Abstract
Metal ion assimilation is essential for all forms of life. However, organisms must properly control the availability of these nutrients within the cell to avoid inactivating proteins by mismetallation. To safeguard against an imbalance between supply and demand in eukaryotes, intracellular compartments contain metal transporters that load and unload metals. Although the vacuoles of Saccharomyces cerevisiae and Arabidopsis thaliana are well established locales for the storage of copper, zinc, iron, and manganese, related compartments are emerging as important mediators of metal homeostasis. Here we describe these compartments and review their metal transporter complement.
Keywords: Algae, Chlamydomonas, Plant, Subcellular Organelle, Transport, Acidocalcisome, Permease, Vacuolar
Introduction
Metal ions are simple yet remarkably versatile protein cofactors. Because of this versatility, copper, zinc, iron, and manganese, in particular, occupy numerous and often essential positions within the chemical framework of the cell. They expand the repertoire of protein-catalyzed reactions and enable difficult chemistry, such as dinitrogen reduction and water oxidation. The latter reaction, which enabled bioenergetic diversification, was a monumental event in the evolution of modern metabolic capacity.
To utilize metal ions as catalysts, the cell has had to overcome the unique challenge of specificity and selectively. With few exceptions, proteins have evolved to exploit the properties of a specific metal, and the interaction between a protein and a different metal is invariably unproductive. However, many metalloproteins bind Cu2+ with higher affinity than they do Zn2+, followed in descending order by Fe2+ then Mn2+ (1). Thus, if these metals were presented in equimolar amounts, many apoproteins would consistently “pick” copper.
As a result, mechanisms evolved to ensure that each protein binds the correct metal to the exclusion of others. Apoprotein mismetallation in a non-native environment, either in a different organism (2–4) or in the wrong compartment within the cell (5, 6), demonstrates that when imbalance between metal availability and apoprotein abundance occurs, mismetallation is inevitable.
A key determinant of cell-imposed metal specificity is the orchestrated uptake and distribution of individual metal ions. In addition to regulated transport of metals into/out of cells, protein-to-protein ligand-exchange pathways, as described for Cu1+ transport (7, 8), and involvement of metal-storage proteins, such as ferritin and metallothionein, serve to control the interaction between metals and proteins. Eukaryotes have the benefit of intracellular storage organelles, which provide an additional means to maintain metal homeostasis. In this minireview, we present a synopsis of our current understanding on the role of intracellular transporters in modulating storage and mobilization of metal ions. Although plant and yeast vacuoles are well established organelles for sequestering and mobilizing metal ions, compartments, such as the acidocalcisome and other lysosome-related organelles, are also emerging as important mediators of metal homeostasis.
Metal Homeostasis and Organelles of the Endomembrane System
The endomembrane system is a complex network of membrane-bound compartments that includes the nuclear envelope, the endoplasmic reticulum, the Golgi apparatus, organism- or cell type-specific post-Golgi organelles, and the plasma membrane. A distinguishing feature of this system is the dynamic vesicle-mediated trafficking of lipids, proteins, metabolites, and ions between membranes. In addition to mediating endocytosis, secretion, and excretion, the coordinated exchange of membranes and cargo provides the means to establish a myriad of specialized compartments in response to the needs of the cell.
One such need is the balancing of metal concentrations. Several relatively well characterized compartments house metal transporters that are involved in both the metal-excess and the metal-deficient situations (Fig. 1). In microbes and plants, these compartments (specifically vacuoles) are generally constitutively present in the cell because they carry out functions in addition to metal homeostasis, but the abundance of metal transporters in their membranes changes in response to need (see below). There are other compartments whose presence may be transient and are typically either observed with metal-binding fluorescent probes or identified based on the localization of particular metal transporters.
FIGURE 1.
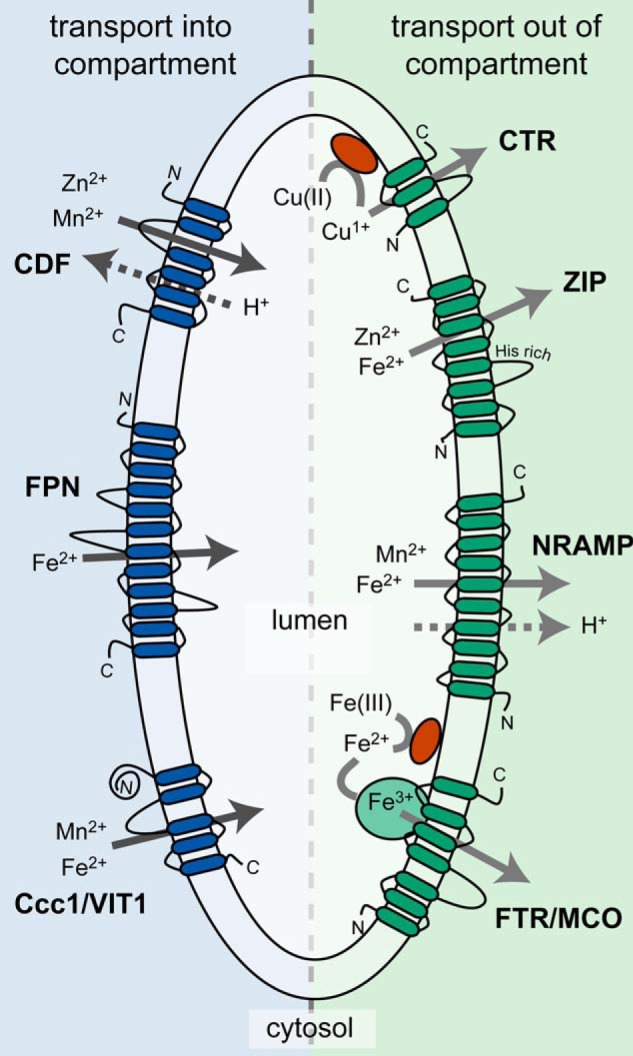
Schematic of common transporter families found in the membranes of vacuoles and other lysosome-related organelles. Predicted membrane topologies and common substrates for each transporter family are shown. Solid arrows represent the direction of metal ion transport. Metal reductases are represented as orange balls. Abbreviations: FPN, ferroportin; FTR, Fe transporter; MCO, multicopper oxidase.
The Vacuole in Saccharomyces cerevisiae
The vacuole of S. cerevisiae is frequently described as an organelle analogous to the lysosome in animals but is a jack-of-all-trades with respect to function. Because the vacuole is the largest organelle in the cell and is easily visualized by light microscopy, the localization of transporters to the vacuolar membrane is readily achieved by immunofluorescence (native or tagged protein) or by imaging a GFP (or other fluorescent protein) fusion.
The involvement of the vacuole in metal homeostasis is reliant in large part on the acidity of its lumen, which is maintained by vacuolar proton-translocating ATPases (V-type ATPases).2 Defective vacuole acidification impacts a wide range of cellular activities including the homeostasis of transition metals (9, 10). At least two metal transporters in the vacuolar membrane are dependent on the proton gradient for activity (members of the NRAMP (metal/proton symporter) and CDF families (metal/proton antiporter)) (Fig. 1).
Although trafficking of metal transporters to the vacuole is uncharacterized, newly synthesized integral membrane proteins are expected to take one of two main routes from the Golgi to the vacuole. The carboxypeptidase Y (CPY) pathway takes cargo through the prevacuolar/late endosomal compartment, whereas vacuolar targeting by the alkaline phosphatase (ALP) pathway is dependent on the adaptor protein complex-3 (AP-3) and bypasses the prevacuolar compartment (11).
Plant Vacuoles
Depending on the developmental stage of the plant and the cell type, at least three different types of vacuoles are described in Arabidopsis thaliana: protein storage, senescent, and lytic. Protein storage vacuoles accumulate primarily in developing seeds and are less acidic than are the other two types but can contain membrane-bound compartments called globoids, which share characteristics with lytic vacuoles (12). The globoids are rich in phytic acid, which is likely responsible for chelating metals (13, 14). A second type of vacuole is the senescence-associated vacuole (SAV), which as the name implies, is involved in degradation of macromolecules during senescence (15). The liberation of metal ions during the degradation of metalloproteins would be expected to occur in SAVs, but the contribution of SAVs to metal homeostasis is unexplored. Lytic vacuoles (also referred to as central vacuoles (CVs)) are the main vacuoles found in vegetative tissue and are the major site of metal sequestration.
As with yeast, the plant CV is not just a site for macromolecule degradation. The contents of the CV reflect the specialized function of each tissue, with pigments in flower vacuoles and some defense metabolites concentrated in the vacuoles of epidermal cells. Both V-type ATPases and proton-translocating pyrophosphatases (V-type PPase) maintain the acidic pH of the lumen (16). The CV contains many different molecules that can bind metals. Glutathione and phytochelatins, also found in the yeast vacuole, have garnered much attention for their role in detoxifying metals such as cadmium and mercury (17, 18).
There are at least two separate routes for the trafficking of newly synthesized transporters to the vacuolar membrane: Ap-3-dependent and Ap-3-independent routes (19). Recently, a trafficking route that bypasses the Golgi has also been described in meristematic root cells (20).
Acidocalcisomes
First characterized in trypanosomatids, the acidocalcisome is a storage organelle defined by the presence of pyrophosphate and polyphosphate complexed with calcium and other divalent metal ions that appears as an electron-dense granule by transmission electron microscopy (21). Acidocalcisomes have been purified from parasitic protozoa, the green alga Chlamydomonas reinhardtii, the red alga Cyanidioschyzon merolae, and the amoeba Dictyostelium discoideum and are thought to be functionally equivalent to a ubiquitous group of polyphosphate-containing compartments formerly referred to as volutin granules or polyphosphate vacuoles. Analogous to the plant vacuole, acidocalcisomes contain both the V-type ATPases and the V-type pyrophosphatases that acidify this compartment.
These organelles contain zinc and iron, and transporters that are predicted to facilitate their uptake (CDF and Ccc1p/VIT1 families, respectively) have been detected by proteomic analysis (22, 23). The presence of copper in these organelles had not been documented until recently because researchers typically determine metal content with x-ray microanalysis of thin sections of biological material using copper grids.
The trafficking of resident proteins to the acidocalcisome is poorly understood and likely varies between organisms. As with the yeast and plant vacuoles, a role for AP-3 has been established in both Trypanosoma brucei (24) and Leishmania major (25), but although AP-3 is essential for acidocalcisome biogenesis in T. brucei, this adaptor complex was important but not essential for L. major acidocalcisomes.
Zincosomes
In fungi and mammals, Zn2+ can be sequestered in small cytoplasmic compartments, which are typically visualized with the fluorescently labeled, zinc-binding probes Zinquin or FluoZin-3. In fungi, these zinc bodies are distinct from the vacuole (26–28) and correspond to an uncharacterized compartment. Because the predominant store of Zn2+ is in the vacuole (29), these compartments may be involved in a transient flux of cytosolic Zn2+. In mammals, the term “zincosome” was coined to refer to similar cytoplasmic foci corresponding to accumulated Zn2+ (30). These foci have different functions depending on the cell type. Zn2+-loaded “storage granules” function in Zn2+ loading of milk (31), neuron function (32), and insulin secretion (33). Zinc bodies also form in response to excess Zn2+ supplementation alone (30, 34) or in combination with either the overexpression or the silencing of intracellularly localized Zn2+ transporters (35, 36).
Gut Granules
Gut granules are acidic organelles that have lysosome-like characteristics and are found in the intestines of the nematode Caenorhabditis elegans. As shown for Zn2+ (37), these organelles may be able to mediate the uptake and distribution of metals from the diet, analogous to what the vacuoles do in plant roots. A high Zn2+ diet induces the formation of a specialized structure that sequesters Zn2+; the gut granules take on a bilobed appearance, and one side of the granule is loaded with Zn2+ by the Zn2+ transporter CDF-2 (37). As shown for the yeast vacuole, whereas these Zn2+ compartments provide resistance to excess Zn2+, the sequestered Zn2+ can be distributed to the rest of the organism when needed.
Metal Transporters
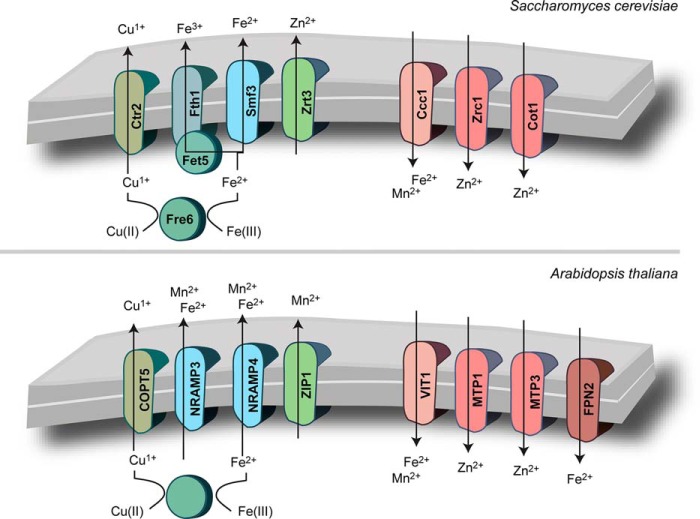
The ability of intracellular organelles to mediate metal availability is dependent on the transporters in their membranes (Fig. 2). In this review, we limit our discussion to those transporters that facilitate the transfer of uncomplexed metal ions across the vacuole membrane, but it should be pointed out that metal conjugates, such as complexes of phytochelatin (38), are also transported into the vacuole. Most of the transporter families were initially recognized for their involvement in the transport of metal ions across the plasma membrane (or assimilation). Subsequently, members of the family were localized to an intracellular compartment (for storage and distribution). In general, for a given transporter type, the direction of transport, i.e. into or out of the cytoplasm, is presumed to be conserved, regardless of the membrane in which the transporter resides.
FIGURE 2.
Metal ion transporters in A. thaliana and S. cerevisiae that enable vacuole-mediated metal storage and mobilization. Transporters from the same family are given the same color.
Transport into the Cytoplasm
NRAMP
These permeases are typically divalent cation/proton symporters (as described for mammalian NRAMP2 and the yeast homolog Smf1p (39, 40)), whereas one NRAMP was shown to have a physiological role in Al3+ transport (41). Although NRAMPs can transport a broad range of divalent cations, as determined mainly with heterologous expression in Xenopus laevis oocytes, these transporters are most often implicated in the transport of Fe2+ and, to a lesser extent, Mn2+ (for a current review, we recommend Ref. 42).
The S. cerevisiae genome encodes three members of the NRAMP family, Smf1p, Smf2p, and Smf3p. Smf1p and Smf2p are involved in Mn2+ transport and are localized to the plasma membrane and small intracellular compartments of unknown function, respectively (43, 44). Smf3p has a steady-state localization to the vacuolar membrane (43). Based on regulation of gene expression (45) and higher iron content of vacuoles from a smf3Δ mutant (46), Smf3 is proposed to mobilize Fe2+ from this compartment in response to iron deficiency.
Of the six NRAMP homologs in A. thaliana, AtNRAMP3 and AtNRAMP4 localize to the vacuolar membrane, and as with SMF3, expression of both genes is increased by iron depletion (47, 48). In seeds, these transporters localize to the globoids, and nramp3 nramp4 double mutant displays defects in mobilization of iron stored in globoids, which is critical during seed germination in iron-depleted soil (47). AtNRAMP3 and AtNRAMP4 are also located in mesophyll cells, the major site for photosynthesis, where they are involved in the mobilization of Mn2+ from vacuoles (49). This source of Mn2+ is required for photosystem II activity when plants face suboptimal manganese nutrition in the soil. Surprisingly, manganese nutrition affects neither the expression nor the abundances of these transporters.
The green alga C. reinhardtii provides a convenient single-celled reference system for studying metal metabolism that is comparable with the mesophyll cell in land plants. Of the four NRAMP members in C. reinhardtii, CrNRAMP4 is both orthologous to AtNRAMP4, and expression of the corresponding gene is increased during growth in iron-deficient medium (50), suggesting that CrNRAMP4 also resides in the membrane of an intracellular compartment and can transport Fe2+ into the cytosol.
ZIP
Members of the ZIP family are characterized as divalent cation transporters (for a current review, we recommend Ref. 51). Zn2+ transport is enhanced by the addition of bicarbonate to transfected mammalian cell lines, suggesting that these permeases are metal/bicarbonate symporters (52, 53). However, based on kinetics of a bacterial homolog in reconstituted proteoliposomes, ZIPs are proposed to mediate Zn2+ electrodiffusion (54).
Of the four ZIPs in S. cerevisiae, Zrt3p localizes to the vacuole. The corresponding gene is a direct target of the Zn2+-responsive regulator Zap1p, and expression is accordingly increased by zinc depletion (55). ZRT3 is not associated with a growth defect in Zn2+-depleted medium unless the genes encoding the two major assimilatory transporters are also deleted (56). Even in the triple knock-out, an obvious growth phenotype is only observed within a narrow window of supplemental Zn2+. This phenotype is typical for vacuole-localized transporters because the transporter is only able to confer a fitness advantage if there is an adequate supply of metal in the vacuole to be mobilized. At low concentrations of Zn2+, there is not enough Zn2+ available to support growth and be stored, whereas at high concentrations of Zn2+, an internal supply is not necessary to support growth. If the vacuole is first loaded with Zn2+ by exposing cells to high levels of Zn2+ in the medium, this store of Zn2+ can sustain growth in the absence of an external supply (29).
In contrast to yeast, the A. thaliana genome encodes 18 members of the ZIP family (57). Of these, only AtZIP1 has been localized to the vacuole so far (58). As with ZRT3, the abundance of AtZIP1 mRNA is higher in response to Zn2+ depletion (59, 60). Not surprising, given the possible redundancy of Zn2+ transport pathways, a growth phenotype linking AtZIP1 to Zn2+ homeostasis has yet to be found (58). However, AtZIP1 is associated with manganese homeostasis because disruption of AtZIP1 causes a growth defect on low manganese and defective root-to-shoot translocation of manganese (58).
CTR
Trimeric CTR is proposed to form a channel-like pore through the membrane; Cu1+ is likely to transverse this pore by a succession of ligand-exchange reactions (61). A negative charge at the extracellular face attracts Cu1+ ions, whereas a positive charge at the cytoplasmic face promotes the exit of Cu1+ (62). As proposed for the ZIP transporters, transport is further facilitated by the concentration gradient across the plasma membrane that is maintained by Cu1+ chelation within the cell. Unlike ZIP transporters, which can transport a broad spectrum of divalent metal cations, CTRs transport monovalent cations, lending substrate specificity to Cu1+ (for a current review, we recommend Ref. 63). Accordingly, transport by CTR requires Cu2+ reduction either at the plasma membrane for assimilation or at the vacuole membrane for mobilization.
CTRs at the vacuole membrane lack the extracellular methionine extensions (ectodomain) that are characteristic of CTRs in the plasma membrane. Intriguingly, cleavage of the ectodomain in mammalian Ctr1 is required for Cu1+ efflux from an endosomal compartment (64), suggesting that the acidic pH of the lumen likely alters the affinity of the ectodomain for Cu1+ (65), hence inhibiting transport.
In S. cerevisiae, Ctr2p (66–68) and the reductase Fre6p are responsible for Cu1+ transport from the vacuole. In the absence of the assimilatory transporters Ctr1p and Ctr3p, Ctr2p is required for Cu,Zn-Sod activity and Fe3+ uptake (because Cu1+ is needed for Fet3p) (68). In A. thaliana, COPT5 is needed during severe copper limitation (69, 70), but unlike the situation in S. cerevisiae (61), neither COPT5 mRNA abundance nor the protein amount is affected by copper (69). In C. reinhardtii, CrCOPT1 is predicted to localize to an intracellular compartment, but as with AtCOPT5, the mRNA abundance of CrCOPT1 is not affected by copper deficiency.
FTR (Fe Transporter)/MCO (Multicopper Oxidase) Complex
S. cerevisiae does not contain ferritin and relies on iron sequestration in the vacuole to balance iron levels in the cell (71). Paralogs of the iron-specific transporter Ftr1p/Fet3p are found in the vacuole membrane, and these function together to mobilize sequestered iron in a situation of iron deficiency (72). Although Smf3p also localizes to the vacuole and mediates Fe2+ transport into the cytosol, SMF3 is negatively regulated by oxygen (73). Therefore, Smf3p is required for Fe2+ mobilization when Ftr1p/Fet3p is inactive due to the absence of oxygen, which is required for ferrous oxidation and transport by this complex. The low affinity Fe2+ transporter in the plasma membrane Fet4p is co-regulated with Smf3p. Fet4p and Smf3p may represent an Fe2+ transport pathway that predates the evolution of Ftr1/Fet3 and Fth1/Fet5. The former evolved under anoxic conditions, whereas the latter was recently acquired.
Transport Out of the Cytoplasm
CDF
CDF transporters catalyze metal/proton antiport, and the acidic lumen of the vacuole therefore facilitates metal transport out of the cytosol. Based on sequence similarity, the CDF family can be divided into functional subgroups that correspond to characterized physiological substrates (57). Within the Zn2+ subgroup, the S. cerevisiae transporters Zrc1p and Cot1p are responsible for resistance to excess Zn2+ accumulation and are also induced by Zn2+ depletion in anticipation of Zn2+ shock, which is likely a more relevant pathway for excess Zn2+ to enter the cell rather than toxic levels in the environment (74). Indeed, this mechanism is probably conserved in C. reinhardtii (CrMTP1) (75) and A. thaliana (AtMTP2) (60), but these transporters have not yet been localized to an intracellular compartment. Two additional Zn2+-type CDF transporters, AtMTP1 (76) and AtMTP3 (77), do localize to the tonoplast and can transport Zn2+ out of the cytosol, whereas a Mn2+-type CDF, AtMTP11, localizes to a trans-Golgi compartment and mediates manganese tolerance (78).
Ccc1/VIT1
This transport family has not yet been characterized biochemically. Proteins containing a domain related to Ccc1p from S. cerevisiae and VIT1 from A. thaliana are found in all three kingdoms of life, but this domain is absent in animals (based on a PSI-BLAST search of GenBankTM; note that Vit1-like sequences found in Lipotes vexillifer and Pantholops hodgsonii genomes are likely from a contaminating source of bacterial DNA given the high degree of similarity between these proteins and ones from Acinetobacter). Several bacterial proteins that contain the Vit1 domain (pfam01988) also contain a ferritin-like domain, which is predicted to extend into the cytosol (79). In bacteria, these homologs are involved in iron efflux from the cell (80, 81), whereas in eukaryotes they are involved in iron loading of the vacuole (82, 83). The Vit1 domain is also found in proteins that reside in endoplasmic reticulum bodies (84) and root nodules (85), but the function of these proteins remains to be determined.
In order for AtNRAMP3 and AtNRAMP4 to mobilize Fe2+ within the seed, Fe2+ must be preloaded into the compartment by AtVIT1. AtVIT1 is highly expressed in developing seeds, and AtVIT1 localizes to the vacuole membrane (86). The absence of this transporter does not affect the absolute concentration of iron in seeds but does lead to an aberrant distribution of iron within the seed (86), which is detrimental when seeds from the mutant are germinated on alkaline soil (because iron is less soluble in alkaline soil, plant iron assimilation is affected). Because of its constitutive role in seed maturation, AtVIT1 expression does not change in response to iron nutrition.
Expression of the two VIT1 homologs in C. reinhardtii is increased under iron deficiency (50) in contrast to the S. cerevisiae homolog, CCC1, whose expression is induced by iron excess (83). Whether these C. reinhardtii homologs also localize to the vacuole and mediate iron transport is unknown, but the differential expression between land plants, algae, and yeast suggests that members of this family have evolved to perform different physiological roles.
Ferroportin
Members of the ferroportin family are well characterized iron exporters in animals that mediate iron mobilization between tissues (87). In A. thaliana, FPN2/IREG2 transports Fe2+ (and other metal ions (88, 89)) into the vacuole. Unlike AtVIT1, FPN2 is expressed in the two outermost layers of the root in response to iron deficiency (88). FPN2 may either buffer the influx of Fe2+ from the soil or serve to protect the plant from iron shock. The protein and its response to iron deficiency is conserved in C. reinhardtii (50).
A Shifting Perspective on Metal Homeostasis
Our picture of metal economy (the uptake and distribution of metal ions) has largely focused on one metal at a time. When faced with metal deficiency, the goal is to increase supply relative to demand (90, 91), while prioritizing the distribution of the limiting cofactor to essential metalloproteins.
However, metal homeostasis can be viewed another way. Deficiency of one metal causes a relative excess of the other metal ions in the cell. Consequently, those proteins lacking their cofactor due to the insufficiency are more susceptible to mismetallation. In several organisms, iron deficiency can lead to the misincorporation of Zn2+ into protoporphyrin IX (92), whereas in yeast, manganese deficiency (generated by deletion of a Mn2+ transporter) can cause misincorporation of Fe2+ into Sod2p (93). Because these “errors” are analogous to those that occur in the metal overload situation resulting from toxic levels in the environment (94–96), the compartmentalization of conditionally excess metal ions is a relatively unexplored mechanism of metal economy.
Metal Sequestration during Deficiency
Poor nutrition of a single metal often affects the cell quota of one or more other metals. The molecular basis for this apparent cross-talk is not completely understood. In several cases, unexpected perturbations to the cellular metal content are attributed to the promiscuity of transport pathways. As an example, the vacuoles of iron-deficient roots of A. thaliana over-accumulate manganese, zinc, and cobalt. IRT1, the major Fe2+ transporter in roots during iron deficiency, can also transport these metals based on 1) heterologous expression in yeast, and 2) the dependency of metal hyper-accumulation on IRT1 expression in A. thaliana (97, 98).
C. reinhardtii hyper-accumulates metal ions specifically during Zn2+ limitation. The metal quota for iron, manganese, and copper is increased to differing degrees (75). Unexpectedly, Zn2+-limited cells are phenotypically copper-deficient as indicated by expression of the copper deficiency regulon and absence of plastocyanin (the major copper-dependent protein in the cell), but not iron-deficient based on reduced abundance of known iron-regulated transcripts (75). Therefore, the copper within the cell is relatively inaccessible to both plastocyanin and the copper regulator CRR1.
An explanation for these observations was attained by analyzing the copper distribution within the cell. Hong-Hermesdorf et al.3 observed the accumulation of copper in punctate cellular bodies in live cells using a fluorescently labeled copper probe, Coppersensor-3, and in fixed cells with nano-scale secondary ion mass spectrometry (NanoSIMS). As with acidocalcisomes, these compartments are electron-dense when viewed by transmission electron microscopy and contain polyphosphate and calcium.
Copper sequestration may be a mechanism to balance the ratio of copper and zinc, thus preventing mismetallation of Zn2+-dependent apoproteins. Plastocyanin, an essential protein for electron transfer in photosynthesis, is dispensable in C. reinhardtii because of the presence of cytochrome c6, a functionally equivalent heme-dependent protein. Therefore, to balance the Cu-Zn ratio, the cell can sequester the plastocyanin-accessible copper pool with little consequence. Cytochrome c oxidase in the mitochondrion, which does not have a copper-independent backup, does not appear to be affected by copper sequestration during zinc limitation. Copper hyper-accumulation (in contrast to copper sequestration) is likely a consequence of compartmentalization and the activation of the CRR1 regulon, which includes the assimilatory Cu1+ transporters CTR1 and CTR2. Because plastocyanin places a relatively large intracellular demand on C. reinhardtii for Cu1+ acquisition (estimated to be 8 × 106 atoms/cell (99)), copper hyper-accumulation provides a ready supply of copper when zinc homeostasis is restored. Indeed, upon Zn2+ resupply, the copper within these bodies is mobilized and used to metallate plastocyanin.
Looking Forward
Metal homeostasis serves to ensure that the accessibility of metal ions is controlled, and apo-proteins only interact with the right metal. One such mechanism is the fine-tuning of metal balance afforded by the ability to partition metal ions through compartmentalization. Presently accessible imaging techniques such as x-ray fluorescence are being successfully employed for the simultaneous analysis of multiple metal ions in tissues with resolution at the single cell level (100). With increased resolution, and with the development of more sophisticated fluorescence-based metal sensors, we expect that many novel aspects of intracellular metal sequestration and distribution will be illuminated.
This work was supported by the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the U. S. Department of Energy (DE-FD02-04ER15529) (to David Eisenberg) and the National Institutes of Health (GM42143) (to S. M.), respectively. This is the fifth article in the Thematic Minireview series “Metals in Biology 2014.”
A. Hong-Hermesdorf, M. Miethke, S. Gallaher, J. Kropat, S. C. Dodani, J. Chan, D. Barupala, D. W. Domaille, D. I. Shirasaki, J. A. Loo, P. K. Weber, J. Pett-Ridge, T. L. Stemmler, C. J. Chang, and S. S. Merchant, submitted for publication.
- V-type ATPases
- vacuolar proton-translocating ATPases
- SAV
- senescence-associated vacuole
- CV
- central vacuole
- NRAMP
- natural resistance-associated macrophage protein
- CDF
- cation diffusion facilitator
- ZIP
- ZRT, IRT-like protein
- CTR
- copper transporter.
REFERENCES
- 1. Williams R. (2001) Chemical selection of elements by cells. Coordin. Chem. Rev. 216, 583–595 [Google Scholar]
- 2. Nar H., Huber R., Messerschmidt A., Filippou A. C., Barth M., Jaquinod M., van de Kamp M., Canters G. W. (1992) Characterization and crystal structure of zinc azurin, a by-product of heterologous expression in Escherichia coli of Pseudomonas aeruginosa copper azurin. Eur. J. Biochem. 205, 1123–1129 [DOI] [PubMed] [Google Scholar]
- 3. Aguirre J. D., Clark H. M., McIlvin M., Vazquez C., Palmere S. L., Grab D. J., Seshu J., Hart P. J., Saito M., Culotta V. C. (2013) A manganese-rich environment supports superoxide dismutase activity in a Lyme disease pathogen, Borrelia burgdorferi. J. Biol. Chem. 288, 8468–8478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tripp B. C., Bell C. B., 3rd, Cruz F., Krebs C., Ferry J. G. (2004) A role for iron in an ancient carbonic anhydrase. J. Biol. Chem. 279, 6683–6687 [DOI] [PubMed] [Google Scholar]
- 5. Tottey S., Waldron K. J., Firbank S. J., Reale B., Bessant C., Sato K., Cheek T. R., Gray J., Banfield M. J., Dennison C., Robinson N. J. (2008) Protein-folding location can regulate manganese-binding versus copper- or zinc-binding. Nature 455, 1138–1142 [DOI] [PubMed] [Google Scholar]
- 6. Luk E., Yang M., Jensen L. T., Bourbonnais Y., Culotta V. C. (2005) Manganese activation of superoxide dismutase 2 in the mitochondria of Saccharomyces cerevisiae. J. Biol. Chem. 280, 22715–22720 [DOI] [PubMed] [Google Scholar]
- 7. Padilla-Benavides T., George Thompson A. M., McEvoy M. M., Argüello J. M. (2014) Mechanism of ATPase-mediated Cu+ export and delivery to periplasmic chaperones: the interaction of Escherichia coli CopA and CusF. J. Biol. Chem. 289, 20492–20501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. González-Guerrero M., Argüello J. M. (2008) Mechanism of Cu+-transporting ATPases: soluble Cu+ chaperones directly transfer Cu+ to transmembrane transport sites. Proc. Natl. Acad. Sci. U.S.A. 105, 5992–5997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eide D. J., Clark S., Nair T. M., Gehl M., Gribskov M., Guerinot M. L., Harper J. F. (2005) Characterization of the yeast ionome: a genome-wide analysis of nutrient mineral and trace element homeostasis in Saccharomyces cerevisiae. Genome Biol. 6, R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramsay L. M., Gadd G. M. (1997) Mutants of Saccharomyces cerevisiae defective in vacuolar function confirm a role for the vacuole in toxic metal ion detoxification. FEMS Microbiol. Lett. 152, 293–298 [DOI] [PubMed] [Google Scholar]
- 11. Cowles C. R., Odorizzi G., Payne G. S., Emr S. D. (1997) The AP-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell 91, 109–118 [DOI] [PubMed] [Google Scholar]
- 12. Rogers J. C. (2011) Internal membranes in maize aleurone protein storage vacuoles: beyond autophagy. Plant Cell 23, 4168–4171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wada T., Lott J. (1997) Light and electron microscopic and energy dispersive x-ray microanalysis studies of globoids in protein bodies of embryo tissues and the aleurone layer of rice (Oryza sativa L.) grains. Can. J. Bot. 75, 1137–1147 [Google Scholar]
- 14. Regvar M., Eichert D., Kaulich B., Gianoncelli A., Pongrac P., Vogel-Mikus K., Kreft I. (2011) New insights into globoids of protein storage vacuoles in wheat aleurone using synchrotron soft x-ray microscopy. J. Exp. Bot. 62, 3929–3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Otegui M. S., Noh Y. S., Martínez D. E., Vila Petroff M. G., Staehelin L. A., Amasino R. M., Guiamet J. J. (2005) Senescence-associated vacuoles with intense proteolytic activity develop in leaves of Arabidopsis and soybean. Plant J. 41, 831–844 [DOI] [PubMed] [Google Scholar]
- 16. Kim E. J., Zhen R. G., Rea P. A. (1994) Heterologous expression of plant vacuolar pyrophosphatase in yeast demonstrates sufficiency of the substrate-binding subunit for proton transport. Proc. Natl. Acad. Sci. U.S.A. 91, 6128–6132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krämer U. (2005) Phytoremediation: novel approaches to cleaning up polluted soils. Curr. Opin. Biotechnol. 16, 133–141 [DOI] [PubMed] [Google Scholar]
- 18. Howe G., Merchant S. (1992) Heavy metal-activated synthesis of peptides in Chlamydomonas reinhardtii. Plant Physiol. 98, 127–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wolfenstetter S., Wirsching P., Dotzauer D., Schneider S., Sauer N. (2012) Routes to the tonoplast: the sorting of tonoplast transporters in Arabidopsis mesophyll protoplasts. Plant Cell 24, 215–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Viotti C., Krüger F., Krebs M., Neubert C., Fink F., Lupanga U., Scheuring D., Boutté Y., Frescatada-Rosa M., Wolfenstetter S., Sauer N., Hillmer S., Grebe M., Schumacher K. (2013) The endoplasmic reticulum is the main membrane source for biogenesis of the lytic vacuole in Arabidopsis. Plant Cell 25, 3434–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Docampo R., de Souza W., Miranda K., Rohloff P., Moreno S. N. (2005) Acidocalcisomes: conserved from bacteria to man. Nat. Rev. Microbiol. 3, 251–261 [DOI] [PubMed] [Google Scholar]
- 22. Ferella M., Nilsson D., Darban H., Rodrigues C., Bontempi E. J., Docampo R., Andersson B. (2008) Proteomics in Trypanosoma cruzi: localization of novel proteins to various organelles. Proteomics 8, 2735–2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yagisawa F., Nishida K., Yoshida M., Ohnuma M., Shimada T., Fujiwara T., Yoshida Y., Misumi O., Kuroiwa H., Kuroiwa T. (2009) Identification of novel proteins in isolated polyphosphate vacuoles in the primitive red alga Cyanidioschyzon merolae. Plant J. 60, 882–893 [DOI] [PubMed] [Google Scholar]
- 24. Huang G., Fang J., Sant'Anna C., Li Z. H., Wellems D. L., Rohloff P., Docampo R. (2011) Adaptor protein-3 (AP-3) complex mediates the biogenesis of acidocalcisomes and is essential for growth and virulence of Trypanosoma brucei. J. Biol. Chem. 286, 36619–36630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Besteiro S., Tonn D., Tetley L., Coombs G. H., Mottram J. C. (2008) The AP3 adaptor is involved in the transport of membrane proteins to acidocalcisomes of Leishmania. J. Cell Sci. 121, 561–570 [DOI] [PubMed] [Google Scholar]
- 26. Eide D. J. (2006) Zinc transporters and the cellular trafficking of zinc. Biochim. Biophys. Acta 1763, 711–722 [DOI] [PubMed] [Google Scholar]
- 27. Devirgiliis C., Murgia C., Danscher G., Perozzi G. (2004) Exchangeable zinc ions transiently accumulate in a vesicular compartment in the yeast Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 323, 58–64 [DOI] [PubMed] [Google Scholar]
- 28. Li L., Kaplan J. (2001) The yeast gene MSC2, a member of the cation diffusion facilitator family, affects the cellular distribution of zinc. J. Biol. Chem. 276, 5036–5043 [DOI] [PubMed] [Google Scholar]
- 29. Simm C., Lahner B., Salt D., LeFurgey A., Ingram P., Yandell B., Eide D. J. (2007) Saccharomyces cerevisiae vacuole in zinc storage and intracellular zinc distribution. Eukaryot. Cell 6, 1166–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haase H., Beyersmann D. (1999) Uptake and intracellular distribution of labile and total Zn(II) in C6 rat glioma cells investigated with fluorescent probes and atomic absorption. Biometals 12, 247–254 [DOI] [PubMed] [Google Scholar]
- 31. McCormick N., Velasquez V., Finney L., Vogt S., Kelleher S. L. (2010) X-ray fluorescence microscopy reveals accumulation and secretion of discrete intracellular zinc pools in the lactating mouse mammary gland. PLoS One 5, e11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khan M., Goldsmith C. R., Huang Z., Georgiou J., Luyben T. T., Roder J. C., Lippard S. J., Okamoto K. (2014) Two-photon imaging of Zn2+ dynamics in mossy fiber boutons of adult hippocampal slices. Proc. Natl. Acad. Sci. U.S.A. 111, 6786–6791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kambe T., Narita H., Yamaguchi-Iwai Y., Hirose J., Amano T., Sugiura N., Sasaki R., Mori K., Iwanaga T., Nagao M. (2002) Cloning and characterization of a novel mammalian zinc transporter, zinc transporter 5, abundantly expressed in pancreatic β cells. J. Biol. Chem. 277, 19049–19055 [DOI] [PubMed] [Google Scholar]
- 34. Haase H., Beyersmann D. (2002) Intracellular zinc distribution and transport in C6 rat glioma cells. Biochem. Biophys. Res. Commun. 296, 923–928 [DOI] [PubMed] [Google Scholar]
- 35. Palmiter R. D., Cole T. B., Findley S. D. (1996) ZnT-2, a mammalian protein that confers resistance to zinc by facilitating vesicular sequestration. EMBO J. 15, 1784–1791 [PMC free article] [PubMed] [Google Scholar]
- 36. Jeong J., Walker J. M., Wang F., Park J. G., Palmer A. E., Giunta C., Rohrbach M., Steinmann B., Eide D. J. (2012) Promotion of vesicular zinc efflux by ZIP13 and its implications for spondylocheiro dysplastic Ehlers-Danlos syndrome. Proc. Natl. Acad. Sci. U.S.A. 109, E3530–E3538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roh H. C., Collier S., Guthrie J., Robertson J. D., Kornfeld K. (2012) Lysosome-related organelles in intestinal cells are a zinc storage site in C. elegans. Cell Metab. 15, 88–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Song W. Y., Park J., Mendoza-Cózatl D. G., Suter-Grotemeyer M., Shim D., Hörtensteiner S., Geisler M., Weder B., Rea P. A., Rentsch D., Schroeder J. I., Lee Y., Martinoia E. (2010) Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc. Natl. Acad. Sci. U.S.A. 107, 21187–21192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gunshin H., Mackenzie B., Berger U. V., Gunshin Y., Romero M. F., Boron W. F., Nussberger S., Gollan J. L., Hediger M. A. (1997) Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388, 482–488 [DOI] [PubMed] [Google Scholar]
- 40. Sacher A., Cohen A., Nelson N. (2001) Properties of the mammalian and yeast metal-ion transporters DCT1 and Smf1p expressed in Xenopus laevis oocytes. J. Exp. Biol. 204, 1053–1061 [DOI] [PubMed] [Google Scholar]
- 41. Xia J., Yamaji N., Kasai T., Ma J. F. (2010) Plasma membrane-localized transporter for aluminum in rice. Proc. Natl. Acad. Sci. U.S.A. 107, 18381–18385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cellier M. F. (2012) Nramp: from sequence to structure and mechanism of divalent metal import. Curr. Top. Membr. 69, 249–293 [DOI] [PubMed] [Google Scholar]
- 43. Portnoy M. E., Liu X. F., Culotta V. C. (2000) Saccharomyces cerevisiae expresses three functionally distinct homologues of the NRAMP family of metal transporters. Mol. Cell. Biol. 20, 7893–7902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Luk E. E., Culotta V. C. (2001) Manganese superoxide dismutase in Saccharomyces cerevisiae acquires its metal co-factor through a pathway involving the Nramp metal transporter, Smf2p. J. Biol. Chem. 276, 47556–47562 [DOI] [PubMed] [Google Scholar]
- 45. Portnoy M. E., Jensen L. T., Culotta V. C. (2002) The distinct methods by which manganese and iron regulate the Nramp transporters in yeast. Biochem. J. 362, 119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Singh A., Kaur N., Kosman D. J. (2007) The metalloreductase Fre6p in Fe-efflux from the yeast vacuole. J. Biol. Chem. 282, 28619–28626 [DOI] [PubMed] [Google Scholar]
- 47. Lanquar V., Lelièvre F., Bolte S., Hamès C., Alcon C., Neumann D., Vansuyt G., Curie C., Schröder A., Krämer U., Barbier-Brygoo H., Thomine S. (2005) Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J. 24, 4041–4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thomine S., Lelièvre F., Debarbieux E., Schroeder J. I., Barbier-Brygoo H. (2003) AtNRAMP3, a multispecific vacuolar metal transporter involved in plant responses to iron deficiency. Plant J. 34, 685–695 [DOI] [PubMed] [Google Scholar]
- 49. Lanquar V., Ramos M. S., Lelièvre F., Barbier-Brygoo H., Krieger-Liszkay A., Krämer U., Thomine S. (2010) Export of vacuolar manganese by AtNRAMP3 and AtNRAMP4 is required for optimal photosynthesis and growth under manganese deficiency. Plant Physiol. 152, 1986–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Urzica E. I., Casero D., Yamasaki H., Hsieh S. I., Adler L. N., Karpowicz S. J., Blaby-Haas C. E., Clarke S. G., Loo J. A., Pellegrini M., Merchant S. S. (2012) Systems and trans-system level analysis identifies conserved iron deficiency responses in the plant lineage. Plant Cell 24, 3921–3948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jeong J., Eide D. J. (2013) The SLC39 family of zinc transporters. Mol. Aspects Med. 34, 612–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. He L., Girijashanker K., Dalton T. P., Reed J., Li H., Soleimani M., Nebert D. W. (2006) ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: characterization of transporter properties. Mol. Pharmacol. 70, 171–180 [DOI] [PubMed] [Google Scholar]
- 53. Gaither L. A., Eide D. J. (2000) Functional expression of the human hZIP2 zinc transporter. J. Biol. Chem. 275, 5560–5564 [DOI] [PubMed] [Google Scholar]
- 54. Lin W., Chai J., Love J., Fu D. (2010) Selective electrodiffusion of zinc ions in a Zrt-, Irt-like protein, ZIPB. J. Biol. Chem. 285, 39013–39020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lyons T. J., Gasch A. P., Gaither L. A., Botstein D., Brown P. O., Eide D. J. (2000) Genome-wide characterization of the Zap1p zinc-responsive regulon in yeast. Proc. Natl. Acad. Sci. U.S.A. 97, 7957–7962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. MacDiarmid C. W., Gaither L. A., Eide D. (2000) Zinc transporters that regulate vacuolar zinc storage in Saccharomyces cerevisiae. EMBO J. 19, 2845–2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Blaby-Haas C. E., Merchant S. S. (2012) The ins and outs of algal metal transport. Biochim. Biophys. Acta 1823, 1531–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Milner M. J., Seamon J., Craft E., Kochian L. V. (2013) Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. J. Exp. Bot. 64, 369–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Grotz N., Fox T., Connolly E., Park W., Guerinot M. L., Eide D. (1998) Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc. Natl. Acad. Sci. U.S.A. 95, 7220–7224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. van de Mortel J. E., Almar Villanueva L., Schat H., Kwekkeboom J., Coughlan S., Moerland P. D., Ver Loren van Themaat E., Koornneef M., Aarts M. G. (2006) Large expression differences in genes for iron and zinc homeostasis, stress response, and lignin biosynthesis distinguish roots of Arabidopsis thaliana and the related metal hyperaccumulator Thlaspi caerulescens. Plant Physiol. 142, 1127–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. De Feo C. J., Aller S. G., Siluvai G. S., Blackburn N. J., Unger V. M. (2009) Three-dimensional structure of the human copper transporter hCTR1. Proc. Natl. Acad. Sci. U.S.A. 106, 4237–4242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tsigelny I. F., Sharikov Y., Greenberg J. P., Miller M. A., Kouznetsova V. L., Larson C. A., Howell S. B. (2012) An all-atom model of the structure of human copper transporter 1. Cell Biochem. Biophys 63, 223–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pope C. R., Flores A. G., Kaplan J. H., Unger V. M. (2012) Structure and function of copper uptake transporters. Curr. Top. Membr. 69, 97–112 [DOI] [PubMed] [Google Scholar]
- 64. Öhrvik H., Nose Y., Wood L. K., Kim B. E., Gleber S. C., Ralle M., Thiele D. J. (2013) Ctr2 regulates biogenesis of a cleaved form of mammalian Ctr1 metal transporter lacking the copper- and cisplatin-binding ecto-domain. Proc. Natl. Acad. Sci. U.S.A. 110, E4279–E4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rubino J. T., Chenkin M. P., Keller M., Riggs-Gelasco P., Franz K. J. (2011) A comparison of methionine, histidine and cysteine in copper(I)-binding peptides reveals differences relevant to copper uptake by organisms in diverse environments. Metallomics 3, 61–73 [PubMed] [Google Scholar]
- 66. Rees E. M., Lee J., Thiele D. J. (2004) Mobilization of intracellular copper stores by the Ctr2 vacuolar copper transporter. J. Biol. Chem. 279, 54221–54229 [DOI] [PubMed] [Google Scholar]
- 67. Rees E. M., Thiele D. J. (2007) Identification of a vacuole-associated metalloreductase and its role in Ctr2-mediated intracellular copper mobilization. J. Biol. Chem. 282, 21629–21638 [DOI] [PubMed] [Google Scholar]
- 68. Portnoy M. E., Schmidt P. J., Rogers R. S., Culotta V. C. (2001) Metal transporters that contribute copper to metallochaperones in Saccharomyces cerevisiae. Mol. Genet. Genomics 265, 873–882 [DOI] [PubMed] [Google Scholar]
- 69. Garcia-Molina A., Andrés-Colás N., Perea-García A., Del Valle-Tascón S., Peñarrubia L., Puig S. (2011) The intracellular Arabidopsis COPT5 transport protein is required for photosynthetic electron transport under severe copper deficiency. Plant J. 65, 848–860 [DOI] [PubMed] [Google Scholar]
- 70. Klaumann S., Nickolaus S. D., Fürst S. H., Starck S., Schneider S., Ekkehard Neuhaus H., Trentmann O. (2011) The tonoplast copper transporter COPT5 acts as an exporter and is required for interorgan allocation of copper in Arabidopsis thaliana. New Phytol. 192, 393–404 [DOI] [PubMed] [Google Scholar]
- 71. Raguzzi F., Lesuisse E., Crichton R. R. (1988) Iron storage in Saccharomyces cerevisiae. FEBS Lett. 231, 253–258 [DOI] [PubMed] [Google Scholar]
- 72. Urbanowski J. L., Piper R. C. (1999) The iron transporter Fth1p forms a complex with the Fet5 iron oxidase and resides on the vacuolar membrane. J. Biol. Chem. 274, 38061–38070 [DOI] [PubMed] [Google Scholar]
- 73. Jensen L. T., Culotta V. C. (2002) Regulation of Saccharomyces cerevisiae FET4 by oxygen and iron. J. Mol. Biol. 318, 251–260 [DOI] [PubMed] [Google Scholar]
- 74. MacDiarmid C. W., Milanick M. A., Eide D. J. (2002) Biochemical properties of vacuolar zinc transport systems of Saccharomyces cerevisiae. J. Biol. Chem. 277, 39187–39194 [DOI] [PubMed] [Google Scholar]
- 75. Malasarn D., Kropat J., Hsieh S. I., Finazzi G., Casero D., Loo J. A., Pellegrini M., Wollman F. A., Merchant S. S. (2013) Zinc deficiency impacts CO2 assimilation and disrupts copper homeostasis in Chlamydomonas reinhardtii. J. Biol. Chem. 288, 10672–10683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kawachi M., Kobae Y., Mori H., Tomioka R., Lee Y., Maeshima M. (2009) A mutant strain Arabidopsis thaliana that lacks vacuolar membrane zinc transporter MTP1 revealed the latent tolerance to excessive zinc. Plant Cell Physiol. 50, 1156–1170 [DOI] [PubMed] [Google Scholar]
- 77. Arrivault S., Senger T., Krämer U. (2006) The Arabidopsis metal tolerance protein AtMTP3 maintains metal homeostasis by mediating Zn exclusion from the shoot under Fe deficiency and Zn oversupply. Plant J. 46, 861–879 [DOI] [PubMed] [Google Scholar]
- 78. Peiter E., Montanini B., Gobert A., Pedas P., Husted S., Maathuis F. J., Blaudez D., Chalot M., Sanders D. (2007) A secretory pathway-localized cation diffusion facilitator confers plant manganese tolerance. Proc. Natl. Acad. Sci. U.S.A. 104, 8532–8537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Andrews S. C. (2010) The Ferritin-like superfamily: evolution of the biological iron storeman from a rubrerythrin-like ancestor. Biochim. Biophys. Acta 1800, 691–705 [DOI] [PubMed] [Google Scholar]
- 80. Bhubhanil S., Chamsing J., Sittipo P., Chaoprasid P., Sukchawalit R., Mongkolsuk S. (2014) Roles of Agrobacterium tumefaciens membrane-bound ferritin (MbfA) in iron transport and resistance to iron under acidic conditions. Microbiology 160, 863–871 [DOI] [PubMed] [Google Scholar]
- 81. Sankari S., O'Brian M. R. (2014) A bacterial iron exporter for maintenance of iron homeostasis. J. Biol. Chem. 289, 16498–16507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Li L., Chen O. S., McVey Ward D., Kaplan J. (2001) CCC1 is a transporter that mediates vacuolar iron storage in yeast. J. Biol. Chem. 276, 29515–29519 [DOI] [PubMed] [Google Scholar]
- 83. Li L., Bagley D., Ward D. M., Kaplan J. (2008) Yap5 is an iron-responsive transcriptional activator that regulates vacuolar iron storage in yeast. Mol. Cell. Biol. 28, 1326–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yamada K., Nagano A. J., Nishina M., Hara-Nishimura I., Nishimura M. (2013) Identification of two novel endoplasmic reticulum body-specific integral membrane proteins. Plant Physiol. 161, 108–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hakoyama T., Niimi K., Yamamoto T., Isobe S., Sato S., Nakamura Y., Tabata S., Kumagai H., Umehara Y., Brossuleit K., Petersen T. R., Sandal N., Stougaard J., Udvardi M. K., Tamaoki M., Kawaguchi M., Kouchi H., Suganuma N. (2012) The integral membrane protein SEN1 is required for symbiotic nitrogen fixation in Lotus japonicus nodules. Plant Cell Physiol. 53, 225–236 [DOI] [PubMed] [Google Scholar]
- 86. Kim S. A., Punshon T., Lanzirotti A., Li L., Alonso J. M., Ecker J. R., Kaplan J., Guerinot M. L. (2006) Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science 314, 1295–1298 [DOI] [PubMed] [Google Scholar]
- 87. Ward D. M., Kaplan J. (2012) Ferroportin-mediated iron transport: expression and regulation. Biochim. Biophys. Acta 1823, 1426–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Schaaf G., Honsbein A., Meda A. R., Kirchner S., Wipf D., von Wirén N. (2006) AtIREG2 encodes a tonoplast transport protein involved in iron-dependent nickel detoxification in Arabidopsis thaliana roots. J. Biol. Chem. 281, 25532–25540 [DOI] [PubMed] [Google Scholar]
- 89. Morrissey J., Baxter I. R., Lee J., Li L., Lahner B., Grotz N., Kaplan J., Salt D. E., Guerinot M. L. (2009) The ferroportin metal efflux proteins function in iron and cobalt homeostasis in Arabidopsis. Plant Cell 21, 3326–3338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Merchant S. S., Helmann J. D. (2012) Elemental economy: microbial strategies for optimizing growth in the face of nutrient limitation. Adv. Microb. Physiol. 60, 91–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Blaby-Haas C., Merchant S. (2013) Sparing and salvaging metals in chloroplasts. in Encyclopedia of Inorganic and Bioinorganic Chemistry, pp. 51–64, John Wiley & Sons, Chichester, West Sussex, United Kingdom [Google Scholar]
- 92. Labbé R. F., Rettmer R. L., Shah A. G., Turnlund J. R. (1987) Zinc protoporphyrin: past, present, and future. Ann. N.Y. Acad. Sci. 514, 7–14 [DOI] [PubMed] [Google Scholar]
- 93. Yang M., Cobine P. A., Molik S., Naranuntarat A., Lill R., Winge D. R., Culotta V. C. (2006) The effects of mitochondrial iron homeostasis on cofactor specificity of superoxide dismutase 2. EMBO J. 25, 1775–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Macomber L., Imlay J. A. (2009) The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. U.S.A. 106, 8344–8349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Macomber L., Hausinger R. P. (2011) Mechanisms of nickel toxicity in microorganisms. Metallomics 3, 1153–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gu M., Imlay J. A. (2013) Superoxide poisons mononuclear iron enzymes by causing mismetallation. Mol. Microbiol. 89, 123–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Korshunova Y. O., Eide D., Clark W. G., Guerinot M. L., Pakrasi H. B. (1999) The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Mol. Biol. 40, 37–44 [DOI] [PubMed] [Google Scholar]
- 98. Vert G., Grotz N., Dédaldéchamp F., Gaymard F., Guerinot M. L., Briat J. F., Curie C. (2002) IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14, 1223–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Merchant S., Hill K., Howe G. (1991) Dynamic interplay between two copper-titrating components in the transcriptional regulation of cyt c6. EMBO J. 10, 1383–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhao F. J., Moore K. L., Lombi E., Zhu Y. G. (2014) Imaging element distribution and speciation in plant cells. Trends Plant Sci. 19, 183–192 [DOI] [PubMed] [Google Scholar]