Abstract
Metalloregulatory proteins allow cells to sense metal ions and appropriately adjust the expression of metal uptake, storage, and efflux pathways. Bacillus subtilis provides a model for the coordinate regulation of iron and manganese homeostasis that involves three key regulators: Fur senses iron sufficiency, MntR senses manganese sufficiency, and PerR senses the intracellular Fe/Mn ratio. Here, I review the structural and physiological bases of selective metal perception, the effects of non-cognate metals, and mechanisms that may serve to coordinate iron and manganese homeostasis.
Keywords: Bacillus, Gene Regulation, Iron, Iron Metabolism, Manganese, Metal Homeostasis, Metal Ion-Protein Interaction, Repressor Protein, Zinc
Manganese and Iron: Chemically Similar Elements with Distinct Roles in Cell Physiology
Manganese and iron are both predominantly present as divalent cations in the reducing environment of the cytoplasm and are maintained at overall concentrations (averaged over the cell) that can approach 0.5 mm (1–3). Much of this manganese and iron is bound by enzymes that acquire metal from a kinetically accessible (labile) pool buffered in the low μm range (4–6). These two metals are not only important from the perspective of individual microbial cells, but have a global impact due to their central roles in photosynthesis, nitrogen fixation, and other key steps in elemental cycling.
Nearly all cellular life requires significant amounts of iron. The sole documented exceptions are the lactobacilli and the spirochete Borrelia burgdorferi, which have little if any requirement for iron (3). Coincident with this disregard for iron, these same organisms have high requirements for manganese and have been described as manganese-centric (7). Conversely, other organisms have little demonstrable requirement for manganese, although they may use manganese when available. The best characterized is Escherichia coli, which has a largely iron-centric metabolism, but conditionally imports manganese in response to oxidative stress (8).
Here, I review the key factors regulating iron and Mn homeostasis in the model organism Bacillus subtilis. B. subtilis encodes three members of the Fur2 family (Fur, PerR, and Zur) and one member of the DtxR/MntR family (MntR) that regulate the import of nutrient metal ions (9–11). Structural and biochemical studies have provided insights into the mechanisms of selective activation of these repressors by their cognate metal ions (12–14). B. subtilis requires both iron and manganese for growth, a property shared with many pathogens for which the ability to obtain these metals from the host is a critical determinant of virulence (7, 15–17).
Metalloregulators as a Window into Cellular Physiology
Metalloregulators function as the arbiters of metal ion sufficiency and excess (6, 12, 18). For those that sense sufficiency, the affinity (measured as Kd) for their cognate metal(s) defines the level of metal judged to be sufficient. When free metal concentrations rise above this level, the regulator transitions from its inactive (apo-) form to its activated (holo-) form and represses expression of metal import. In B. subtilis, Fur serves as the primary sensor of iron sufficiency (19), and MntR monitors manganese sufficiency (11), with both Mn2+ and Fe2+ pools buffered to the low μm range (4).
The ability of metalloregulators to respond selectively to their cognate metals relies on a complex interplay of multiple factors that can be succinctly described as affinity, access, and allostery (5). Affinity is largely determined by the nature and geometry of the metal-binding ligands. However, in agreement with the Irving-Williams series, sensors of Mn2+ and Fe2+ typically bind Zn2+ with an affinity greater than their cognate metals. Nevertheless, Zn2+ does not elicit repression because cellular levels of free Zn2+ are maintained far below those for Fe2+ and Mn2+ (4). This highlights the second key determinant of selectivity: response to metals depends on access, and this can vary drastically between organisms. Finally, non-cognate metals may be discriminated against after binding: they may fail to elicit a genetic response due to an inability to trigger allostery.
Biochemical measurements can provide insights into the affinity of metalloregulators for metal ions. Direct measurements of metal binding in the absence of DNA are likely most relevant for metal-activated repressors that bind DNA subsequent to metal binding. Because of the thermodynamic coupling (coupling free energy) between metal and DNA binding, binding to DNA influences metal affinity to the same degree that metal binding influences DNA affinity (6). This is of particular relevance when considering sensors of metal excess that bind DNA as apo-proteins and dissociate upon metal binding. In this case, the functional metal sensor (DNA-bound apo-protein) will bind metal with an affinity significantly lower than measured for the apo-protein in solution.
Studies of metalloregulators, and in particular the identification of their target genes, also provide insights into metal physiology. In addition to the derepression of uptake systems, metal limitation may activate pathways to repress the synthesis of some metalloenzymes and to induce alternative enzymes to replace those whose activity becomes compromised when their metal cofactor is limiting (20). For example, when B. subtilis is zinc-limited, derepression of the Zur regulon leads to induction of an alternative, non-zinc-dependent enzyme required for folate biosynthesis (21). Indeed, sophisticated mechanisms to bypass what would otherwise be metabolic bottlenecks created by limitation for specific elements are legion (20).
Metal Ion Speciation in the Cell
The total metal content (quota) of the cell is distributed among ligands with a wide range of affinities and kinetic lability, from comparatively tight binding metalloproteins to both low molecular weight (LMW) and macromolecular anions. In both E. coli and Bacillus anthracis, the largest pools of protein-associated iron are in iron-superoxide dismutase (Fe-SOD) and oxidized to ferric hydroxide within ferritins and mini-ferritins (Dps family proteins) (22, 23). Once oxidized, stored iron no longer contributes to the labile iron pool. Mn-SOD may be a dominant pool of protein-bound manganese in Bacilli (23).
The labile pool can be defined as that portion of the metal quota that is available for equilibration with metalloregulators. The labile manganese pool is likely buffered as an LMW pool bound to phosphate, nucleotides, peptides, and organic acids (24, 25), including the abundant metabolite fructose 1,6-diphosphate (26). The labile iron pool is thought to be largely chelated by LMW thiols such as glutathione, with a lesser contribution from citrate and other organic acids (27).
The labile manganese and iron pools may represent a significant fraction of the total metal quota. Indeed, metallated enzymes may comprise part of the labile pool for these metals. For example, the metallation state of both Fe-SOD and Mn-SOD can be very sensitive to metal availability in the cell, with binding of the non-cognate ion typically leading to enzyme inhibition (8, 23, 28, 29) In addition, both iron and manganese present in mononuclear enzymes may dissociate readily during purification, suggestive of weak binding (30, 31). Direct measurements of Mn2+ dissociation from the B. anthracis MntR ortholog reveal a half-time of 6 s (32), and exchange reactions on this time scale are likely common in the cytosol. To the extent that Mn2+- and Fe2+-cofactored enzymes rapidly exchange their cofactor, these ions may be considered part of the labile pool.
B. subtilis Fur, MntR, and PerR Coordinate Iron and Manganese Homeostasis
Fur, MntR, and PerR are the key regulators of iron and manganese homeostasis in B. subtilis (13). Fur senses the labile iron pool, whereas MntR monitors the free manganese pool (Fig. 1). PerR is a Fur paralog best known for its role in the regulation of peroxide stress genes. When metallated by iron, PerR is exquisitely sensitive to H2O2, which oxidizes either of two His ligands to 2-oxo-His (33, 34). In addition, PerR has a second function as sensor of the Fe/Mn ratio. In addition to Fur and PerR, a third Fur paralog, Zur, functions to maintain zinc homeostasis (10, 35).
FIGURE 1.
Schematic diagram illustrating the three interconnected metalloregulatory circuits that control iron and manganese homeostasis in B. subtilis. The intracellular levels of manganese and iron are regulated by MntR and Fur, respectively. PerR senses the Fe/Mn ratio and represses peroxide stress genes, fur, and perR itself. However, only the PerR:Zn,Mn form represses fur expression. Representative structures are shown for the activated metalloregulators including PerR:Zn,Mn (66) (representative also of the PerR:Zn,Fe form), for MntR:Mn2 (55), and for a homology model of the Fur:Zn,Fe2 repressor (4).
The Fur Regulon and Iron Homeostasis
Fur is the central regulator of iron homeostasis in B. subtilis (9). Indeed, nearly all of the genes induced by the Fe2+ chelator dipyridyl are equivalently derepressed in a fur mutant (19). Fur represses synthesis of the bacillibactin siderophore together with pathways involved in iron uptake (36, 37). In the stationary phase, and in response to oxidative stress, excess cytosolic iron may be incorporated into two mini-ferritins, Dps and MrgA (38, 39). Fur also indirectly regulates many more genes as mediated by a small RNA (FsrA) in collaboration with FbpA, FbpB, and FbpC (putative RNA chaperones) (40). FsrA down-regulates low priority iron enzymes as part of an iron-sparing response with far-reaching impacts on central metabolism (41, 42).
The Precarious Nature of Iron-selective Recognition by Fur
Fur family proteins provide an instructive example of how cells can adapt a common protein scaffold for sensing of diverse metals including iron (Fur), zinc (Zur), manganese (Mur), and nickel (Nur) (43, 44). Fur in particular plays a critical role in the biology of many pathogens (45, 46). B. subtilis Fur responds with high selectivity to Fe2+, but in other cases, metallosensing may be more promiscuous.
A model for metal recognition by B. subtilis Fur has been developed using homology modeling, competition metal binding, and mutational studies (4). Fur is a dimeric DNA-binding protein with a structural Cys4:Zn site (site 1) required for protein stability (Fig. 2). Fur contains two other metal-sensing sites per monomer (sites 2 and 3). Metal binds first at site 3 with subsequent binding to the key allosteric site (site 2) leading to a >100-fold increase in DNA binding affinity. In vitro, ∼1 μm free iron activates DNA binding (4).
FIGURE 2.
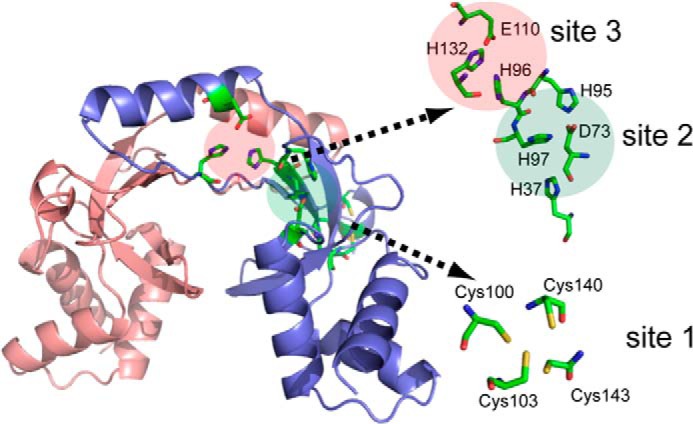
Structural insights into iron recognition by B. subtilis Fur based on a homology model using Streptomyces coelicolor Zur as template (Protein Data Bank (PDB) 3MWM; sequence identity: 33.3%) (92). Three metal-binding sites are highlighted with conserved putative metal-binding ligands shown in stick configuration. Putative metal-binding ligands that are not conserved between ScZur and BsFur are not shown. Site 1 is the structural zinc site required for protein folding. Both site 2 and site 3 are occupied by metal in the functional holo-repressor, with an overall apparent affinity (Kd) of ∼1 μm Fe2+ in a coupled DNA binding assay. Adapted from Ref. 4.
Binding of Mn2+ can also activate Fur with somewhat lower affinity (Kd ∼24 μm), which is above the estimated level of free Mn2+ as normally monitored by MntR (Kd ∼6 μm). Thus, Mn2+ does not activate Fur under most conditions. This contrasts with enteric bacteria where selection for manganese resistance leads to recovery of mutations in fur (47). This might suggest that manganese toxicity arises from inappropriate repression of iron import. In Salmonella enterica, the mntH gene is repressed by both MntR (in response to manganese) and Fur (in response to iron), but both metalloregulators can also mediate repression (albeit less efficiently) in response to the non-cognate metal (48).
Although B. subtilis Fur is highly specific for Fe2+ under most conditions, cross-talk may sometimes occur. For example, the Fur regulon is moderately repressed by high levels of Cd2+ and Zn2+ (49). Furthermore, when Fur protein levels are elevated ∼2-fold (by mutation of the PerR repressor) Fur is constitutively activated by ambient Mn2+, leading to a severe iron starvation phenotype (4, 50). This suggests that this system is delicately poised, and conditions that transiently elevate the level of either Fur protein or Mn2+ will, by mass action, lead to manganese-cofactored Fur and repression of iron import. Cross-talk between manganese and iron clearly occurs in E. coli (48), but this may be less of an issue for this iron-centric bacterium because ambient manganese is generally maintained at very low levels.
The MntR Regulon and Manganese Homeostasis
MntR is a Mn2+-specific metalloregulator structurally related to DtxR/IdeR Fe2+ sensors (11, 51, 52). Members of this large and diverse protein family function to sense iron, manganese, or both, and are key regulators in several pathogens (53). B. subtilis MntR represses two manganese uptake systems: the ATP-dependent MntABCD transporter and MntH, a proton-coupled symporter (54). An mntR mutant is extremely sensitive to manganese, largely due to an inability to repress MntH (11). Unlike wild type, which tolerates 1 mm Mn2+, an mntR mutant is unable to grow with 5 μm Mn2+. Thus, the ability to repress Mn2+ uptake is critical for cell physiology.
Selective Recognition of Manganese by MntR
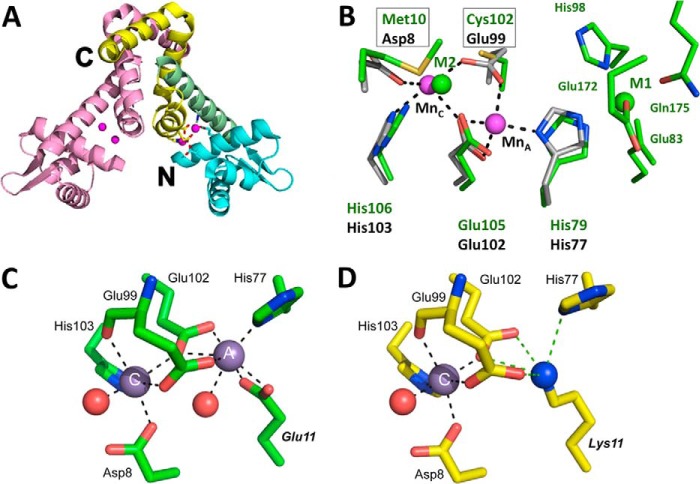
From a structural perspective, B. subtilis MntR is one of the most thoroughly studied metalloregulators and provides an interesting comparison with the iron-sensing DtxR/IdeR homologs (51) (Fig. 3). MntR binds Mn2+ at two sites per monomer, A and C (55). Formation of this metal cluster orients the two DNA-binding recognition helices at the appropriate distance for binding to operator DNA (55, 56). Although various Mn2+ binding affinities have been measured for MntR, recent studies reveal an overall Kd of ∼6 μm (4). Cd2+ can also bind to MntR to repress the MntR regulon (49, 57). Although Cd2+ is considered a non-cognate metal with respect to Mn2+ homeostasis, Cd2+ may be a physiologically relevant agonist; MntH is a major route of entry for the toxic cadmium ion (11).
FIGURE 3.
Structural insights into manganese recognition by B. subtilis MntR. A, one subunit of the dimer is colored pink, and the other is colored according to region: cyan for the N-terminal DNA-binding domain, yellow for the dimerization domain, and green for the linking helix (α4). The N and C termini of the multicolored subunit are labeled. Bound manganese ions are colored magenta (55). B, overlay of the two manganese-binding sites in MntR (51, 55) with the two metal-binding sites of cobalt-bound IdeR (PDB entry 1FX7; green). Amino acid residues are labeled in black for MntR and in green for IdeR. For MntR, carbon, nitrogen, and oxygen atoms are gray, blue, and red, respectively, and the two manganese ions are magenta spheres. For IdeR, the two metal ions are pink. Metal-ligand interactions for MntR are denoted by dashed lines (Glu-11 of MntR omitted for clarity). Note that the activating manganese (MnC site) in MntR aligns with the key iron-sensing site (M2) of IdeR. Boxes highlight the superposition of Asp-8(MntR) and Met-10(IdeR) and Glu-99(MntR) and Cys-102(IdeR). C and D, comparison of manganese binding by wild-type MntR (PDB entry 2F5D) in the AC conformation (55) (C) and E11K MntR (PDB entry 4HX4) (57) (D) (adapted from Ref. 59). All residues are shown as sticks. Coordination bonds are represented by black dashed lines, with potential electrostatic interactions for the ϵNH2 group of Lys-11 in E11K MntR shown as green dashed lines. Note that all residues are conserved in S. pneumoniae PsaR with identical residue numbers (except that His-77 is His-76 in PsaR). Thus, the MntR E11K mutant is an excellent model for manganese sensing by the streptococcal homologs including S. pneumoniae PsaR (59) and S. gordonii ScaR (58).
Numerous structures are available for B. subtilis MntR as an apo-protein (56), in various metallated states (51, 55, 57), and for mutant proteins altered in metal recognition (57). Collectively, these structures lead to a model for manganese-selective metallosensing in which manganese binds first to the A site with heptacoordinate ligation, and this organizes the C site to enable binding of a second manganese and stabilization of the active repressor conformation (Fig. 3C). MntR also binds tightly to non-cognate metals (Fe2+, Co2+, and Zn2+), but these fail to allosterically activate DNA binding due to an inability to form a binuclear cluster (57). Fe2+ binds at the A site but with a different geometry than manganese, and this distorts the C site such that the second metal binding event (required for DNA binding) is inhibited (57). As a result, Fe2+ is an antagonist for MntR (11, 52, 54). Indeed, an mntR-regulated gene is not repressed even when fur mutant cells, derepressed for iron import, are grown in high iron conditions (52). In contrast, in S. enterica, MntR can respond to both Mn2+ and Fe2+ (48), although the structural differences that allow this wider effector response are not understood.
Insights into the origins of metal specificity have been obtained from structural comparisons of manganese-specific representatives such as B. subtilis MntR and Streptococcus gordonii ScaR with iron-specific family members such as Corynebacterium diphtheriae DtxR (52, 57–59). Comparison of MntR and DtxR/IdeR proteins revealed two notable differences in the first coordination sphere for the activating metal (Fig. 3B). Specifically, Met-10 in DtxR/IdeR is replaced by Asp-8 in MntR, and Cys-102 is replaced by Glu-99 (52). This suggests that sulfur-containing ligands tip the balance in favor of iron activation by altering the affinity of the metalloregulator for its activating metal(s). Studies with mutant versions of both DtxR and MntR provide partial support for this notion. For example, a D8M mutant of MntR responds to both iron and manganese in B. subtilis, suggesting that the presence of a thioether ligand (Met) in site C is sufficient to allow activation by iron (52).
To explore the biochemical determinants for the high iron selectivity of DtxR, this metalloregulator was expressed in B. subtilis. Unexpectedly, DtxR responded to both iron and manganese with comparable sensitivity in B. subtilis (52). This suggests that the failure of DtxR to respond to manganese in its native environment is due to a lack of access: ambient levels of manganese that are maintained (presumably by the C. diphtheriae ortholog of MntR (60)) at levels below those needed for activation. If this model is correct, C. diphtheriae MntR should bind Mn2+ with substantially higher affinity than B. subtilis MntR. DtxR could be converted to a Mn2+-selective repressor by replacement of two ligands corresponding to the MntR C site; the DtxR M10D/C102E double mutant responds to manganese but not to iron in B. subtilis. However, a robust response to iron is recovered in a fur mutant strain derepressed for iron import, indicating that these two amino acid substitutions have reduced, but not eliminated, the ability of iron to activate DNA binding (52). Thus, metalloregulators are finely tuned to function within their specific host milieu and, not surprisingly, evolution has optimized selectivity only to the point where a sufficiently selective response is obtained under ambient metal concentrations (5).
Collectively, these results suggest that formation of a binuclear cluster enables selective Mn2+ sensing in MntR. However, some MntR orthologs can selectively sense Mn2+ with a single binding site. This is the case for S. gordonii ScaR (58) and Streptococcus pneumoniae PsaR (59), which have a Lys residue positioned to replace the need for metal binding in the A site (Fig. 3D), as visualized in the structure of a manganese-bound EllK variant of B. subtilis MntR (57).
PerR and the PerR Regulon
B. subtilis PerR is a member of the Fur family of proteins and plays a central role in regulation of the adaptive response to H2O2 (61–63). Like its two paralogs (Fur and Zur), it forms a stable dimer containing one structural Zn2+ per monomer (in a Cys4 site) (64). Binding of one additional metal ion per monomer (in a site homologous to site 2 of Fur) activates DNA binding (33, 65, 66) (Fig. 4). B. subtilis PerR regulates peroxide defense functions including the major vegetative catalase (KatA), heme biosynthesis, alkyl hydroperoxide reductase (AhpCF), and MrgA, a homolog of the E. coli DNA-binding and iron sequestration protein, Dps (9, 38, 67). It is notable that several PerR-regulated genes (MrgA, Fur, and ZosA) have roles in metal ion homeostasis. Indeed, peroxide stress commonly elicits adaptive changes in metal homeostasis that conspire to reduce the largely iron-dependent toxicity (68).
FIGURE 4.
Structural insights into iron and manganese recognition by B. subtilis PerR. A, superposition of the global dimer structures of the apo-repressor (PerR:Zn; brown) and holo-repressor (PerR:Zn,Mn; green). The structural zinc ions are in black, and the regulatory manganese ions are in yellow (adapted from Ref. 66). B, close-up of the PerR regulatory site with His-37, His-91, His-93, Asp-85, and Asp-104 coordinating manganese (yellow). C, close-up of the regulatory metal-binding site (metal in blue) and relationship to putative site 3 residues (Glu-114 and His-128) in PerR:Zn,Mn (PDB entry 3F8N; adapted from Ref. 93). Coordination of His-37 from the DNA-binding domain, to the bound metal ion, triggers the allosteric reorientation of the two DNA-binding domains. Oxidation of this same residue by H2O2 leads to derepression of the PerR regulon. D, top view of the regulatory site highlighting (in red) the hydrophilic environment that facilitates metal exchange and reaction of bound iron with H2O2 (adapted from Ref. 66). Note that the size of the Asp-104 side chain affects the size of the hydrophilic channel and thereby influences H2O2 reactivity. A D104E mutant of PerR reduces H2O2 sensitivity, and a reverse mutation in Fur (E108D) increases H2O2 reactivity (70).
Most studies to date of the PerR regulon have focused on the mrgA gene as a model. Repression of mrgA requires PerR and a divalent cation as a corepressor (33, 69). Both Mn2+ and Fe2+ activate PerR to bind DNA with high affinity, thereby allowing repression of the PerR regulon. Conversely, resuspension of growing cells in minimal medium lacking both manganese and iron leads to rapid derepression of the PerR regulon, whereas omission of either metal alone does not (69).
Under most growth conditions, PerR is predominantly in the Fe2+ form (designated PerR:Zn,Fe). Indeed, in vitro, DNA binding is activated by Fe2+ (Kd = 0.1 μm) at lower concentrations than Mn2+ (Kd = 2.8 μm) (64), and when both metals are present at equivalent concentrations, the iron form predominates (33). However, in medium supplemented with manganese, and deficient for iron, PerR is in the PerR:Zn,Mn form and is insensitive to H2O2 (67–69). It is not entirely clear why some Fur family members, such as PerR, are highly sensitive to H2O2, whereas others are not, even when bound with iron. One factor that influences the H2O2 reactivity of bound iron may be the solvent accessibility (Fig. 4D), as inferred from the effects of amino acid substitutions in PerR and Fur (70).
Importantly, not all PerR-regulated genes respond the same to metal ion availability (69). Although manganese leads to strong repression of all PerR-regulated genes, iron only represses a subset including those functions most critical under conditions of peroxide stress such as katA, ahpCF, and mrgA. Other PerR-regulated genes, including fur and perR itself, are repressed by PerR:Zn,Mn, but not by PerR:Zn,Fe (69). For these genes, PerR does not function as a peroxide sensor (and these genes are not induced by H2O2), but instead acts as a sensor of the Fe/Mn ratio. The molecular basis for this differential regulation is an active area of research.
Possible Mechanisms for Coordinating Iron and Manganese Homeostasis
The high selectivity of the Fur and MntR metalloregulators, as well as the minimal cross-talk observed between the iron and manganese starvation responses, has led to a general model in which iron and manganese homeostasis are largely independent (Fig. 1). However, several of the observations summarized above suggest that they may in fact be more closely integrated than heretofore appreciated.
In addition to regulating manganese uptake via MntR, manganese may, at least under some conditions, also regulate iron homeostasis. In vitro, Mn2+ can activate B. subtilis Fur to bind DNA, and recent results reveal that ambient Mn2+ levels are sufficient to support constitutive (and inappropriate) repression of iron uptake when Fur protein levels are elevated from ∼10,000 to ∼22,000 monomers per cell due to loss of PerR repression (50). This cross-talk likely reflects the fact that the affinity of Fur for Mn2+ (Kd∼24 μm) is only modestly less than the MntR affinity for Mn2+ (Kd∼6 μm) that presumably governs intracellular manganese levels. It is possible that similar mass action effects are normally at play to fine-tune iron responsiveness in the cell. Specifically, when the Fe/Mn ratio is high (as sensed by PerR), fur will be derepressed, and this higher level of Fur protein may serve to decrease the level of iron needed to repress the Fur regulon. Conversely, when manganese is abundant, fur transcription will be reduced by PerR:Zn,Mn and higher levels of iron will be needed for the efficient repression of iron uptake. A similar coordination is apparent in the α-proteobacterium Bradyrhizobium japonicum; manganese limitation directly regulates iron homeostasis by antagonizing the heme-dependent degradation of the key iron regulator Irr (71).
There are also several plausible mechanisms by which iron might modulate manganese homeostasis. For example, Fe2+ serves as an antagonist of MntR-dependent repression in vitro. Thus, under conditions of transient iron overload (such as may occur upon shift of iron-deficient cells to iron-sufficient medium or as a consequence of oxidative damage to iron-containing enzymes (72–74)), iron may compete for the MntR A site and thereby impede formation of the functional repressor. One consequence would be to increase manganese uptake under conditions of iron excess, a plausible mechanism to ensure a balance between these two competitive metal ions. It has also been observed that a fur mutant strain displays down-regulation of the two major Mn2+ uptake systems (mntH and mntABCD), and this effect required components of the iron-sparing response (the FbpAB proteins and FsrA) (41, 42). This raises the possibility that conditions of iron deprivation lead to both an induction of iron uptake and a concomitant down-regulation of manganese uptake. Such mechanisms may represent a strategy to balance the Fe/Mn ratio.
Physiological Consequences of Imbalances in the Fe/Mn Ratio
Bacteria have evolved a wide variety of mechanisms to ensure that iron and manganese levels are properly regulated, including the recent discovery of manganese efflux systems (71, 75). In E. coli, metabolism seems to be relatively iron-centric with only conditional use of manganese (8). Indeed, Mn-SOD, a representative manganese-dependent enzyme, is largely unmetallated in unstressed cells (8). In response to oxidative stress, manganese import is derepressed, Mn-SOD acquires its cofactor, and several iron-containing enzymes switch to a less redox-sensitive manganese form (31). Unlike E. coli, B. subtilis is reliant on manganese for growth and normally maintains comparable pools of both total and labile iron and manganese.
The complexity of the interplay between iron and manganese has been explored from several directions. For example, most manganese- and iron-cofactored SODs are active only with their cognate metal due to ligand-mediated tuning of the metal redox potential (76). Proper metallation of SODs appears to be particularly sensitive to metal availability (29, 77). Interestingly, heterologous expression studies with Mn-SOD from the manganese-centric B. burgdorferi indicate that it is only metallated when the cytosol contains high levels of Mn2+, consistent with the ambient conditions in its native host (28). Other enzymes may also vary between Fe2+- and Mn2+-cofactored forms, as shown for E. coli peptide deformylase, threonine dehydrogenase, cytosine deaminase, and 3-deoxy-d-arabinoheptulosonate 7-phosphate synthase (31). It is notable that some essential mononuclear enzymes in B. subtilis, including peptide deformylase and methionine aminopeptidase, have multiple isozymes with apparently distinct metal selectivity (78, 79). The cellular Fe/Mn ratio has also emerged as a critical determinant of radioresistance (1), and the LMW pool of labile manganese is thought to have significant protective effects under oxidative stress conditions (25).
One important, but still poorly understood, area of physiology relates to the consequences of metal imbalances. When metal ions are deficient, cells will cease growth, but the nature of the resulting defects are generally not known. Some insights into the processes that fail when metals are limited can be gleaned from genes induced in response to metal starvation. For example, derepression of a manganese-cofactored ribonucleotide reductase (RNR) in E. coli in response to iron limitation suggests that this is one essential process that can be compromised by metal limitation (80). B. subtilis encodes a single manganese-ribonucleotide reductase (81) and a manganese-dependent phosphoglycerate mutase (82), a key enzyme in glycolysis, that might each contribute to the manganese requirement for growth. Similarly, in B. japonicum, pyruvate kinase has been defined as a key manganese-dependent enzyme (83).
The molecular basis for manganese toxicity is also poorly understood. Manganese import into manganese-starved B. subtilis cells leads to a transient growth arrest (associated with manganese hyperaccumulation), and growth resumes only after manganese levels return to normal (84). Presumably, elevated manganese interferes with one or more critical iron-dependent enzymes. Recent results in E. coli suggest that ferrochelatase may be one such enzyme (85), consistent with biochemical studies suggesting that Mn2+ can serve as a competitive inhibitor of Fe2+ loading into protoporphyrin IX (86). In Neisseria meningitidis, manganese toxicity is exacerbated under low iron conditions. As a countermeasure, the MntX protein exports Mn2+ and increases the intracellular Fe/Mn ratio (75). This and related Mn2+ efflux systems are important in several bacterial pathogens (75, 87–89), underlining the importance of manganese homeostasis for pathogenicity.
Additional insights into the physiological effects of high manganese in B. subtilis have emerged from transcriptomic studies (54). Shifting cells from manganese-limited to manganese-sufficient conditions led to a significant induction of the σB regulon apparently due to activation of the manganese-dependent RsbU phosphatase. This suggests that RsbU activity is normally limited by cofactor availability. A second notable effect was significant induction of the nitrogen limitation stress response regulated by TnrA. This was hypothesized to result from a shift of glutamine synthetase from a largely magnesium-cofactored form to a manganese-cofactored form known, from in vitro studies, to be less sensitive to feedback inhibition by glutamine (54).
The consequences of iron starvation and iron overload have also been challenging to unravel. Starvation for iron can activate sophisticated iron-sparing responses that repress synthesis of some iron-utilizing enzymes and may lead to mobilization of iron either from storage proteins or by proteolysis of dispensable metalloenzymes. The identity of the critical step(s) that ultimately fail is unclear and is likely dependent on growth conditions. In B. subtilis, and many other organisms, iron starvation leads to induction of flavodoxins that can functionally replace ferredoxins (19, 90, 91), thereby implicating the latter as being sensitive to iron deprivation. Conversely, the adverse consequences of iron overload are not well understood, although it is clear that high iron levels can enhance sensitivity to oxidative stress (72). Another possible effect of iron overload in a manganese-centric organism such as B. subtilis is interference with one or more of the critical, manganese-dependent enzymes needed for growth.
Conclusions
As this brief survey illustrates, B. subtilis provides an excellent model system for investigating the complex interactions between iron and manganese homeostasis, informed by an abundance of structural data for the cognate metalloregulators, transcriptome data revealing the responses to metal deprivation and excess, and molecular genetic studies of the corresponding gene regulatory circuitry. Studies in this and other microbial systems promise to help elucidate the complex bioinorganic chemistry of the cell, which plays a central role in host-pathogen interactions as well as elemental cycling on a global scale.
Acknowledgments
I thank members of the Helmann laboratory for critical reading of the manuscript and Dr. Pete Chandrangsu for assistance with the preparation of figures.
This work was supported, in whole or in part, by National Institutes of Health Grant GM059323 (to J. D. H.). This is the third article in the Thematic Minireview series “Metals in Biology 2014.”
- Fur
- ferric uptake regulator
- Zur
- zinc uptake regulator
- MntR
- manganese transport regulator
- LMW
- low molecular weight
- SOD
- superoxide dismutase.
REFERENCES
- 1. Daly M. J., Gaidamakova E. K., Matrosova V. Y., Vasilenko A., Zhai M., Venkateswaran A., Hess M., Omelchenko M. V., Kostandarithes H. M., Makarova K. S., Wackett L. P., Fredrickson J. K., Ghosal D. (2004) Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science 306, 1025–1028 [DOI] [PubMed] [Google Scholar]
- 2. Outten C. E., O'Halloran T. V. (2001) Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292, 2488–2492 [DOI] [PubMed] [Google Scholar]
- 3. Posey J. E., Gherardini F. C. (2000) Lack of a role for iron in the Lyme disease pathogen. Science 288, 1651–1653 [DOI] [PubMed] [Google Scholar]
- 4. Ma Z., Faulkner M. J., Helmann J. D. (2012) Origins of specificity and cross-talk in metal ion sensing by Bacillus subtilis Fur. Mol. Microbiol. 86, 1144–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Waldron K. J., Rutherford J. C., Ford D., Robinson N. J. (2009) Metalloproteins and metal sensing. Nature 460, 823–830 [DOI] [PubMed] [Google Scholar]
- 6. Guerra A. J., Giedroc D. P. (2012) Metal site occupancy and allosteric switching in bacterial metal sensor proteins. Arch. Biochem. Biophys. 519, 210–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lisher J. P., Giedroc D. P. (2013) Manganese acquisition and homeostasis at the host-pathogen interface. Front. Cell. Infect. Microbiol. 3, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anjem A., Varghese S., Imlay J. A. (2009) Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol. Microbiol. 72, 844–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bsat N., Herbig A., Casillas-Martinez L., Setlow P., Helmann J. D. (1998) Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol. Microbiol. 29, 189–198 [DOI] [PubMed] [Google Scholar]
- 10. Gaballa A., Helmann J. D. (1998) Identification of a zinc-specific metalloregulatory protein, Zur, controlling zinc transport operons in Bacillus subtilis. J. Bacteriol. 180, 5815–5821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Que Q., Helmann J. D. (2000) Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol. Microbiol. 35, 1454–1468 [DOI] [PubMed] [Google Scholar]
- 12. Helmann J. D., Soonsanga S., Gabriel S. (2007) Metalloregulators: arbiters of metal sufficiency. in Molecular Microbiology of Heavy Metals (Nies D. H., Silver S., eds), pp. 37–71, Springer-Verlag, Berlin [Google Scholar]
- 13. Ma Z., Helmann J. D. (2013) Metal homeostasis and oxidative stress in Bacillus subtilis. in Encyclopedia of Inorganic and Bioinorganic Chemistry (Scott R. A., ed), pp. eibc2129, John Wiley & Sons, Ltd., Chichester, UK [Google Scholar]
- 14. Moore C. M., Helmann J. D. (2005) Metal ion homeostasis in Bacillus subtilis. Curr. Opin. Microbiol. 8, 188–195 [DOI] [PubMed] [Google Scholar]
- 15. Papp-Wallace K. M., Maguire M. E. (2006) Manganese transport and the role of manganese in virulence. Annu. Rev. Microbiol. 60, 187–209 [DOI] [PubMed] [Google Scholar]
- 16. Kehl-Fie T. E., Skaar E. P. (2010) Nutritional immunity beyond iron: a role for manganese and zinc. Curr. Opin. Chem. Biol. 14, 218–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caza M., Kronstad J. W. (2013) Shared and distinct mechanisms of iron acquisition by bacterial and fungal pathogens of humans. Front. Cell. Infect. Microbiol. 3, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ma Z., Jacobsen F. E., Giedroc D. P. (2009) Coordination chemistry of bacterial metal transport and sensing. Chem. Rev. 109, 4644–4681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baichoo N., Wang T., Ye R., Helmann J. D. (2002) Global analysis of the Bacillus subtilis Fur regulon and the iron starvation stimulon. Mol. Microbiol. 45, 1613–1629 [DOI] [PubMed] [Google Scholar]
- 20. Merchant S. S., Helmann J. D. (2012) Elemental economy: microbial strategies for optimizing growth in the face of nutrient limitation. Adv. Microb. Physiol. 60, 91–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sankaran B., Bonnett S. A., Shah K., Gabriel S., Reddy R., Schimmel P., Rodionov D. A., de Crécy-Lagard V., Helmann J. D., Iwata-Reuyl D., Swairjo M. A. (2009) Zinc-independent folate biosynthesis: genetic, biochemical, and structural investigations reveal new metal dependence for GTP cyclohydrolase IB. J. Bacteriol. 191, 6936–6949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sevcenco A. M., Pinkse M. W., Wolterbeek H. T., Verhaert P. D., Hagen W. R., Hagedoorn P. L. (2011) Exploring the microbial metalloproteome using MIRAGE. Metallomics 3, 1324–1330 [DOI] [PubMed] [Google Scholar]
- 23. Tu W. Y., Pohl S., Gray J., Robinson N. J., Harwood C. R., Waldron K. J. (2012) Cellular iron distribution in Bacillus anthracis. J. Bacteriol. 194, 932–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sharma A., Gaidamakova E. K., Matrosova V. Y., Bennett B., Daly M. J., Hoffman B. M. (2013) Responses of Mn2+ speciation in Deinococcus radiodurans and Escherichia coli to γ-radiation by advanced paramagnetic resonance methods. Proc. Natl. Acad. Sci. U.S.A. 110, 5945–5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Culotta V. C., Daly M. J. (2013) Manganese complexes: diverse metabolic routes to oxidative stress resistance in prokaryotes and yeast. Antioxid. Redox Signal. 19, 933–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tabares L. C., Un S. (2013) In situ determination of manganese(II) speciation in Deinococcus radiodurans by high magnetic field EPR: detection of high levels of Mn(II) bound to proteins. J. Biol. Chem. 288, 5050–5055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hider R. C., Kong X. (2013) Iron speciation in the cytosol: an overview. Dalton Trans. 42, 3220–3229 [DOI] [PubMed] [Google Scholar]
- 28. Aguirre J. D., Clark H. M., McIlvin M., Vazquez C., Palmere S. L., Grab D. J., Seshu J., Hart P. J., Saito M., Culotta V. C. (2013) A manganese-rich environment supports superoxide dismutase activity in a Lyme disease pathogen, Borrelia burgdorferi. J. Biol. Chem. 288, 8468–8478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aguirre J. D., Culotta V. C. (2012) Battles with iron: manganese in oxidative stress protection. J. Biol. Chem. 287, 13541–13548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cotruvo J. A., Jr., Stubbe J. (2012) Metallation and mismetallation of iron and manganese proteins in vitro and in vivo: the class I ribonucleotide reductases as a case study. Metallomics 4, 1020–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anjem A., Imlay J. A. (2012) Mononuclear iron enzymes are primary targets of hydrogen peroxide stress. J. Biol. Chem. 287, 15544–15556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sen K. I., Sienkiewicz A., Love J. F., vanderSpek J. C., Fajer P. G., Logan T. M. (2006) Mn(II) binding by the anthracis repressor from Bacillus anthracis. Biochemistry 45, 4295–4303 [DOI] [PubMed] [Google Scholar]
- 33. Lee J. W., Helmann J. D. (2006) The PerR transcription factor senses H2O2 by metal-catalysed histidine oxidation. Nature 440, 363–367 [DOI] [PubMed] [Google Scholar]
- 34. Traoré D. A., El Ghazouani A., Jacquamet L., Borel F., Ferrer J. L., Lascoux D., Ravanat J. L., Jaquinod M., Blondin G., Caux-Thang C., Duarte V., Latour J. M. (2009) Structural and functional characterization of 2-oxo-histidine in oxidized PerR protein. Nat. Chem. Biol. 5, 53–59 [DOI] [PubMed] [Google Scholar]
- 35. Ma Z., Gabriel S. E., Helmann J. D. (2011) Sequential binding and sensing of Zn(II) by Bacillus subtilis Zur. Nucleic Acids Res. 39, 9130–9138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gaballa A., Helmann J. D. (2007) Substrate induction of siderophore transport in Bacillus subtilis mediated by a novel one-component regulator. Mol. Microbiol. 66, 164–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gaballa A., MacLellan S., Helmann J. D. (2012) Transcription activation by the siderophore sensor Btr is mediated by ligand-dependent stimulation of promoter clearance. Nucleic Acids Res. 40, 3585–3595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen L., Helmann J. D. (1995) Bacillus subtilis MrgA is a Dps(PexB) homologue: evidence for metalloregulation of an oxidative stress gene. Mol. Microbiol. 18, 295–300 [DOI] [PubMed] [Google Scholar]
- 39. Zeth K. (2012) Dps biomineralizing proteins: multifunctional architects of nature. Biochem. J. 445, 297–311 [DOI] [PubMed] [Google Scholar]
- 40. Gaballa A., Antelmann H., Aguilar C., Khakh S. K., Song K. B., Smaldone G. T., Helmann J. D. (2008) The Bacillus subtilis iron-sparing response is mediated by a Fur-regulated small RNA and three small, basic proteins. Proc. Natl. Acad. Sci. U.S.A. 105, 11927–11932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smaldone G. T., Antelmann H., Gaballa A., Helmann J. D. (2012) The FsrA sRNA and FbpB protein mediate the iron-dependent induction of the Bacillus subtilis lutABC iron-sulfur-containing oxidases. J. Bacteriol. 194, 2586–2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smaldone G. T., Revelles O., Gaballa A., Sauer U., Antelmann H., Helmann J. D. (2012) A global investigation of the Bacillus subtilis iron-sparing response identifies major changes in metabolism. J. Bacteriol. 194, 2594–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fillat M. F. (2014) The FUR (ferric uptake regulator) superfamily: diversity and versatility of key transcriptional regulators. Arch. Biochem. Biophys. 546, 41–52 [DOI] [PubMed] [Google Scholar]
- 44. Lee J. W., Helmann J. D. (2007) Functional specialization within the Fur family of metalloregulators. Biometals 20, 485–499 [DOI] [PubMed] [Google Scholar]
- 45. Troxell B., Hassan H. M. (2013) Transcriptional regulation by Ferric Uptake Regulator (Fur) in pathogenic bacteria. Front. Cell. Infect. Microbiol. 3, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carpenter B. M., Whitmire J. M., Merrell D. S. (2009) This is not your mother's repressor: the complex role of fur in pathogenesis. Infect. Immun. 77, 2590–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hantke K. (1987) Selection procedure for deregulated iron transport mutants (fur) in Escherichia coli K12: fur not only affects iron metabolism. Mol. Gen. Genet. 210, 135–139 [DOI] [PubMed] [Google Scholar]
- 48. Ikeda J. S., Janakiraman A., Kehres D. G., Maguire M. E., Slauch J. M. (2005) Transcriptional regulation of sitABCD of Salmonella enterica serovar Typhimurium by MntR and Fur. J. Bacteriol. 187, 912–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moore C. M., Gaballa A., Hui M., Ye R. W., Helmann J. D. (2005) Genetic and physiological responses of Bacillus subtilis to metal ion stress. Mol. Microbiol. 57, 27–40 [DOI] [PubMed] [Google Scholar]
- 50. Faulkner M. J., Ma Z., Fuangthong M., Helmann J. D. (2012) Derepression of the Bacillus subtilis PerR peroxide stress response leads to iron deficiency. J. Bacteriol. 194, 1226–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Glasfeld A., Guedon E., Helmann J. D., Brennan R. G. (2003) Structure of the manganese-bound manganese transport regulator of Bacillus subtilis. Nat. Struct. Biol. 10, 652–657 [DOI] [PubMed] [Google Scholar]
- 52. Guedon E., Helmann J. D. (2003) Origins of metal ion selectivity in the DtxR/MntR family of metalloregulators. Mol. Microbiol. 48, 495–506 [DOI] [PubMed] [Google Scholar]
- 53. Merchant A. T., Spatafora G. A. (2014) A role for the DtxR family of metalloregulators in gram-positive pathogenesis. Mol. Oral Microbiol. 29, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Guedon E., Moore C. M., Que Q., Wang T., Ye R. W., Helmann J. D. (2003) The global transcriptional response of Bacillus subtilis to manganese involves the MntR, Fur, TnrA and σB regulons. Mol. Microbiol. 49, 1477–1491 [DOI] [PubMed] [Google Scholar]
- 55. Kliegman J. I., Griner S. L., Helmann J. D., Brennan R. G., Glasfeld A. (2006) Structural basis for the metal-selective activation of the manganese transport regulator of Bacillus subtilis. Biochemistry 45, 3493–3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. DeWitt M. A., Kliegman J. I., Helmann J. D., Brennan R. G., Farrens D. L., Glasfeld A. (2007) The conformations of the manganese transport regulator of Bacillus subtilis in its metal-free state. J. Mol. Biol. 365, 1257–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McGuire A. M., Cuthbert B. J., Ma Z., Grauer-Gray K. D., Brunjes Brophy M., Spear K. A., Soonsanga S., Kliegman J. I., Griner S. L., Helmann J. D., Glasfeld A. (2013) Roles of the A and C sites in the manganese-specific activation of MntR. Biochemistry 52, 701–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stoll K. E., Draper W. E., Kliegman J. I., Golynskiy M. V., Brew-Appiah R. A., Phillips R. K., Brown H. K., Breyer W. A., Jakubovics N. S., Jenkinson H. F., Brennan R. G., Cohen S. M., Glasfeld A. (2009) Characterization and structure of the manganese-responsive transcriptional regulator ScaR. Biochemistry 48, 10308–10320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lisher J. P., Higgins K. A., Maroney M. J., Giedroc D. P. (2013) Physical characterization of the manganese-sensing pneumococcal surface antigen repressor from Streptococcus pneumoniae. Biochemistry 52, 7689–7701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schmitt M. P. (2002) Analysis of a DtxR-like metalloregulatory protein, MntR, from Corynebacterium diphtheriae that controls expression of an ABC metal transporter by an Mn2+-dependent mechanism. J. Bacteriol. 184, 6882–6892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Duarte V., Latour J. M. (2010) PerR vs OhrR: selective peroxide sensing in Bacillus subtilis. Mol. Biosyst. 6, 316–323 [DOI] [PubMed] [Google Scholar]
- 62. Dubbs J. M., Mongkolsuk S. (2012) Peroxide-sensing transcriptional regulators in bacteria. J. Bacteriol. 194, 5495–5503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zuber P. (2009) Management of oxidative stress in Bacillus. Annu. Rev. Microbiol. 63, 575–597 [DOI] [PubMed] [Google Scholar]
- 64. Lee J. W., Helmann J. D. (2006) Biochemical characterization of the structural Zn2+ site in the Bacillus subtilis peroxide sensor PerR. J. Biol. Chem. 281, 23567–23578 [DOI] [PubMed] [Google Scholar]
- 65. Herbig A. F., Helmann J. D. (2001) Roles of metal ions and hydrogen peroxide in modulating the interaction of the Bacillus subtilis PerR peroxide regulon repressor with operator DNA. Mol. Microbiol. 41, 849–859 [DOI] [PubMed] [Google Scholar]
- 66. Jacquamet L., Traoré D. A., Ferrer J. L., Proux O., Testemale D., Hazemann J. L., Nazarenko E., El Ghazouani A., Caux-Thang C., Duarte V., Latour J. M. (2009) Structural characterization of the active form of PerR: insights into the metal-induced activation of PerR and Fur proteins for DNA binding. Mol. Microbiol. 73, 20–31 [DOI] [PubMed] [Google Scholar]
- 67. Chen L., Keramati L., Helmann J. D. (1995) Coordinate regulation of Bacillus subtilis peroxide stress genes by hydrogen peroxide and metal ions. Proc. Natl. Acad. Sci. U.S.A. 92, 8190–8194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Faulkner M. J., Helmann J. D. (2011) Peroxide stress elicits adaptive changes in bacterial metal ion homeostasis. Antioxid. Redox Signal. 15, 175–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fuangthong M., Herbig A. F., Bsat N., Helmann J. D. (2002) Regulation of the Bacillus subtilis fur and perR genes by PerR: not all members of the PerR regulon are peroxide inducible. J. Bacteriol. 184, 3276–3286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Parent A., Caux-Thang C., Signor L., Clémancey M., Sethu R., Blondin G., Maldivi P., Duarte V., Latour J. M. (2013) Single glutamate to aspartate mutation makes ferric uptake regulator (Fur) as sensitive to H2O2 as peroxide resistance regulator (PerR). Angew. Chem. Int. Ed. Engl. 52, 10339–10343 [DOI] [PubMed] [Google Scholar]
- 71. Puri S., Hohle T. H., O'Brian M. R. (2010) Control of bacterial iron homeostasis by manganese. Proc. Natl. Acad. Sci. U.S.A. 107, 10691–10695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Imlay J. A. (2013) The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat. Rev. Microbiol. 11, 443–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Keyer K., Imlay J. A. (1996) Superoxide accelerates DNA damage by elevating free-iron levels. Proc. Natl. Acad. Sci. U.S.A. 93, 13635–13640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Keyer K., Imlay J. A. (1997) Inactivation of dehydratase [4Fe-4S] clusters and disruption of iron homeostasis upon cell exposure to peroxynitrite. J. Biol. Chem. 272, 27652–27659 [DOI] [PubMed] [Google Scholar]
- 75. Veyrier F. J., Boneca I. G., Cellier M. F., Taha M. K. (2011) A novel metal transporter mediating manganese export (MntX) regulates the Mn to Fe intracellular ratio and Neisseria meningitidis virulence. PLoS Pathog. 7, e1002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Miller A. F. (2012) Superoxide dismutases: ancient enzymes and new insights. FEBS Lett. 586, 585–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Culotta V. C., Yang M., O'Halloran T. V. (2006) Activation of superoxide dismutases: putting the metal to the pedal. Biochim. Biophys. Acta 1763, 747–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Haas M., Beyer D., Gahlmann R., Freiberg C. (2001) YkrB is the main peptide deformylase in Bacillus subtilis, a eubacterium containing two functional peptide deformylases. Microbiology 147, 1783–1791 [DOI] [PubMed] [Google Scholar]
- 79. You C., Lu H., Sekowska A., Fang G., Wang Y., Gilles A. M., Danchin A. (2005) The two authentic methionine aminopeptidase genes are differentially expressed in Bacillus subtilis. BMC Microbiol. 5, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Martin J. E., Imlay J. A. (2011) The alternative aerobic ribonucleotide reductase of Escherichia coli, NrdEF, is a manganese-dependent enzyme that enables cell replication during periods of iron starvation. Mol. Microbiol. 80, 319–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Parker M. J., Zhu X., Stubbe J. (2014) Bacillus subtilis class Ib ribonucleotide reductase: high activity and dynamic subunit interactions. Biochemistry 53, 766–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Vasantha N., Freese E. (1979) The role of manganese in growth and sporulation of Bacillus subtilis. J. Gen. Microbiol. 112, 329–336 [DOI] [PubMed] [Google Scholar]
- 83. Hohle T. H., O'Brian M. R. (2012) Manganese is required for oxidative metabolism in unstressed Bradyrhizobium japonicum cells. Mol. Microbiol. 84, 766–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fisher S., Buxbaum L., Toth K., Eisenstadt E., Silver S. (1973) Regulation of manganese accumulation and exchange in Bacillus subtilis W23. J. Bacteriol. 113, 1373–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sobota J. M., Gu M., Imlay J. A. (2014) Intracellular hydrogen peroxide and superoxide poison 3-deoxy-d-arabinoheptulosonate 7-phosphate synthase, the first committed enzyme in the aromatic biosynthetic pathway of Escherichia coli. J. Bacteriol. 196, 1980–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dailey H. A. (1987) Metal inhibition of ferrochelatase. Ann. N.Y. Acad. Sci. 514, 81–86 [DOI] [PubMed] [Google Scholar]
- 87. Li C., Tao J., Mao D., He C. (2011) A novel manganese efflux system, YebN, is required for virulence by Xanthomonas oryzae pv. oryzae. PLoS One 6, e21983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rosch J. W., Gao G., Ridout G., Wang Y. D., Tuomanen E. I. (2009) Role of the manganese efflux system mntE for signalling and pathogenesis in Streptococcus pneumoniae. Mol. Microbiol. 72, 12–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jakubovics N. S., Valentine R. A. (2009) A new direction for manganese homeostasis in bacteria: identification of a novel efflux system in Streptococcus pneumoniae. Mol. Microbiol. 72, 1–4 [DOI] [PubMed] [Google Scholar]
- 90. Lawson R. J., von Wachenfeldt C., Haq I., Perkins J., Munro A. W. (2004) Expression and characterization of the two flavodoxin proteins of Bacillus subtilis, YkuN and YkuP: biophysical properties and interactions with cytochrome P450 BioI. Biochemistry 43, 12390–12409 [DOI] [PubMed] [Google Scholar]
- 91. Chazarreta-Cifre L., Martiarena L., de Mendoza D., Altabe S. G. (2011) Role of ferredoxin and flavodoxins in Bacillus subtilis fatty acid desaturation. J. Bacteriol. 193, 4043–4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Shin J. H., Jung H. J., An Y. J., Cho Y. B., Cha S. S., Roe J. H. (2011) Graded expression of zinc-responsive genes through two regulatory zinc-binding sites in Zur. Proc. Natl. Acad. Sci. U.S.A. 108, 5045–5050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ma Z., Lee J. W., Helmann J. D. (2011) Identification of altered function alleles that affect Bacillus subtilis PerR metal ion selectivity. Nucleic Acids Res. 39, 5036–5044 [DOI] [PMC free article] [PubMed] [Google Scholar]