Abstract
Background
Right ventricular (RV) dysfunction in ischemic cardiomyopathy (ICM) is associated with poor prognosis, but RV assessment by conventional echocardiography remains difficult. We sought to validate RV global longitudinal strain (RVGLS) and global longitudinal strain rate (RVGLSR) against cardiac magnetic resonance (CMR) and outcome in ICM.
Methods
In 57 patients (43 men, 64 ± 12 years) with ICM who underwent conventional and strain echocardiography and CMR, RVGLS and RVGLSR were measured off-line. RV dysfunction was determined by CMR [RV ejection fraction (RVEF) < 50%]. Patients were followed over 15 ± 9 months for a composite of death and hospitalization for worsening heart failure.
Results
RVGLS showed significant correlations with CMR RVEF (r = -0.797, p < 0.01), RV fractional area change (RVFAC, r = -0.530, p < 0.01), and tricuspid annular plane systolic excursion (TAPSE, r = -0.547, p < 0.01). RVGLSR showed significant correlations between CMR RVEF (r = -0.668, p < 0.01), RVFAC (r = -0.394, p < 0.01), and TAPSE (r = -0.435, p < 0.01). RVGLS and RVGLSR showed significant correlations with pulmonary vascular resistance (r = 0.527 and r = 0.500, p < 0.01, respectively). The best cutoff value of RVGLS for detection of RV dysfunction was -15.4% [areas under the curve (AUC) = 0.955, p < 0.01] with a sensitivity of 81% and specificity 95%. The best cutoff value for RVGLSR was -0.94 s-1 (AUC = 0.871, p < 0.01), sensitivity 72%, specificity 86%. During follow-up, there were 12 adverse events. In Cox-proportional hazard regression analysis, impaired RVGLS [hazard ratio (HR) = 5.46, p = 0.030] and impaired RVGLSR (HR = 3.95, p = 0.044) were associated with adverse clinical outcome.
Conclusion
Compared with conventional echocardiographic parameters, RVGLS and RVGLSR correlate better with CMR RVEF and outcome.
Keywords: Right ventricle, Systolic dysfunction, Strain echocardiography, Cardiac magnetic resonance image
Introduction
Right ventricular (RV) dysfunction is associated with reduced exercise capacity and poor prognosis in patients with dilated1),2),3) and ischemic cardiomyopathy (ICM).4) Assessment of RV function and the identification of RV dysfunction are important for the prediction of prognosis.5) However, RV assessment by conventional 2-dimensional imaging is difficult because of the complex shape of this chamber. Attempts to overcome this challenge with non-echocardiographic techniques have been based on gathering sufficient 2-dimensional images to allow measurement independent of geometric assumptions (cardiac magnetic resonance), 3-dimensional acquisition and analysis (single photon computed tomography) and measurement techniques independent of tracing the RV cavity.6),7),8)
However, echocardiography remains the most common first-line test in these patients. Despite the validation of 3-dimensional echocardiography for this purpose, it remains technically difficult, in part because of the difficulty in gathering the entire RV in a 3-dimensional data set.8) Strain imaging has been used to evaluate global left ventricular (LV) function,9) and could provide a non-geometric approach to RV assessment. Unlike tissue velocity-based strain and strain rate imaging, 2-dimensional strain imaging using speckle tracking can provide an angle-independent quantitative measurement.10) In this study, we sought to validate RV global longitudinal strain (RVGLS) and RV global longitudinal strain rate (RVGLSR) against cardiac magnetic resonance imaging (CMR) and find its association with outcomes in ICM patients.
Methods
Patient characteristics
We retrospectively screened 72 consecutive patients with ICM who underwent conventional and strain echocardiography and CMR from September 2009 to October 2011. Inclusion criteria of ICM patients were 1) dyspnea on exertion (New York Heart Association functional class ≥ II), 2) impaired LV function (LV ejection fraction < 40%), and 3) significant epicardial coronary artery stenosis (≥ 50%) by the conventional coronary angiography. All patients had a history of previous myocardial infarction (MI). Exclusion criteria included age > 80 years, severe tricuspid regurgitation (TR), atrial fibrillation, RV pacing, or poor image quality of RV echocardiographic images without inclusion of whole RV free wall. The study was approved by the Institutional Review Board of Cleveland Clinic.
Echocardiography
Standard echocardiographic examinations with Doppler studies were performed using commercially available echocardiographic systems (Vivid 7, GE Vingmed, Horten, Norway or ie33, Philips, Andover, MA, USA). The echocardiographic images of all subjects were obtained from the parasternal and apical views. Studies were stored digitally and analyzed off-line. RV fractional area change (RVFAC) was calculated from the apical 4-chamber view using the percentage change in areas of the end-diastolic and end-systolic areas of the RV.11) Tricuspid annular plane systolic excursion (TAPSE) was measured the distance of systolic movement of RV annular segment along its longitudinal plane.11) RV myocardial performance (Tei) index was defined as the ratio of isovolumic relaxation time (IVRT) and isovolumic contraction time (IVCT) divided by ejection time (ET) of RV, or (IVRT + IVCT) / ET.11)
Pulmonary artery systolic pressure was estimated from the maximal continuous-wave Doppler velocity of the TR jet plus estimated central venous pressure.11) An index of pulmonary vascular resistance (PVR) was derived by dividing the maximal velocity of the TR jet by the RV outflow tract velocity-time integral.12) An average of 3 measurements was used.
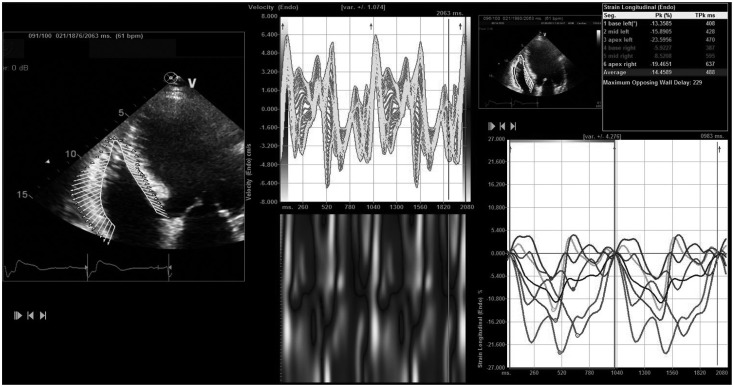
RVGLS was measured off-line with velocity vector imaging (VVI; Axius, Siemens Medical Solutions, Mountain View, CA, USA). The B-mode images of apical 4-chamber views were used for strain measurement. They were obtained and digitally stored in cineloop format with about 60 frames/sec for the offline analysis. After manual tracing of the endocardial border of RV over one frame, then endocardial borders were automatically tracked throughout the cardiac cycle (Fig. 1). Myocardial velocity is derived as the ratio between frame-to-frame displacement of the speckles and the time interval.10) RVGLS was obtained and averaged for all 6 segments of RV free wall and septum. Global longitudinal strain of RV free wall was measured for 3 RV segments (basal, mid, and apex) and ventricular septum for 3 septal segments. The same apical 4-chamber images used for conventional RV measurements were analyzed with VVI by an investigator who was blinded to the conventional echocardiography and CMR data.
Fig. 1.
Representative figure for the measurement of right ventricular longitudinal strain with velocity vector imaging software.
CMR studies
CMR examinations in the patient with sinus rhythm were performed on standard 1.5 T MR scanners (Sonata or Avanto, Siemens Medical Solutions, Erlangen, Germany) with electrocardiographic gating. The time difference between echocardiography and CMR acquisition was 6 ± 10 days. Scout images were acquired initially to identify the cardiac axes. Subsequently, we assessed global RV ejection fraction (RVEF) using multiple long- and short-axis views. RV dysfunction determined by CMR was defined as RVEF < 50%.
Subsequently, delayed hyperenhancement images were obtained to identify scar lesion of the RV in the same long- and short-axis orientations as the above-described images approximately 20 minutes after injection of 0.2 mmol/kg of gadolinium dimeglumine.
Outcomes
Patients were followed over 15 ± 9 months for a composite of death, cardiac hospitalization due to heart failure, and heart transplantation.
Reproducibility
Intraobserver and interobserver variabilities of the RVGLS were evaluated in 15 random subjects by two investigators (J.P. and K.N.) and measured by calculating the intraclass correlation coefficients.
Statistical analysis
The data were analyzed using standard software (SPSS version 18.0, SPSS Inc., Chicago, IL, USA). Summary data were expressed as mean values ± standard deviation or percentage of patients. Linear regression analysis was performed to evaluate the relationship between RVGLS and RVGLSR and other variables. The optimal cutoff value for predicting RV dysfunction defined by CMR RVEF was determined by the receiver-operating characteristic curve analysis. Comparison of areas under the curve (AUC) was done with the method suggested by Hanley and McNeil.13) To assess prognostic variables, we performed a Cox proportional hazards regression with the combined end point of death, hospital admission for heart failure and heart transplantation. To exclude over-fit of the analysis model, we calculated EuroSCORE II in these patients and used it to the analysis.14)
Cumulative event-free survival curves for adverse events were constructed according to the Cox-proportional hazard regression analysis. A p value less than 0.05 was considered statistically significant.
Results
Patient characteristics
Initially, we screened 72 patients with ICM. Of them, 11 patients were excluded for poor echocardiographic image quality and 4 were excluded because of the presence of atrial fibrillation. A total 57 patients (43 males, mean age 64 ± 12 years old) were included in this study. Their baseline clinical, routine echocardiographic and CMR data (Table 1) are typical for an ICM population, with multiple risk factors, LV enlargement and moderate to severe LV dysfunction. RV dysfunction was present in 63% of patients.
Table 1.
Baseline characteristics
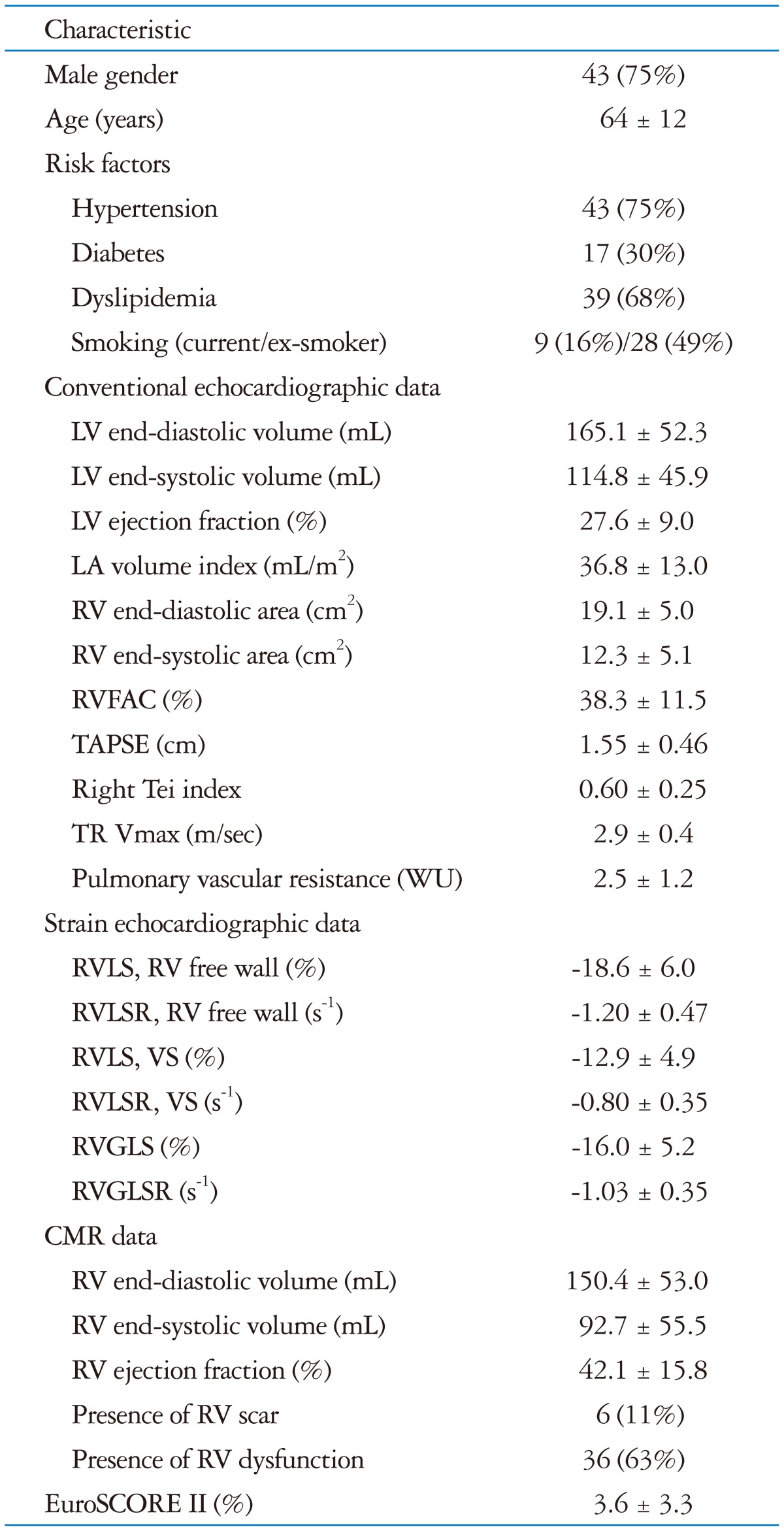
LV: left ventricle, LA: left atrial, RV: right ventricle, RVFAC: right ventricular fractional area change, TAPSE: tricuspid annular plane systolic excursion, TR: tricuspid regurgitation, RVLS: right ventricular longitudinal strain, RVLSR: right ventricular longitudinal strain rate, RVGLS: right ventricular global longitudinal strain, RVGLSR: right ventricular global longitudinal strain rate, VS: ventricular septum, CMR: cardiac magnetic resonance imaging, WU: wood unit
RV strain findings
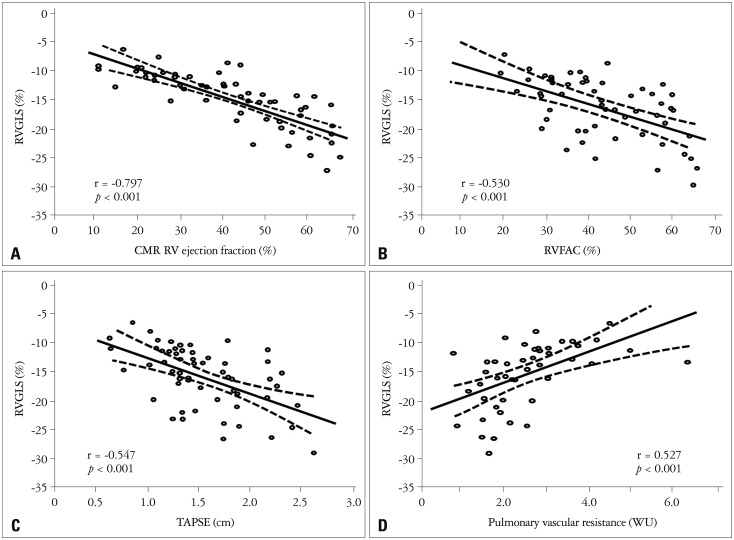
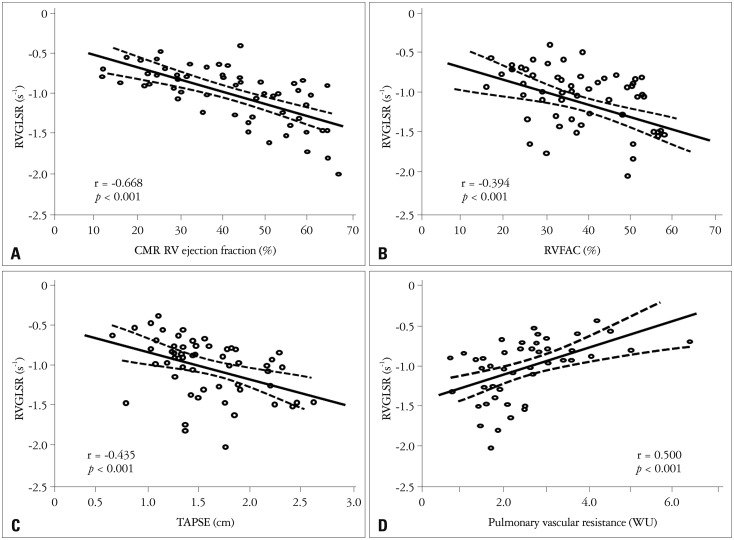
Global and regional two-dimensional strain measurements are listed in Table 1. RVGLS showed significant correlations with CMR RVEF (r = -0.797, p < 0.001), RVFAC (r = -0.530, p < 0.001), and TAPSE (r = -0.547, p < 0.001) (Fig. 2). RVGLSR showed significant correlations between CMR RVEF (r = -0.668, p < 0.01), RVFAC (r = -0.394, p < 0.01), and TAPSE (r = -0.435, p < 0.01) (Fig. 3A, B, and C). RVGLS and RVGLSR showed significant correlations with PVR (r = 0.527, Fig. 2D and r = 0.500, p < 0.01, Fig. 3D, respectively), but not pulmonary artery systolic pressure.
Fig. 2.
Correlations between right ventricular global longitudinal strain (RVGLS) and cardiac magnetic resonance image (CMR) and echocardiographic parameters. RVGLS shows good negative correlation with CMR RV ejection fraction (A), RV fractional area change (RVFAC, B), and tricuspid annular plane systolic excursion (TAPSE, C). Also, RVGLS demonstrates significant correlation with pulmonary vascular resistance (D). WU: wood unit.
Fig. 3.
Correlations between right ventricular global longitudinal strain rate (RVGLSR) and cardiac magnetic resonance image (CMR) and echocardiographic parameters. RVGLSR shows good negative correlation with CMR RV ejection fraction (A), RV fractional area change (RVFAC, B), and tricuspid annular plane systolic excursion (TAPSE, C). Also, RVGLSR demonstrates significant correlation with pulmonary vascular resistance (D). WU: wood unit.
CMR findings
CMR RVEF showed significant correlations with conventional echocardiographic RV parameters including RVFAC (r = 0.500, p < 0.01), RV Tei index (r = -0.446, p < 0.01) and TAPSE (r = 0.360, p < 0.01). There were 36 patients with RV dysfunction determined by CMR (RVEF less than 50%). Patients with RV scar on CMR showed poor RV systolic function, lower strain and strain rate (Table 2). The best cutoff value of RVGLS for detection of RV dysfunction was -15.4% (AUC = 0.955, p < 0.01) with a sensitivity of 81% and specificity 95%. The best cutoff value for RVGLSR was -0.94 (AUC = 0.871, p < 0.01), sensitivity 72%, specificity 86%. RVGLS was more accurate in the detection of RV dysfunction than RVFAC [difference = 0.229, 95% confidential interval (CI): 0.094-0.364, p < 0.01], TAPSE (difference = 0.215, 95% CI: 0.086-0.344, p < 0.01), RV Tei index (difference = 0.252, 95% CI: 0.097-0.406, p < 0.01), and RVGLSR (difference = 0.083, 95% CI: 0.010-0.157, p = 0.03) (Table 3).
Table 2.
Comparison of conventional and strain echocardiographic data according to the presence of scar in the right ventricle
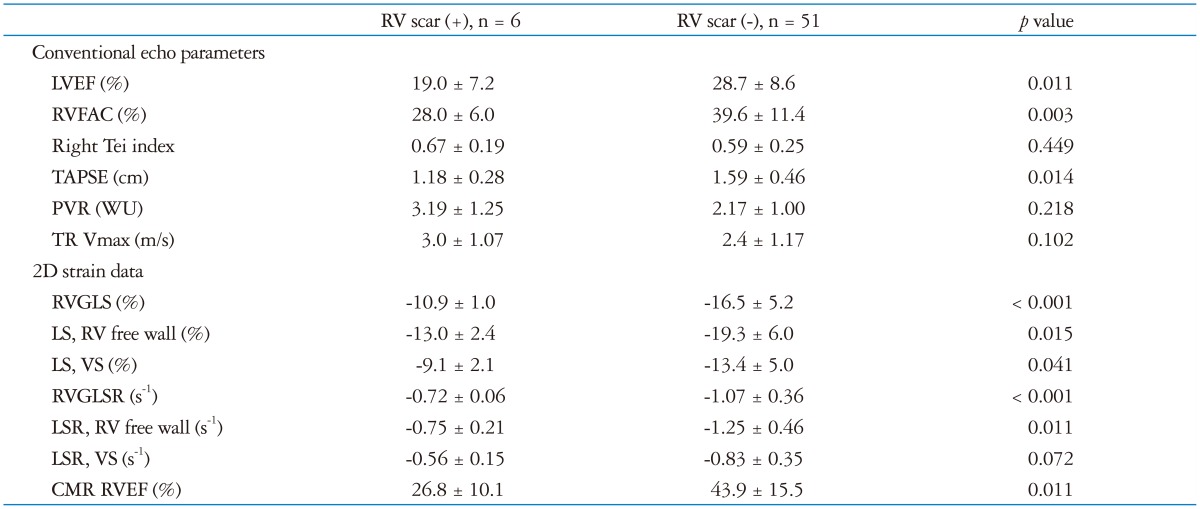
LVEF: left ventricular ejection fraction, RVFAC: right ventricular fractional area change, TAPSE: tricuspid annular plane systolic excursion, PVR: pulmonary vascular resistance, TR: tricuspid regurgitation, 2D: 2-dimensional, RVGLS: right ventricular global longitudinal strain, LS: longitudinal strain, VS: ventricular septum, RVGLSR: right ventricular global longitudinal strain rate, LSR: longitudinal strain rate, CMR: cardiac magnetic resonance imaging, RVEF: right ventricular ejection fraction, WU: wood unit
Table 3.
Best cut off value and area under the curve to detect right ventricular dysfunction according to the parameters
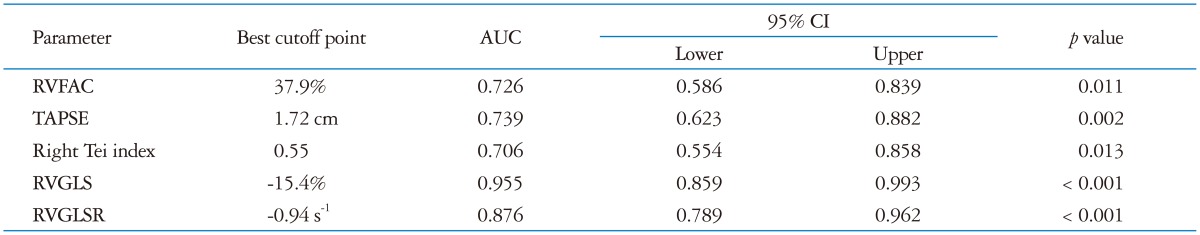
AUC: areas under the curve, CI: confidential interval, RVFAC: right ventricular fractional area change, TAPSE: tricuspid annular plane systolic excursion, RVGLS: right ventricular global longitudinal strain, RVGLSR: right ventricular global longitudinal strain rate
Follow-up
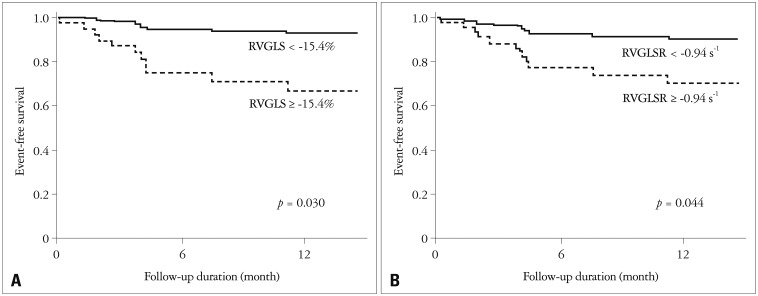
During the follow-up of 15 ± 9 months, there were 12 adverse events including 5 deaths, and 7 hospitalization for heart failure. After univariate analysis by Cox-proportional hazard regression analysis, PVR, decreased TAPSE (< 1.72 cm), and impaired RVGLS (≥ -15.4%) were associated with adverse clinical outcome. After adjustment with EuroSCORE II, decreased RVGLS and RVGLSR were statistically significant (Table 4). Patients with decreased RVGLS and RVGLSR showed significantly lower 1 year event-free survival rate (Fig. 4).
Table 4.
Unadjusted hazard ratio and EuroSCORE II adjusted hazard ratio in the prediction of adverse clinical events
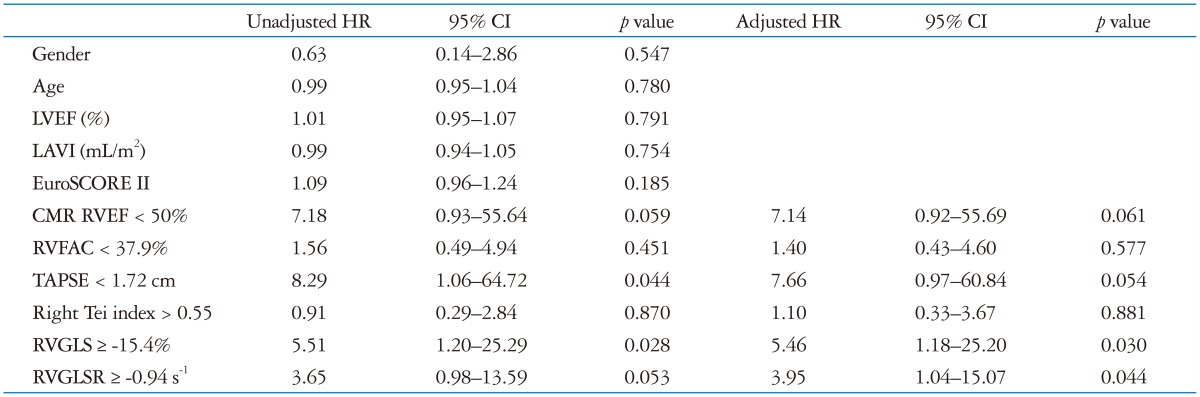
HR: hazard ratio, CI: confidential interval, LVEF: left ventricular ejection fraction, LAVI: left atrial volume index, CMR: cardiac magnetic resonance imaging, RVEF: right ventricular ejection fraction, RVFAC: right ventricular fractional area change, TAPSE: tricuspid annular plane systolic excursion, RVGLS: right ventricular global longitudinal strain, RVGLSR: right ventricular global longitudinal strain rate
Fig. 4.
EuroSCORE II adjusted event free survival by Cox proportional regression analysis. Patients with impaired right ventricular global longitudinal strain (RVGLS) shows lower 1 year event-free survival (93.0% vs. 67.2%, p = 0.030, A). Also, impaired right ventricular global longitudinal strain rate (RVGLSR) group reveals lower 1 year event-free survival (90.5% vs. 67.4%, p = 0.044, B).
Variability
Interobserver variability of RVGLS was small [intraclass correlation coefficient was 0.909 (95% CI = 0.753-0.968), p < 0.001], and similar to intraobserver [0.904 (95% CI = 0.746-0.966), p < 0.01].
Discussion
The main findings of this study are that RVGLS and RVGLSR are the good echocardiographic correlates of RVEF measured by CMR, and decreased RVGLS and RVGLSR are associated with lower 1 year event-free survival.
Detection of RV dysfunction
LV systolic function is a well-known predictor of future cardiovascular morbidity and mortality in patients with MI.15) The presence of RV dysfunction is associated with poor clinical outcome in patients with LV dysfunction after an acute MI.4) Moreover, quantitative measurement of RV size and function is important in the prediction of clinical outcomes in a number of pulmonary diseases.11) However, quantitative assessment of RV function is often neglected and the assessment of RV by conventional echocardiography remains difficult because of the complex shape of the chamber.11) Although several two-dimensional echocardiographic measurements of RV function have been proposed, including RV diameter and two-dimensional area.16) RVFAC is a widely used index of RV function, but despite its relatively good correlation with CMR and radionuclide RVEF,16),17) the feasibility of RVFAC is limited because of the challenge of tracing the entire RV endocardium in end-diastole and end-systole.16),17),18) In our study, RVFAC showed significant correlations with RVEF by CMR, but the degree of correlation was moderate (r = 0.500, p < 0.001).
In the present study, CMR was used as reference in the assessment of RV function. CMR has been validated in several previous studies.19),20) CMR demonstrates an excellent reproducibility for assessment of RV function and RV volumes.6),20) Despite the excellent image quality and reproducibility, long acquisition time, time-consuming analysis and not suitable for some patients unable to take examination are major disadvantages of CMR.21)
Strain imaging
The application of speckle tracking to assessment of global chamber function is based on the understanding that GLS is the result of the sum of all segmental deformation. The use of speckle tracking for the assessment of regional and global LV function has been validated by sonomicrometry,22),23) and this method has been shown to provide prognostic information that is incremental to EF.24)
Speckle strain has been introduced for the assessment of the RV in some disease categories,10),25),26) but has not previously been validated in patients with ICM. Because the muscle fibers of the RV predominantly run in a longitudinal direction and the major contractility of RV occurs in the longitudinal plane,6),27) GLS can represent RV function accurately. Moreover, strain has the advantage of distinguishing true contractility of the free wall rather than tethering or translational motion which can influence indices like TAPSE. This may be important not only in ICM, but also in idiopathic dilated cardiomyopathy with longitudinal rotation of the heart (swinging motion of the heart).25),28) In this study, we found RVGLS of -15.4% and RVGLSR of -0.94 s-1 to be the optimal cut-point for the detection of RV systolic dysfunction. Using these criteria, the detection of RV systolic dysfunction was more accurate than using other conventional RV echocardiographic parameters including RVFAC, TAPSE, and RV Tei index. Decreased RVGLS (≥ -15.4%) and RVGLSR (≥ -0.94 s-1) was associated with lower 1 year event free survivals compared with preserved group irrespective of the LV systolic function.
There are several proprietary approaches to the measurement of myocardial strain. VVI is a validated technique for assessment of LV function,10) which has several advantages in the assessment of ventricular function. Like all 2-dimensional strain approaches, its measurement of myocardial contractility is angle-independent.10),22) It can determine global and regional myocardial function simultaneously and is able to analyze archived digital images.10) VVI seems to be less sensitive than other methods to wall thickness, possibly because of a greater contribution of wall tracking to the strain algorithm. We have previously shown that VVI may be superior to other speckle strain techniques in the assessment of atrial function,29) perhaps reflecting less dependence of tracking on a thick wall.
Like all contraction-phase measurements, RVGLS is significantly influenced by afterload. In this study, this was evidenced by correlation with PVR. Increased PVR, as a result of increased afterload, is associated with increases in RV size and RV dysfunction. Indeed, these findings may be concordant with previously published data showing decreased event-free survival in patients with increased PVR.7)
Limitations
This is an observational study with analysis of relatively small numbers of stored digital images. Bias may have been introduced from patient selection. Analysis may have been affected by image quality-to minimize this potential contribution to error, we excluded the patients with poor image quality and analyzed standard apical four chamber images. Second, we used echocardiographic images of different machines from two vendors in this strain analysis. Therefore, cut-off value of RVGLS and RVGLSR provided in this study cannot be applied to all speckle tracking analysis results in other clinical situations. Nonetheless, a prospective study with large number of patients will be needed to confirm the correlations and the clinical impact of this measurement.
Conclusion
RVGLS and RVGLSR correlate better with CMR RVEF than conventional echocardiographic parameters. Lower RVGLS and RVGLSR are associated with adverse clinical outcome in patients with ICM.
References
- 1.Di Salvo TG, Mathier M, Semigran MJ, Dec GW. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol. 1995;25:1143–1153. doi: 10.1016/0735-1097(94)00511-n. [DOI] [PubMed] [Google Scholar]
- 2.Juillière Y, Barbier G, Feldmann L, Grentzinger A, Danchin N, Cherrier F. Additional predictive value of both left and right ventricular ejection fractions on long-term survival in idiopathic dilated cardiomyopathy. Eur Heart J. 1997;18:276–280. doi: 10.1093/oxfordjournals.eurheartj.a015231. [DOI] [PubMed] [Google Scholar]
- 3.de Groote P, Millaire A, Foucher-Hossein C, Nugue O, Marchandise X, Ducloux G, Lablanche JM. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol. 1998;32:948–954. doi: 10.1016/s0735-1097(98)00337-4. [DOI] [PubMed] [Google Scholar]
- 4.Zornoff LA, Skali H, Pfeffer MA, St John Sutton M, Rouleau JL, Lamas GA, Plappert T, Rouleau JR, Moyé LA, Lewis SJ, Braunwald E, Solomon SD SAVE Investigators. Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction. J Am Coll Cardiol. 2002;39:1450–1455. doi: 10.1016/s0735-1097(02)01804-1. [DOI] [PubMed] [Google Scholar]
- 5.Oldershaw P. Assessment of right ventricular function and its role in clinical practice. Br Heart J. 1992;68:12–15. doi: 10.1136/hrt.68.7.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tandri H, Daya SK, Nasir K, Bomma C, Lima JA, Calkins H, Bluemke DA. Normal reference values for the adult right ventricle by magnetic resonance imaging. Am J Cardiol. 2006;98:1660–1664. doi: 10.1016/j.amjcard.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 7.Mannaerts HF, van der Heide JA, Kamp O, Stoel MG, Twisk J, Visser CA. Early identification of left ventricular remodelling after myocardial infarction, assessed by transthoracic 3D echocardiography. Eur Heart J. 2004;25:680–687. doi: 10.1016/j.ehj.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 8.Kjaergaard J, Petersen CL, Kjaer A, Schaadt BK, Oh JK, Hassager C. Evaluation of right ventricular volume and function by 2D and 3D echocardiography compared to MRI. Eur J Echocardiogr. 2006;7:430–438. doi: 10.1016/j.euje.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Jamal F, Bergerot C, Argaud L, Loufouat J, Ovize M. Longitudinal strain quantitates regional right ventricular contractile function. Am J Physiol Heart Circ Physiol. 2003;285:H2842–H2847. doi: 10.1152/ajpheart.00218.2003. [DOI] [PubMed] [Google Scholar]
- 10.Pirat B, McCulloch ML, Zoghbi WA. Evaluation of global and regional right ventricular systolic function in patients with pulmonary hypertension using a novel speckle tracking method. Am J Cardiol. 2006;98:699–704. doi: 10.1016/j.amjcard.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 11.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 786-8. [DOI] [PubMed] [Google Scholar]
- 12.Abbas AE, Fortuin FD, Schiller NB, Appleton CP, Moreno CA, Lester SJ. A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol. 2003;41:1021–1027. doi: 10.1016/s0735-1097(02)02973-x. [DOI] [PubMed] [Google Scholar]
- 13.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 14.Biancari F, Vasques F, Mikkola R, Martin M, Lahtinen J, Heikkinen J. Validation of EuroSCORE II in patients undergoing coronary artery bypass surgery. Ann Thorac Surg. 2012;93:1930–1935. doi: 10.1016/j.athoracsur.2012.02.064. [DOI] [PubMed] [Google Scholar]
- 15.Risk stratification and survival after myocardial infarction. N Engl J Med. 1983;309:331–336. doi: 10.1056/NEJM198308113090602. [DOI] [PubMed] [Google Scholar]
- 16.Kaul S, Tei C, Hopkins JM, Shah PM. Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J. 1984;107:526–531. doi: 10.1016/0002-8703(84)90095-4. [DOI] [PubMed] [Google Scholar]
- 17.Schenk P, Globits S, Koller J, Brunner C, Artemiou O, Klepetko W, Burghuber OC. Accuracy of echocardiographic right ventricular parameters in patients with different end-stage lung diseases prior to lung transplantation. J Heart Lung Transplant. 2000;19:145–154. doi: 10.1016/s1053-2498(99)00121-7. [DOI] [PubMed] [Google Scholar]
- 18.Hinderliter AL, Willis PW, 4th, Long WA, Clarke WR, Ralph D, Caldwell EJ, Williams W, Ettinger NA, Hill NS, Summer WR, de Boisblanc B, Koch G, Li S, Clayton LM, Jöbsis MM, Crow JW PPH Study Group. Frequency and severity of tricuspid regurgitation determined by Doppler echocardiography in primary pulmonary hypertension. Am J Cardiol. 2003;91:1033–1037. A9. doi: 10.1016/s0002-9149(03)00136-x. [DOI] [PubMed] [Google Scholar]
- 19.Beygui F, Furber A, Delépine S, Helft G, Metzger JP, Geslin P, Le Jeune JJ. Routine breath-hold gradient echo MRI-derived right ventricular mass, volumes and function: accuracy, reproducibility and coherence study. Int J Cardiovasc Imaging. 2004;20:509–516. doi: 10.1007/s10554-004-1097-7. [DOI] [PubMed] [Google Scholar]
- 20.Grothues F, Moon JC, Bellenger NG, Smith GS, Klein HU, Pennell DJ. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am Heart J. 2004;147:218–223. doi: 10.1016/j.ahj.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Bleeker GB, Steendijk P, Holman ER, Yu CM, Breithardt OA, Kaandorp TA, Schalij MJ, van der Wall EE, Nihoyannopoulos P, Bax JJ. Assessing right ventricular function: the role of echocardiography and complementary technologies. Heart. 2006;92(Suppl 1):i19–i26. doi: 10.1136/hrt.2005.082503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amundsen BH, Helle-Valle T, Edvardsen T, Torp H, Crosby J, Lyseggen E, Støylen A, Ihlen H, Lima JA, Smiseth OA, Slørdahl SA. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol. 2006;47:789–793. doi: 10.1016/j.jacc.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 23.Toyoda T, Baba H, Akasaka T, Akiyama M, Neishi Y, Tomita J, Sukmawan R, Koyama Y, Watanabe N, Tamano S, Shinomura R, Komuro I, Yoshida K. Assessment of regional myocardial strain by a novel automated tracking system from digital image files. J Am Soc Echocardiogr. 2004;17:1234–1238. doi: 10.1016/j.echo.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2:356–364. doi: 10.1161/CIRCIMAGING.109.862334. [DOI] [PubMed] [Google Scholar]
- 25.Verhaert D, Mullens W, Borowski A, Popović ZB, Curtin RJ, Thomas JD, Tang WH. Right ventricular response to intensive medical therapy in advanced decompensated heart failure. Circ Heart Fail. 2010;3:340–346. doi: 10.1161/CIRCHEARTFAILURE.109.900134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puwanant S, Park M, Popović ZB, Tang WH, Farha S, George D, Sharp J, Puntawangkoon J, Loyd JE, Erzurum SC, Thomas JD. Ventricular geometry, strain, and rotational mechanics in pulmonary hypertension. Circulation. 2010;121:259–266. doi: 10.1161/CIRCULATIONAHA.108.844340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cresci SG, Goldstein JA. Hemodynamic manifestations of ischemic right heart dysfunction. Cathet Cardiovasc Diagn. 1992;27:28–33. doi: 10.1002/ccd.1810270107. discussion 33-4. [DOI] [PubMed] [Google Scholar]
- 28.Popović ZB, Grimm RA, Ahmad A, Agler D, Favia M, Dan G, Lim P, Casas F, Greenberg NL, Thomas JD. Longitudinal rotation: an unrecognised motion pattern in patients with dilated cardiomyopathy. Heart. 2008;94:e11. doi: 10.1136/hrt.2007.122192. [DOI] [PubMed] [Google Scholar]
- 29.Motoki H, Dahiya A, Bhargava M, Wazni OM, Saliba WI, Marwick TH, Klein AL. Assessment of left atrial mechanics in patients with atrial fibrillation: comparison between two-dimensional speckle-based strain and velocity vector imaging. J Am Soc Echocardiogr. 2012;25:428–435. doi: 10.1016/j.echo.2011.12.020. [DOI] [PubMed] [Google Scholar]