Abstract
OBJECTIVES:
Atrial fibrillation is the most common sustained arrhythmia and is associated with poor outcomes, including stroke. The ability of anticoagulation therapy to reduce the risk of stroke has been well established; however, the prevalence of anticoagulation therapy use in the Public Health System is unknown. The aim of this study is to evaluate both the prevalence of anticoagulation therapy among patients with atrial fibrillation and the indications for the treatment.
METHODS:
In this cross-sectional study, we included consecutive patients who had atrial fibrillation documented by an electrocardiogram performed between September 2011 and March 2012 at a university hospital of the Public Health System. The variables analyzed included the risk of a thromboembolic event and/or bleeding, the use of antiplatelet or anticoagulation therapy, the location where the electrocardiogram report was initially reviewed and the specialty of the physician who initially reviewed it.
RESULTS:
We included 162 patients (mean age 68.9 years, 56% men). Hypertension (90.1%), heart failure (53.4%) and stroke (38.9%) were the most prevalent diseases found. Only 50.6% of the patients knew that they had atrial fibrillation. Regarding the use of therapy, only 37.6% of patients classified as high risk according to the CHADS2 scores and 35.5% according to the CHA2DS2VASc used oral anticoagulation. A presumptive diagnosis of heart failure and the fact that the electrocardiogram was evaluated by a cardiologist were the only independent predictors of the use of anticoagulants.
CONCLUSIONS:
Our study found a low prevalence of oral anticoagulation therapy among patients with atrial fibrillation and an indication for stroke prophylaxis for the use of this therapy, including among those with high CHADS2 and CHA2DS2VASc scores.
Keywords: Atrial Fibrillation, Stroke, Anticoagulation
INTRODUCTION
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia in clinical medicine, affecting approximately 1.5-2% of the population in developed countries (1-5). In Brazil, it is estimated that approximately 1.5 million individuals have AF and that AF is responsible for 33% of all hospitalizations for arrhythmia. Moreover, AF is associated with the occurrence of stroke and heart failure and with an increase in total mortality (6). Although there has been a decrease in the death rate associated with cerebrovascular disease in Brazil over the past few decades, stroke has persisted as the leading cause of death (7). For the prevention of stroke, anticoagulant therapy was established as the therapy of choice for the treatment of patients with AF (8-10).
Anticoagulation therapy using vitamin K inhibitors reduces the rate of stroke by 30% in high-risk patients, indicated by CHADS2 or CHA2DS2VASc risk scores ≥2 (11-12). Recently, new anticoagulants, such as dabigatran, rivaroxaban, apixaban and edoxaban have shown similar or superior results in the prevention of stroke in AF patients compared with warfarin (13-18). The consistent results of anticoagulants have led to the classification of these drugs as indication class I and evidence level A for the prevention of embolic events in AF patients with CHADS2 or CHA2DS2VASc scores ≥2, according to major guidelines dedicated to this subject (4,6,14). However, despite recommendations, studies conducted in developed countries have shown that approximately 20-35% of AF patients with a formal indication for anticoagulation do not use this therapy (19-22). In Brazil in particular, so far, there are no data regarding the use of anticoagulants in AF patients in the Public Health System. Due to the high morbidity and mortality associated with stroke, it is crucial to determine the actual use of appropriate prophylactic therapy in this patient population.
The main objective of our study was to evaluate the prevalence of the use of anticoagulants among AF patients who had a formal indication for stroke prophylaxis.
MATERIALS AND METHODS
Population
The sample studied was provided by the University Hospital ULBRA/Hospital Mãe de Deus (HU-HMD) in Canoas, a city of 323,827 inhabitants located in the metropolitan region of Porto Alegre, Rio Grande do Sul, Brazil. The HU-HMD is a tertiary care institution responsible for servicing the entire municipality and for reviewing electrocardiograms (ECGs) from Basic Healthcare System units.
All ECG reports interpreted at the HU-HMD between September 1, 2011 and March 31, 2012, were initially analyzed. These reports were obtained from the computerized system of the institution. We included consecutive patients whose ECGs showed AF. These patients were identified using hospital registration forms and then contacted by telephone. Those who agreed to participate, completed a questionnaire about their medical condition. These data were used to classify the patients according to thromboembolic events (CHADS2 and CHA2DS2VASc) and bleeding (HAS-BLED) (23) risk scores, the site of care and the use of medications.
We excluded those individuals who did not respond to three calls at different times, those who refused to participate, those who did not clearly answer the questions and those who had died.
The study was approved by the Ethics Committee of the Lutheran University of Brazil and met the provisions of Resolution 196 of the National Health Council.
Variables
Patients with CHADS2 or CHA2DS2VASc risk scores ≥2 were considered to have a formal indication for anticoagulant therapy in the absence of contraindications, such as a history of bleeding, blood dyscrasias or an allergy to anticoagulants. Patients with a HAS-BLED risk score ≥3 were considered to be at high risk for bleeding during anticoagulant therapy.
The specialty of the physician who requested and evaluated the ECG and the location where the patient performed clinical follow-up were determined.
Statistical analysis
Continuous variables are expressed as the mean and standard deviation and Student's t-test was used to assess differences. Categorical variables are reported as percentages and the differences between these variables were evaluated by a chi-square or Fisher's exact test, when appropriate. The outcome-associated variables with a p-value<0.10 or with clinical relevance were selected for multivariate analysis. Logistic regression was then performed to identify independent determinants of outcomes. A p-value<0.05 was considered statistically significant. The results are expressed as odds ratios and 95% confidence intervals (CIs).
The analysis was performed with the Statistical Package for Social Sciences (SPSS), version 20.0.
RESULTS
Population characteristics
During the study period, 6,162 ECGs were interpreted, 295 of them (4.78%) showed AF. These ECGs belonged to 242 patients. Of the total sample, 52 patients (21.4%) could not be found, 23 (9.5%) had died and 5 (2.06%) refused to participate. Thus, 162 patients, representing 67.5% of the initial sample, were ultimately included.
The evaluated patients were predominantly hypertensive men aged >65 years. Nearly half of the patients already knew that they had arrhythmia. The main characteristics of the sample are described in Table 1.
Table 1.
Baseline characteristics.
| Characteristic | n = 162 |
| Age (years) | 68.94±12 |
| Gender (male) | 92 (56.8) |
| Comorbidities | |
| Hypertension | 146 (90.1) |
| DM | 44 (27.2) |
| CHF | 88 (54.3) |
| Stroke | 63 (38.9) |
| ACS | 31 (19.1) |
| Alcohol use | 12 (7.4) |
| Extracardiac vasculopathy | 58 (35.8) |
| Renal disease | 4 (2.5) |
| Liver disease | 3 (1.9) |
| Known to have arrhythmia | 82 (50.6) |
| Medications | |
| ASA | 80 (49.4) |
| Oral anticoagulant | 55 (34) |
| First location where the ECG was reviewed | |
| Basic unit | 57 (35.2) |
| Secondary hospital | 9 (5.6) |
| Tertiary hospital | 51 (31.5) |
| Private clinic | 5 (3.1) |
| Emergency department | 17 (10.5) |
| Outpatient clinic | 20 (12.3) |
| ECG was not checked | 3 (1.9) |
| First doctor who reviewed the ECG | |
| Generalist | 56 (34.6) |
| Family physician | 36 (22.2) |
| Cardiologist | 40 (24.7) |
| Surgeon | 5 (3.1) |
| Emergency physician | 13 (8.0) |
| Neurologist | 4 (2.5) |
| Other | 5 (3.1) |
| ECG checked by a cardiologist at any time | 84 (51.9) |
The data are presented as the median ± standard deviation or n (%). DM: diabetes mellitus; CHF: chronic heart failure; ACS: acute coronary syndrome; ASA: acetylsalicylic acid; ECG: electrocardiogram.
Therapy and the risk of bleeding and thromboembolic events
Table 2 illustrates the distribution of patients according to risk scores. An analysis of CHADS2 and CHA2DS2VASc scores showed that 87% and 95.7%, respectively, of patients had scores ≥2 points.
Table 2.
Distribution of patients according to their CHADS2, CHA2DS2VASc and HAS-BLED scores.
| Score | Score of risk (n (%)) | ||
| CHADS2 | CHA2DS2VASc | HAS-BLED | |
| 0 | 8 (4.9) | 2 (1.2) | 7 (4.3) |
| 1 | 13 (8.0) | 5 (3.1) | 48 (29.6) |
| 2 | 50 (30.9) | 12 (7.4) | 73 (45.1) |
| 3 | 39 (24.1) | 40 (24.7) | 31 (19.1) |
| 4 | 29 (17.9) | 24 (14.8) | 3 (1.9) |
| 5 | 15 (9.3) | 33 (20.4) | 0 (0.0) |
| 6 | 8 (4.9) | 25 (15.4) | 0 (0.0) |
| 7 | - | 15 (9.3) | |
| 8 | - | 6 (3.7) | |
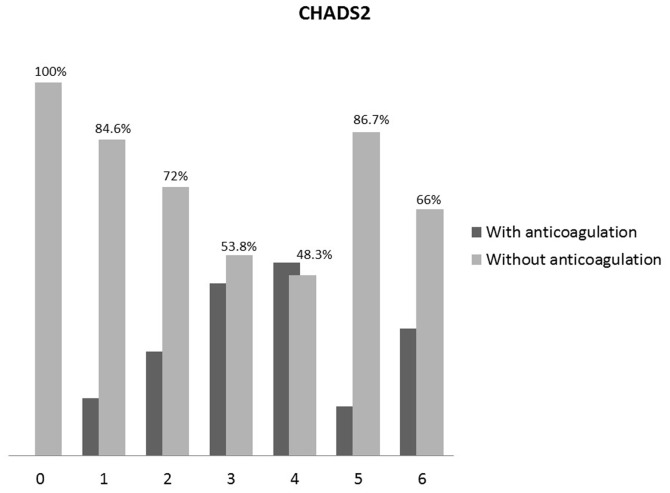
Only 37.6% of patients with a CHADS2 score ≥2 used anticoagulants. When stratified according to CHADS2 score, approximately half of the patients with a CHADS2 score of 3 or 4 used anticoagulants, whereas only 15% of patients with a CHADS2 score >5 used this therapy (Figure 1).
Figure 1.
Prevalence of the use of oral anticoagulants according to the CHADS2 score.
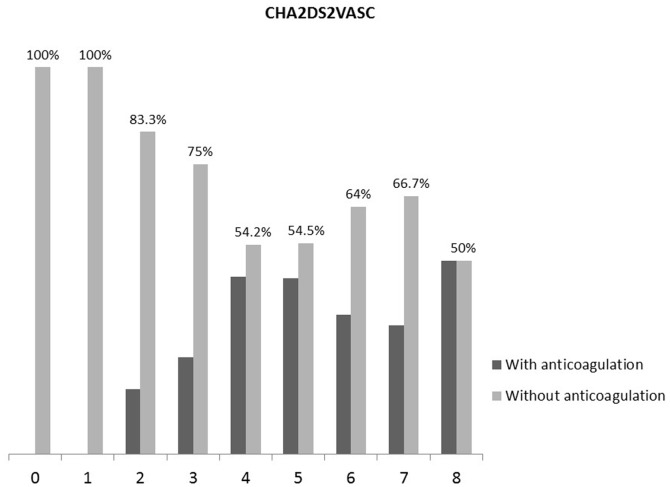
When the CHA2DS2VASc scores were analyzed, we observed similar results; of the patients with a CHA2DS2VASc score ≥2, only 35.5% had been adequately treated with anticoagulants (Figure 2).
Figure 2.
Prevalence of the use of oral anticoagulants according to the CHA2DS2VASc score.
When we compared the two score types, we realized that the CHA2DS2VASc score identified 3% of the patients as belonging to the high-risk group, which would be classified as moderate risk according to the CHADS2 score.
Regarding the use of antiplatelet agents, we observed that 25% of patients with a CHADS2 score of 0 and 61.5% of patients with a score of 1 used this medication. However, none of the patients with a CHA2DS2VASc score of 0 or 1 used antiplatelet therapy.
According to the HAS-BLED score, 34 patients (20.9%) had a score ≥3, which suggested an increase in bleeding risk with the use of oral anticoagulants. Of these patients, 12 (35%) used anticoagulant therapy.
Therapy recommendations based on the specialty of the attending physician
Regarding the specialty of the physician who initially reviewed the ECG revealing the patient's AF diagnosis, we noticed large variation in the percentage of patients with a CHA2DS2VASc score ≥2 who were not using anticoagulant therapy, ranging from 20.6% among those whose ECGs were observed by cardiologists to 100% among those whose ECGs were observed by surgeons or neurologists (p<0.01) (Figure 3).
Figure 3.
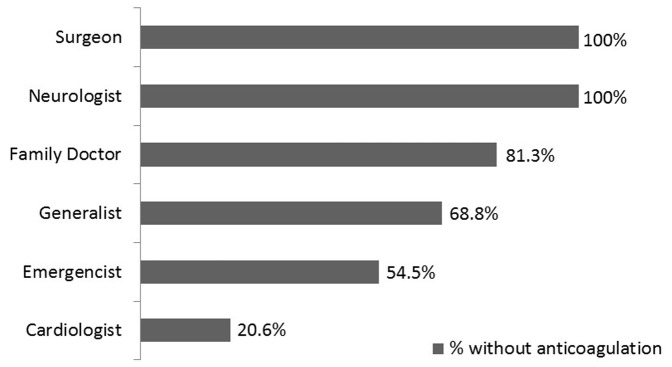
Percentage of patients without anticoagulation therapy according to the specialty of the doctor who treated them.
The location of the ECG review also showed large variation based on the use of anticoagulants (p<0.01) (Figure 4).
Figure 4.
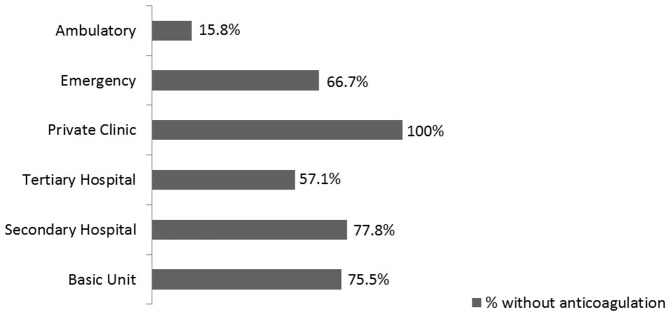
Percentage of patients without anticoagulation therapy according to the location where the ECG was reviewed.
When we analyzed only patients with a history of previous ischemic stroke, we found that only 36.5% chronically used oral anticoagulants.
Heart failure, hypertension, advanced age, diabetes and prior stroke (CHADS2 factors), as well as the specialty of the doctor who initially reviewed the ECG and the location where the ECG was first reviewed, were used as variables in a multivariate analysis. In this analysis, heart failure (OR: 3.07, 95% CI: 1.35-7.00) and the fact the ECG was first reviewed by a cardiologist versus a non-cardiologist (OR: 12.14: 95% CI: 4.85-30.37) were independent predictors of anticoagulation.
DISCUSSION
Our study has shown a low prevalence of oral anticoagulation among patients with AF and an indication for the use of this therapy based on risk scores. An Italian study published in 2000 reported that anticoagulation rates were approximately 70% for moderate-risk patients and greater than 85% for high-risk patients (21). However, a study published in 2002 showed rates closer to 60% for therapy use in the U.S. population (22). The present study has revealed that only 35% of our patients were on anticoagulants, which is a much lower prevalence than values already reported for other countries. Furthermore, our findings highlight the low prevalence (36.5%) of anticoagulant use even in patients with a history of ischemic stroke.
The risk scores used in our study have been widely studied and validated and are directly related to the occurrence of thromboembolic events. The CHADS2 score determines a cumulative risk of stroke of less than 2% per year for patients with a score of 0, of 4% per year for patients with a score of 2 and of up to 18.2% per year for patients with a score of 6 (11). The CHA2DS2VASc score, which was recently incorporated into standard guidelines to more accurately identify patients at moderate risk who would most benefit from the use of anticoagulants, directly correlates with the same outcomes. Additionally, the stroke rate per year varies: 0% for those with a score of 0, 2.2% for those with a score of 2, and 15.2% for those with a score of 9 (12).
The prevalence of AF in our study population was higher than that found in other population-based studies. Whereas the Anticoagulation and Risk Factors In Atrial Fibrillation (ATRIA) study (24) showed a prevalence of 0.95% in the general population and whereas other studies showed values up to 1.49%, our data revealed a 4.78% prevalence, possibly because our patients were selected solely based on the indication for performing an ECG.
Regarding AF prevalence by sex and age our data are similar to data found in the literature, with higher rates in men of all age groups and with a gradual and proportional increase with the aging of the population.
It is estimated that AF patients over 60 years old with no structural heart disease present a cumulative risk of stroke of 1.3% per year (6,25). This rate can be significantly reduced with the proper use of anticoagulant therapy (26,27). In fact, several studies published in recent decades have shown the benefits of anticoagulation in patients with AF, with even better results than those of antiplatelet agents (1-3). Based on current guidelines, the non-indication of anticoagulants in patients with AF and a high risk of thromboembolic events can only be justified by a high risk of bleeding. However, we found no relationship between HAS-BLED bleeding scores and misuse of anticoagulants in our entire study population.
In our study, the location where the patient was monitored seemed to be directly related to misuse of oral anticoagulants, with very low use in basic health units and in private clinics. In contrast, patients in outpatient clinics at a tertiary hospital (HU-HMD) showed a higher prevalence of anticoagulation use when indicated. Another relevant factor was the specialty of the attending physician, with frequent prescription by cardiologists and very infrequent or absent prescription by other specialties.
The most likely explanation for this difference in anticoagulant use based on the location of monitoring may be the difficulty of returning to the same health unit for a dose adjustment of vitamin K inhibitors according to the patient's prothrombin time, causing the doctor to choose not to prescribe anticoagulation therapy. This is especially an issue for elderly patients, who often present with other comorbidities, including a higher risk of falls (28), and reach optimal anticoagulation levels at lower doses of vitamin K inhibitors (29). Therefore, their care is more limited, which may contribute to their doctors not prescribing treatments that they cannot properly monitor.
The use of new anticoagulants that do not require monitoring may help to improve this scenario; however, these drugs are not yet available to the Public Health System. Another potentially much more important factor may be the unawareness of doctors about the importance of this therapeutic intervention to prevent the mortality and morbidity associated with stroke in AF patients.
STUDY LIMITATIONS
Our study has important limitations. For example, the prevalence of AF in the selected population may have been underestimated because the diagnosis was made based on an ECG and not based on continuous monitoring (such as via a Holter monitor or loop recorder). Additionally, there were no data on patients with paroxysmal AF who presented with sinus rhythm on the ECG, for example. In particular, our population was selected because the patients were participating in the Public Health System. We believe that our data suggested a high prevalence of AF, chronic heart failure and stroke, among other conditions, compared with the prevalence in other populations because when the patients sought medical attention, they may have already been carriers of certain changes that could motivate an ECG request.
The data obtained in this study may not represent the whole of Brazil because the sample was limited to one hospital and one municipality, although it is important to note that the HU-HMD provides care to various levels of the Public Health System. However, our analysis may have incurred a measurement bias because the data were collected by telephone. In addition, we have no data regarding poor adherence. Thus, although anticoagulant therapy was prescribed, certain patients may not have used it. Considering the HAS-BLED score, it will be important to evaluate the use of anticoagulant therapy in patients with a high risk of bleeding, not only in the entire population but also in groups stratified by the specialty of the physician and the location where the ECG was first reviewed, given that the present analysis was hampered by the small number of patients within the groups.
Considering that AF is the most prevalent arrhythmia and given the high morbidity and mortality associated with stroke, it is possible that misuse of anticoagulants in this population is a major public health problem associated with the treatment of cardiac arrhythmias.
Our study found a low prevalence of oral anticoagulation therapy among patients with AF and an indication for stroke prophylaxis for the use of this therapy, including among those with high CHADS2 and CHA2DS2VASc scores.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Effect of low-dose varfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation the Boston area anticoagulation trial for atrial fibrillation investigators. N Engl J Med. 1990;323(22):1505–11. doi: 10.1056/NEJM199011293232201. [DOI] [PubMed] [Google Scholar]
- 2.Stroke prevention in atrial fibrillation investigators. Stroke prevention in atrial fibrillation study. Final results. Circulation. 1991;84(2):527–39. doi: 10.1161/01.cir.84.2.527. [DOI] [PubMed] [Google Scholar]
- 3.Petersen P, Boysen G, Godtfredsen J, Andersen ED, Andersen B. Placebo-controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK study. Lancet. 1989;1(8631):175–9. doi: 10.1016/s0140-6736(89)91200-2. [DOI] [PubMed] [Google Scholar]
- 4.Camm AJ, Lip GY, De Caterina R, Savelioeva I, Atar D, Hohnloser S, et al. 2012 focused update of ESC Guidelines for the management of atrial fibrillation – an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Eur Heart J. 2012;33(21):2719–47. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 5.Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104(11):1534–9. doi: 10.1016/j.amjcard.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Zimerman LI, Fenelon G, Martinelli Filho M, Grupi C, Atié J, Lorga Filho A, et al. Sociedade Brasileira de Cardiologia. Diretrizes Brasileiras de Fibrilação Atrial. Arq Bras Cardiol. 2009;92(6 supl.1):1–39. [Google Scholar]
- 7.Mansur AP, Favarato D, Avakian SD, Ramires JA. Trends in ischemic heart disease and stroke death ratios in Brazilian women and men. Clinics. 2010;65(11):1143–7. doi: 10.1590/S1807-59322010001100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singer DE, Chang Y, Fang MC, Borowsky LH, Pomernacki NK, Udaltsova N, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2009;151(5):297–304. doi: 10.7326/0003-4819-151-5-200909010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, et al. A comparasion of rate control and rhythm control in patients with atrial fibrillation. The AFFIRM Investigators. N Engl J Med. 2002;347(23):1825–33. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 10.Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, et al. Rhythm control versus rate control for atrial fibrillation in heart failure. N Engl J Med. 2008;358(25):2667–77. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 11.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 12.Lip GY, Frison L, Helperin JL, Lane DA. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010;41(12):2731–8. doi: 10.1161/STROKEAHA.110.590257. [DOI] [PubMed] [Google Scholar]
- 13.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboon J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 14.Wann LS, Curtis AB, January CT, Ellenbogen KA, Lowe JE, Estes NA, 3rd, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:104–23. doi: 10.1161/CIR.0b013e3181fa3cf4. [DOI] [PubMed] [Google Scholar]
- 15.Connolly SJ, Ezekowitz MD, Yusuf S, Reilly PA, Wallentin L. Newly identified events in the RE-LY trial. N Engl J Med. 2010;363(19):1875–6. doi: 10.1056/NEJMc1007378. [DOI] [PubMed] [Google Scholar]
- 16.ROCKET AF Study Investigators. Rivaroxaban-once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation: rationale and design of the ROCKET AF study. Am Heart J. 2010;159(3):340–7. doi: 10.1016/j.ahj.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 17.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 18.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson GH, Bjorholdt I, Krakau I. Anticoagulant treatment of patients with chronic atrial fibrillation in primary health care in Sweden - a retrospective study of incidence and quality in a registered population. Family Practice. 2004;21(6):612–6. doi: 10.1093/fampra/cmh606. [DOI] [PubMed] [Google Scholar]
- 20.Chae SH, Froehlich J, Morady F, Oral H. Prevalence and predictors of warfarin use in patients with atrial fibrillation at low or intermediate risk and relation to thromboembolic events. Clin. Cardiol. 2011;34(10):640–4. doi: 10.1002/clc.20967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filippi A, Bettoncelli G, Zaninelli A. Detected atrial fibrillation in North Italy: rates, calculated stroke risk and proportion of patients receiving thrombo-prophylaxis. Fam Pract. 2000;17(4):337–9. doi: 10.1093/fampra/17.4.337. [DOI] [PubMed] [Google Scholar]
- 22.Lakshminarayan K, Solid CA, Collins AJ, Anderson DC, Herzog CA. Atrial fibrillation and stroke in the general medicare population: a 10-year prospective (1992 to 2002) Stroke. 2006;37(8):1969–74. doi: 10.1161/01.STR.0000230607.07928.17. [DOI] [PubMed] [Google Scholar]
- 23.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 24.Go AS, Hylek EM, Philips KA, Chang YC, Henault LE, Selby JV, et al. Prevalence of Diagnosed Atrial Fibrillation in Adults. National Implications for Rhythm Management and Stroke Prevention: the AnTicoagulation and Risk Factors In Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 25.Levy S. Classification system of atrial fibrillation. Curr Opin Cardiol. 2000;15(1):54–7. doi: 10.1097/00001573-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Connolly SJ, Laupacis A, Gent M, Roberts RS, Cairns JA, Joyner C, et al. Canadian Atrial Fibrillation Anticoagulation (CAFA) Study. J Am Coll Cardiol. 1991;18(2):349–55. doi: 10.1016/0735-1097(91)90585-w. [DOI] [PubMed] [Google Scholar]
- 27.Gorter JW. Major bleeding during anticoagulation after cerebral ischemia: patterns and risk factors. Stroke Prevention in Reversible Ischemia Trial (SPIRIT) and European Atrial Fibrillation Trial (EAFT) study groups. Neurology. 1999;53(6):1319–25. doi: 10.1212/wnl.53.6.1319. [DOI] [PubMed] [Google Scholar]
- 28.Santos AC, Nobre MR, Nussbacher A, Rodrigues GH, Gebara OC, Azul JB, et al. Predictors of the risk of falls among elderly with chronic atrial fibrillation. Clinics. 2012;67(4):305–11. doi: 10.6061/clinics/2012(04)02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mansur AP, Takada JY, Avakian SD, Strunz CM. Warfarin doses for anticoagulation therapy in elderly patients with chronic atrial fibrillation. Clinics. 2012;67(6):543–6. doi: 10.6061/clinics/2012(06)01. [DOI] [PMC free article] [PubMed] [Google Scholar]