0.0667 | 0.0583 | **** |
* Nei’s genetic identity and genetic distance is indicated above and below diagonals, respectively.
These close genetic identities suggest that all three Saudi Arabian populations had a recent common ancestor, an inference further supported by the results of the UPGMA analysis (Figure 4). Moreover, the three B. salicina populations share similar habitats; such a situation tends to result in similar population characteristics, as a clear association exists between a population’s characteristics and its environment [48,49]. The similar habitats of these populations sharing very close genetic identities also support the hypothesis that these populations have only recently become separated.
Figure 4.
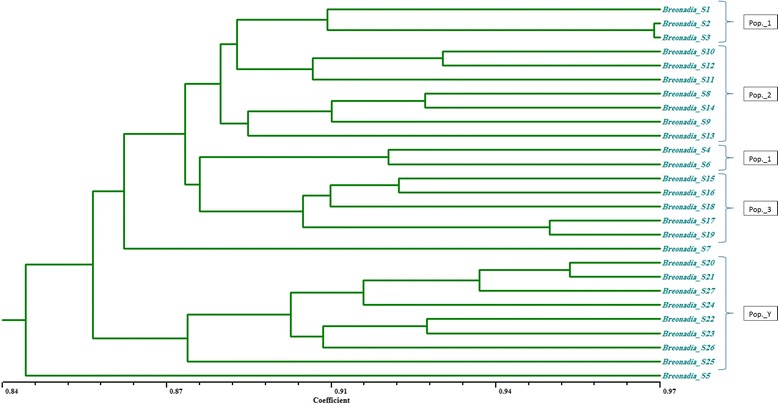
Dendrogram obtained from UPGMA cluster analysis of 27 accessions of Breonadia salicina . The scale bar corresponds to genetic distance based on Jaccard’s similarity coefficient.
We evaluated the genetic relationships of the 19 B. salicina accessions from Saudi Arabia along with the eight Yemeni accessions based on Jaccard’s similarity coefficient using UPGMA cluster analysis [32] (Figure 4). The 19 Saudi Arabian B. salicina accessions were divided into two major clusters. Cluster I comprised 10 accessions from populations 1 and 2, and cluster II contained seven accessions from population 3 and 2 outliers from population 1 (Breonadia_S4 and Breonadia_S6). In addition, a sample from Al-Baha (Breonadia_S7), the geographically most distant locality, was also the most genetically distant accession, forming its own clade. One accession (Breonadia_S5) from population 1 was widely separated from the rest of the Saudi Arabian accessions, suggesting that population 1 was the ancestral population of B. salicina. Although genetic similarity based on Jaccard’s similarity coefficients was highest between populations 1 and 2 and lowest between populations 1 and 3, genetic similarities among all three populations were nearly identical. Jaccard’s similarity coefficients ranged from 82.76 to 94.82% among the B. salicina accessions, indicating a narrow genetic diversity (Table 7). An accession of population 3 (Breonadia_S15) had the lowest genetic similarity (82.76%) to the other studied accessions. The cause of this higher genetic diversity is unknown, but is presumably due to environmental factors prevailing at the higher altitude (1,715 m) of population 3.
Table 7.
Similarity matrix generated using NTSYS-pc software based on ISSR data from 27 accessions of Breonadia salicina
| Breo nadia S1 | Breo nadia S2 | Breo nadia S3 | Breo nadia S4 | Breo nadia S5 | Breo nadia S6 | Breo nadia S7 | Breo nadia S8 | Breo nadia S9 | Breo nadia S10 | Breo nadia S11 | Breo nadia S12 | Breo nadia S13 | Breo nadia S14 | Breo nadia S15 | Breo nadia S16 | Breo nadia S17 | Breo nadia S18 | Breo nadia S19 | Breo nadia S20 | Breo nadia S21 | Breo nadia S22 | Breo nadia S23 | Breo nadia S24 | Breo nadia S25 | Breo nadia S26 | Breo nadia S27 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 1.000 | ||||||||||||||||||||||||||
| S2 | 0.899 | 1.000 | |||||||||||||||||||||||||
| S3 | 0.910 | 0.969 | 1.000 | ||||||||||||||||||||||||
| S4 | 0.884 | 0.874 | 0.875 | 1.000 | |||||||||||||||||||||||
| S5 | 0.847 | 0.864 | 0.866 | 0.849 | 1.000 | ||||||||||||||||||||||
| S6 | 0.893 | 0.873 | 0.884 | 0.916 | 0.848 | 1.000 | |||||||||||||||||||||
| S7 | 0.859 | 0.849 | 0.850 | 0.861 | 0.843 | 0.851 | 1.000 | ||||||||||||||||||||
| S8 | 0.894 | 0.893 | 0.894 | 0.869 | 0.850 | 0.897 | 0.871 | 1.000 | |||||||||||||||||||
| S9 | 0.898 | 0.878 | 0.879 | 0.844 | 0.863 | 0.891 | 0.866 | 0.902 | 1.000 | ||||||||||||||||||
| S10 | 0.889 | 0.898 | 0.899 | 0.864 | 0.855 | 0.892 | 0.858 | 0.913 | 0.878 | 1.000 | |||||||||||||||||
| S11 | 0.878 | 0.878 | 0.879 | 0.872 | 0.853 | 0.871 | 0.874 | 0.892 | 0.857 | 0.907 | 1.000 | ||||||||||||||||
| S12 | 0.888 | 0.888 | 0.879 | 0.835 | 0.863 | 0.891 | 0.847 | 0.902 | 0.916 | 0.927 | 0.896 | 1.000 | |||||||||||||||
| S13 | 0.866 | 0.884 | 0.866 | 0.859 | 0.869 | 0.849 | 0.862 | 0.869 | 0.892 | 0.865 | 0.854 | 0.873 | 1.000 | ||||||||||||||
| S14 | 0.910 | 0.871 | 0.873 | 0.856 | 0.866 | 0.874 | 0.877 | 0.923 | 0.908 | 0.899 | 0.860 | 0.879 | 0.904 | 1.000 | |||||||||||||
| S15 | 0.899 | 0.879 | 0.881 | 0.846 | 0.828 | 0.873 | 0.858 | 0.893 | 0.859 | 0.889 | 0.887 | 0.888 | 0.865 | 0.862 | 1.000 | ||||||||||||
| S16 | 0.881 | 0.871 | 0.881 | 0.865 | 0.856 | 0.903 | 0.868 | 0.884 | 0.860 | 0.909 | 0.869 | 0.869 | 0.856 | 0.900 | 0.918 | 1.000 | |||||||||||
| S17 | 0.885 | 0.853 | 0.867 | 0.879 | 0.833 | 0.888 | 0.891 | 0.870 | 0.855 | 0.866 | 0.873 | 0.846 | 0.889 | 0.876 | 0.894 | 0.904 | 1.000 | ||||||||||
| S18 | 0.886 | 0.876 | 0.877 | 0.852 | 0.861 | 0.870 | 0.882 | 0.881 | 0.866 | 0.867 | 0.884 | 0.847 | 0.881 | 0.877 | 0.914 | 0.896 | 0.910 | 1.000 | |||||||||
| S19 | 0.904 | 0.866 | 0.886 | 0.898 | 0.833 | 0.917 | 0.863 | 0.908 | 0.864 | 0.904 | 0.892 | 0.874 | 0.879 | 0.895 | 0.894 | 0.904 | 0.948 | 0.891 | 1.000 | ||||||||
| S20 | 0.875 | 0.874 | 0.886 | 0.878 | 0.850 | 0.868 | 0.871 | 0.888 | 0.864 | 0.874 | 0.882 | 0.873 | 0.879 | 0.876 | 0.865 | 0.884 | 0.889 | 0.890 | 0.889 | 1.000 | |||||||
| S21 | 0.869 | 0.859 | 0.851 | 0.872 | 0.834 | 0.871 | 0.865 | 0.872 | 0.848 | 0.859 | 0.876 | 0.857 | 0.872 | 0.869 | 0.878 | 0.907 | 0.892 | 0.865 | 0.883 | 0.952 | 1.000 | ||||||
| S22 | 0.853 | 0.824 | 0.835 | 0.837 | 0.809 | 0.836 | 0.840 | 0.876 | 0.841 | 0.843 | 0.850 | 0.832 | 0.856 | 0.872 | 0.843 | 0.872 | 0.886 | 0.849 | 0.886 | 0.876 | 0.919 | 1.000 | |||||
| S23 | 0.863 | 0.834 | 0.836 | 0.838 | 0.819 | 0.837 | 0.850 | 0.876 | 0.851 | 0.853 | 0.860 | 0.851 | 0.867 | 0.873 | 0.843 | 0.854 | 0.858 | 0.850 | 0.877 | 0.896 | 0.910 | 0.924 | 1.000 | ||||
| S24 | 0.829 | 0.838 | 0.839 | 0.832 | 0.832 | 0.840 | 0.835 | 0.870 | 0.836 | 0.838 | 0.845 | 0.827 | 0.851 | 0.848 | 0.847 | 0.867 | 0.871 | 0.845 | 0.881 | 0.890 | 0.914 | 0.897 | 0.878 | 1.000 | |||
| S25 | 0.878 | 0.822 | 0.824 | 0.843 | 0.816 | 0.824 | 0.837 | 0.882 | 0.857 | 0.840 | 0.838 | 0.829 | 0.844 | 0.879 | 0.840 | 0.841 | 0.864 | 0.847 | 0.873 | 0.872 | 0.876 | 0.869 | 0.870 | 0.884 | 1.000 | ||
| S26 | 0.858 | 0.811 | 0.822 | 0.851 | 0.814 | 0.832 | 0.845 | 0.862 | 0.809 | 0.838 | 0.855 | 0.809 | 0.843 | 0.859 | 0.838 | 0.848 | 0.872 | 0.845 | 0.881 | 0.871 | 0.894 | 0.918 | 0.888 | 0.872 | 0.874 | 1.000 | |
| S27 | 0.884 | 0.855 | 0.866 | 0.859 | 0.840 | 0.858 | 0.880 | 0.888 | 0.863 | 0.874 | 0.881 | 0.854 | 0.869 | 0.894 | 0.864 | 0.884 | 0.879 | 0.861 | 0.888 | 0.927 | 0.941 | 0.925 | 0.915 | 0.930 | 0.891 | 0.910 | 1.000 |
On the other hand, based on genetic distance (Tables 6 and 7), the Yemeni population was more differentiated and formed a separate clade in the UPGMA dendrogram (Figures 4 and 5). The lower genetic diversity of the Yemeni population in spite of the species’ wider distribution in that country may be due to the presence of optimal growing conditions for B. salicina (i.e., high escarpments and abundant rainfall) without any external disturbance. In addition, the plant is cultivated in Yemen for wood and timber [50,51].
Figure 5.
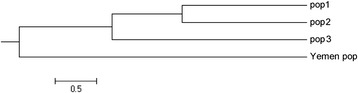
UPGMA dendrogram showing relationships between Saudi Arabian and Yemeni populations of Breonadia salicina .
Implications for B. salicina conservation
Drawing on our study results, we suggest a possible management strategy for the conservation of B. salicina. The first component of our proposed strategy concerns the mating system of B. salicina, suggested in this study to involve self-pollination. To verify this finding, further investigation is urgently needed. The outcome of such a study would have important implications for B. salicina conservation and management, as knowledge of the mating system is a major factor in the elucidation of genetic variability levels at both species and population levels [52].
A second important consideration for restoration of endangered and rare species is population size [53]. Establishment of on-site protection zones for B. salicina to reduce the impact of human activities would allow its habitats to increase in size through natural regeneration to reach effective population sizes. The construction of an in situ conservation area to preserve all extant populations would also maintain most existing genetic variation. Although losses of any one population or individual tree at specific locations may not cause an immediate decrease in genetic diversity, such losses have dangerous long-term consequences.
Third, plants can be introduced from other populations via appropriate propagation and seedling management to increase the chance of gene exchange and recombination and to improve the level of genetic diversity over time. In Tanzania, effective management procedures for B. salicina have included seed germination, which is more successful under moist conditions, and placing cuttings and wildlings in mud, the most frequently used method. Wildlings appear to have a higher survival rate than nursery seedlings, which need to be at least 1 m high to survive transplantation [12].
A fourth suggested management practice is establishment of an ex situ conservation program to capture most of the detected genetic variability and to increase genetic diversity by crossing different populations. Fifth, collection of seeds and germplasm in botanical gardens or other institutions may be of significant practical value for the conservation of genetic diversity. Sixth, establishment of a regeneration system for this endangered plant via tissue culture would not only guarantee its ex situ conservation and sustainable survival, but would also enhance its in situ conservation. A seventh component of the action plan is the education and involvement of local people. Finally, as genetic information alone will still not provide a sufficient solution, demographic and ecological perspectives must be taken into consideration before drawing up and implementing a conservation strategy.
Conclusions
The molecular markers used in this study were found to be effective for the assessment of B. salicina genetic diversity. Observed low levels of within-population genetic diversity coupled with the relatively high genetic differentiation detected among Saudi Arabian B. salicina populations suggest the hypothesis that these populations were formerly part of a single population that has become fragmented as a result of biotic and abiotic factors in the natural habitat. Alternatively, current populations were larger in the past, with intervening, now-extinct populations (e.g., Wadi Fadah) ensuring lower levels of gene diversity. While both Saudi Arabian and Yemeni populations are marked by low genetic diversity, the presence of optimal growing conditions and cultivation in Yemen has contributed to the widespread distribution of B. salicina in that country. To broaden the genetic base of B. salicina and conserved this critically endangered plant species in Saudi Arabia, appropriate management and conservation strategies require implementation.
Acknowledgments
This project was supported by King Saud University, Deanship of Scientific Research, College of Science Research Center. Special thanks are due to Dr. Jacob Pandalayil (curator of the King Saud University herbarium) for his contributions to this endangered plant survey.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AZG, FA and SK, designed the experiments. AZG conducted the experiments. AZG and SK analyzed and interpreted the data. AZG, FA and SK drafted the manuscript. All authors read and approved the manuscript and this submission.
Contributor Information
Abdel-Rhman Z Gaafar, Email: abdou.gaafar@yahoo.com.
Fahad Al-Qurainy, Email: fahad_alqurainy@yahoo.com.
Salim Khan, Email: salimkhan17@yahoo.co.in.
References
- 1.Khan S, Al-Qurainy F, Nadeem M. Biotechnological approaches for conservation and improvement of rare and endangered plants of Saudi Arabia. Saudi J Biol Sci. 2012;19:1–11. doi: 10.1016/j.sjbs.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Farhan A, Al-Turki TA, Basahy RA. Flora of Jizan Region, Final Report of project AR-17-7. King Abdulaziz City for Science and Technology (KACST), KSA. 2005;1:1–545. [Google Scholar]
- 3.Thomas J: Plant Diversity of Saudi Arabia/Endangered plants. In [http://plantdiversityofsaudiarabia.info/]
- 4.Al-Turki TA. An initiative in exploration and management of plant genetic diversity in Saudi Arabia. In: Rao VR, Brown AH, Jackson MT, editors. J.M. Engels, Managing plant genetic diversity. UK: Wallingford: CAB International; 2002. pp. 339–349. [Google Scholar]
- 5.Mahlo SM: Characterization and biological activity of antifungal compounds present inBreonadia salicina(Rubiaceae) leaves. In University of Pretoria, South Africa: PhD thesis, Phytomedicine Programme; 2009.
- 6.Palgrave KC. Trees of Southern Africa, Revised Edition” C. Cape Town/Johannesburg: Struik Publishers; 1988. [Google Scholar]
- 7.Chang P, Koh Y, Geh SL, Soepadmo E, Goh SH, Wong AK. Cradiovascular effects in the rat of dihydrocorynantheine isolated from Uncaria callophylla. J Ethnopharmacol. 1989;25:213–215. doi: 10.1016/0378-8741(89)90023-8. [DOI] [PubMed] [Google Scholar]
- 8.Sibandze GF, Van Zyla RL, Van Vuurena SF. The anti-diarrhoeal properties of Breonadia salicina, Syzygium cordatum and Ozoroa sphaerocarpa when used in combination in Swazi traditional medicine. J Ethnopharmacol. 2010;132:506–511. doi: 10.1016/j.jep.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 9.Al-Qurainy FH, Gaafar AZ, Khan S, Nadeem M, Tarroum M, Alaklabi A, Thomas J. Antibacterial activity of leaf extract of Breonadia salicina (Rubiaceae), an endangered medicinal plant of Saudi Arabia. Genet Mol Res. 2013;12(Suppl 3):3212–3219. doi: 10.4238/2013.August.29.5. [DOI] [PubMed] [Google Scholar]
- 10.Mahlo SM, McGaw LJ, Eloff JN: Antifungal activity and cytotoxicity of isolated compounds from leaves ofBreonadia salicina.J Ethnopharmacol148(Suppl 3):909–913. [DOI] [PubMed]
- 11.Arnold HJ, Gulumian M. Pharmacopoeia of traditional medicine in Venda. J Ethnopharmacol. 1984;12:35–74. doi: 10.1016/0378-8741(84)90086-2. [DOI] [PubMed] [Google Scholar]
- 12.Hines DA, Eckman K: Indigenous multipurpose trees of Tanzania: Uses and economic benefits for people. In [http://www.fao.org/docrep/X5327e/x5327e00.htm]
- 13.Schaal BA, Leverich WJ, Rogstad SH. Comparison of methods for assessing genetic variation in plant conservation biology. In: Falk DA, Holsinger KE, editors. Genetics and Conservation of Rare Plants. New York: Oxford University Press; 1991. pp. 123–134. [Google Scholar]
- 14.Barrett SCH, Kohn JR. Genetic and evolutionary consequences of small population size in plants: implications for conservation. In: Falk DA, Holsinger KE, editors. Genetics and Conservation of Rare Plants. New York: Oxford University Press; 1991. pp. 3–30. [Google Scholar]
- 15.Cruzan MB. Population size and fragmentation thresholds for the maintenance of genetic diversity in the herbaceous endemic Scutellaria Montana (Lamiaceae) Evolution. 2001;55:1569–1580. doi: 10.1111/j.0014-3820.2001.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 16.Wallace LE. Examining the effects of fragmentation on genetic variation in Platanthera leucopheaea (Orchidaceae): Inferences from allozyme and random amplified polymorphic DNA markers. Plant Species Biol. 2002;17:37–49. doi: 10.1046/j.1442-1984.2002.00072.x. [DOI] [Google Scholar]
- 17.Culley TM, Wolfe AD. Population genetic structure of the cleistogamous plant species Viola pubescens Aiton (Violaceae), as indicated by allozyme and ISSR molecular markers. Heredity. 2001;86:545–556. doi: 10.1046/j.1365-2540.2001.00875.x. [DOI] [PubMed] [Google Scholar]
- 18.Gupta M, Chyi YS, Romero-Severson J, Owen JL. Amplification of DNA markers from evolutionarily diverse genomes using single primers of simple-sequence repeats. Theor Appl Genet. 1994;89:998–1006. doi: 10.1007/BF00224530. [DOI] [PubMed] [Google Scholar]
- 19.Yildiz M, Ekbic E, Keles D, Sensoy S, Abak K. Use of ISSR, SRAP, and RAPD markers to assess genetic diversity in Turkish melons. Sci Hort. 2011;130:349–353. doi: 10.1016/j.scienta.2011.06.048. [DOI] [Google Scholar]
- 20.Trindade H, Sena I, Gonçalves S, Romano A. Genetic diversity of wild populations of Tuberaria major (Cistaceae), an endangered species endemic to the Algarve region (Portugal), using ISSR markers. Biochem Syst Ecol. 2012;45:9–56. doi: 10.1016/j.bse.2012.06.028. [DOI] [Google Scholar]
- 21.Zhang QX, Shen YK, Shao RX, He J, Fang YQ, Ren JX, Zheng BS, Chen GJ. Genetic diversity of natural Miscanthus sinensis populations in China revealed by ISSR markers. Biochem Syst Ecol. 2013;48:248–256. doi: 10.1016/j.bse.2012.12.024. [DOI] [Google Scholar]
- 22.Felsenstein J. Phylogenies and the comparative method. Am Nat. 1985;125:1–15. doi: 10.1086/284325. [DOI] [Google Scholar]
- 23.Silvertown J, Dodd M. Comparing plants and connecting traits. Philos Trans R Soc Lond B Biol Sci. 1996;351:1233–1239. doi: 10.1098/rstb.1996.0106. [DOI] [Google Scholar]
- 24.Gitzendanner MA, Soltis PS. Patterns of genetic variation in rare and widespread plant congeners. Am J Bot. 2000;87:783–792. doi: 10.2307/2656886. [DOI] [PubMed] [Google Scholar]
- 25.Khan S, Qureshi MI, Kamaluddin Alam T, Abdin MZ. Protocol for isolation of genomic DNA from dry and fresh roots of medicinal plants suitable for RAPD and restriction digestion. Afr J Biotechnol. 2007;6(Suppl 3):175–178. [Google Scholar]
- 26.Al-Qurainy F. Application of inter simple sequence repeat (ISSR marker) to detect genotoxic effect of heavy metals on Eruca sativa (L) Afr J Biotechnol. 2010;9(Suppl 4):467–474. [Google Scholar]
- 27.Nei M. Analysis of gene diversity in subdivided populations. Proc Natl Acad Sci U S A. 1973;70:3321–3323. doi: 10.1073/pnas.70.12.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeh FC, Yang R, Boyle TJ, Ye Z, Xiyan JM. POPGENE 32, Microsoft windows-based freeware for population genetic analysis. Edmonton: Molecular biology and biotechnology center University of Alberta; 2000. [Google Scholar]
- 29.Peakall R, Smouse PE. GenAlEx 6: genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Notes. 2006;6:288–295. doi: 10.1111/j.1471-8286.2005.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peakall R, Smouse PE. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research – an update. Bioinformatics. 2012;28:2537–2539. doi: 10.1093/bioinformatics/bts460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rohlf FJ: NTSYS-pc: numerical taxonomy system ver.2.21. Setauket, NY: Exeter Publishing Ltd; 2010.
- 32.Sneath PHA, Sokal RR. Numerical taxonomy. San Francisco: Freeman WH and Company; 1973. [Google Scholar]
- 33.Young AG, Brown AHD. Comparative population genetic structure of the rare woodland shrub Daviesia suaveolens and its common congener D. mimosoides. Conserv Biol. 1996;10:1220–1228. doi: 10.1046/j.1523-1739.1996.10041220.x. [DOI] [Google Scholar]
- 34.Pillai PP, Sajan JS, Menon KM, Jayakumar KSP, Subramoniam A. ISSR analysis reveals high intraspecific variation in Rauvolfia serpentina L. – A high value medicinal plant. Biochem Systemat Ecol. 2012;40:192–197. doi: 10.1016/j.bse.2011.10.019. [DOI] [Google Scholar]
- 35.Uysal T, Ozel E, Bzkurt M, Ertuğrul K. Genetic diversity in threatened populations of the endemic species Centaurea lycaonica Boiss. & Heldr. (Asteraceae) Res J Biol. 2012;2(Suppl 3):110–116. [Google Scholar]
- 36.Huang Y, Zhang CQ, Li DZ. Low genetic diversity and high genetic differentiation in the critically endangered Omphalogramma souliei (Primulaceae): implications for its conservation. J Systemat Evol. 2009;47(Suppl 2):103–109. doi: 10.1111/j.1759-6831.2009.00008.x. [DOI] [Google Scholar]
- 37.Rossi AAB, De Oliveira LO, Venturini BA, Silva RS. Genetic diversity and geographic differentiation of disjunct Atlantic and Amazonian populations of Psychotria ipecacuanha (Rubiaceae) Genetica. 2009;136:57–67. doi: 10.1007/s10709-008-9309-z. [DOI] [PubMed] [Google Scholar]
- 38.Cieslak E, Szelaq Z. Genetic diversity of Galium cracoviense ehrend. (Rubiaceae) -the polish endemic plant. Acta Societatis Botanicorum Poloniae. 2009;78:123–129. doi: 10.5586/asbp.2009.016. [DOI] [Google Scholar]
- 39.Barbosa TCS, Sibov ST, Telles MPC, Soares TN. Genetic characterization of natural populations of the medicinal plant Palicourea coriacea (Rubiaceae) with molecular markers. Genet Mol Res. 2010;9(Suppl 2):695–704. doi: 10.4238/vol9-2gmr671. [DOI] [PubMed] [Google Scholar]
- 40.Nybom H. Comparison of different DNA markers for estimating intraspecific genetic variation in plants. Mol Ecol. 2004;13:1143–1155. doi: 10.1111/j.1365-294X.2004.02141.x. [DOI] [PubMed] [Google Scholar]
- 41.Hamrick JL, Godt MJ. Allozyme diversity in plant species. In: Brown AHD, Clegg MT, Kahler AL, Weir BS, editors. Plant population genetics, breeding, and genetic resources. MA: Sinauer, Sunderland; 1990. pp. 43–63. [Google Scholar]
- 42.He YP, Liu JQ. A review on recent advances in the studies on plant breeding system. Acta Phytoecologica Sinicapp. 2003;27(Suppl 2):151–163. [Google Scholar]
- 43.Aguilar R, Quesada M, Ashworth L, Herreriasdiego Y, Lobo J. Genetic consequences of habitat fragmentation in plant populations: susceptible signals in plant traits and methodological approaches. Mol Ecol. 2008;17:5177–5188. doi: 10.1111/j.1365-294X.2008.03971.x. [DOI] [PubMed] [Google Scholar]
- 44.Jennersten O. Pollination in Dianthus deltoids (Caryophyllaceae): Effects of habitat fragmentation on visitation and seed set. Conserv Biol. 1988;2:359–366. doi: 10.1111/j.1523-1739.1988.tb00200.x. [DOI] [Google Scholar]
- 45.Wilcock C, Neiland MRM. Pollination failure in plants: why it happens and when it matters. Trends Plant Sci. 2002;7:270–277. doi: 10.1016/S1360-1385(02)02258-6. [DOI] [PubMed] [Google Scholar]
- 46.Andrieu E, Dornier A, Rouifed S, Schatz B, Cheptou PO. The town Crepis and the country Crepis: How does fragmentation affect a plant-pollinator interaction? Acta Oecologica. 2009;35:1–7. doi: 10.1016/j.actao.2008.07.002. [DOI] [Google Scholar]
- 47.Nei M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics. 1978;89:583–590. doi: 10.1093/genetics/89.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aston JL, Bradshaw AD. Evolution in closely adjacent plant populations II. Agrostis stolonifera in maritime habitats. Heredity. 1966;37:9–25. [Google Scholar]
- 49.Al-Hiyaly SAK, McNeilly T, Bradshaw AD, Mortimer AM. The effect of zinc contamination from electricity pylons. Genetic constraints on selection for zinc tolerance. Heredity. 1993;70:22–32. doi: 10.1038/hdy.1993.4. [DOI] [Google Scholar]
- 50.Al-Khulaidi AA: Flora of Yemen. [http://ye.chm-cbd.net/implementation/documents/1-flora-final-by-dr.-abdul-wali-al-khulaidi-2013-part-1-introduction.pdf]
- 51.Al-Khulaidi AA, Abdullah HA, AbuoGanem A, Rageh A, Al-shurai AA, Al-dalas M, Al-khulani M, Mokred A, Silan A, Al-nassiri M: Second National Report on the State of Plant Genetic Resources for Food and Agriculture: State of plant genetic resources for food and agriculture In Yemen. [http://www.fao.org/docrep/013/i1500e/Yemen.pdf]
- 52.Nybom H, Bartish IV. Effects of life history traits and sampling strategies on genetic diversity estimates obtained with RAPD makers in plants. Perspect Plant Ecol Evol Syst. 2000;3(Suppl 2):93–114. doi: 10.1078/1433-8319-00006. [DOI] [Google Scholar]
- 53.Allendorf FW. Genetic drift and the loss of alleles versus heterozygosity. Zoo Biol. 1986;5:181–190. doi: 10.1002/zoo.1430050212. [DOI] [Google Scholar]


