Background: A set of miRNAs accumulates in polarized macrophages.
Results: The same miRNAs accrued during macrophage differentiation with varied rates depending on exogenous conditions and were regulated at a preprocessing step.
Conclusion: Key monocytic cell miRNAs accumulated in response to activation and differentiation signals.
Significance: Accumulation of key miRNAs was tailored to diverse stimuli leading to customized miRNA expression.
Keywords: Cell Differentiation, Dicer, Gene Expression, Macrophage, MicroRNA (miRNA), Monocyte
Abstract
Circulating monocytes recruited to tissues can differentiate into macrophages and adopt unique gene expression programs in response to environmental cues. We recently described the regulated expression of several microRNAs (miRNAs) in polarized human monocyte-derived macrophages (MDMs). Basal expression of these activation-associated miRNAs was low in monocytes relative to MDMs. As development occurs in the context of specific cellular environments, we hypothesized that the rate of miRNA accumulation would be modified in the presence of microbial or cellular products during monocyte-to-macrophage differentiation. Indeed, LPS treatment augmented the accumulation of miR-146a and miR-155, whereas IL-4 treatment augmented the accumulation of miR-193b and miR-222 during development. In contrast, some stimuli repressed accumulation of specific miRNAs including interferons (IFNs) (miR-27a, miR-125a-5p, and miR-222), IL-4 (miR-125a-5p), and LPS (miR-27a). RT-PCR-based expression profiling of monocytes differentiated with distinct methods showed that activation-associated miRNAs and markers of macrophage polarization were substantially altered in MDMs differentiated in the presence of non-monocytic peripheral blood mononuclear cells due in part to NF-κB and STAT1 pathway activation. Expression of several of these miRNAs was regulated at a preprocessing step because the expression of the primary miRNAs, but not Dicer, correlated with mature miRNA expression. We conclude that a set of miRNAs is regulated during MDM differentiation, and the rate is uniquely modified for each miRNA by environmental factors. The low basal expression of activation-associated miRNAs in monocytes and their dynamic rates of accumulation during MDM differentiation permit monocytes to tailor miRNA profiles in peripheral tissues during differentiation to macrophages.
Introduction
Tissue-resident macrophages established prior to birth from yolk sac- and fetal liver-derived progenitors replicate to maintain populations under homeostatic conditions (1–3). Disruption of tissues due to disturbances such as infection, inflammation, and injury can lead to reduced numbers of tissue-resident macrophages in a phenomenon known as the disappearance reaction (1, 4–6). During this time, bone marrow-derived monocytes are recruited to the affected tissue and differentiate into macrophages. In contrast to the resident macrophages, the infiltrating macrophages often are a transient population. Upon resolution of tissue disturbances, the balance of tissue macrophage populations is restored through both maintenance of some infiltrating macrophages and replication of the remaining original tissue-resident macrophages (2, 3, 5, 7).
Despite their high abundance in most tissues, our understanding of human tissue-resident macrophages is poor due to limitations in obtaining these cells (8). Conversely, infiltrating macrophages can be modeled ex vivo by differentiating peripheral blood monocytes to MDMs.2 Tissue-infiltrating monocytes respond to local environments by dramatically altering their gene expression profiles and consequently their functional phenotypes as they differentiate to macrophages. The addition of cytokines and other activating stimuli ex vivo to monocyte cultures can be utilized to represent possible conditions encountered by monocytes recruited into tissues. Monocytic cell gene expression regulation occurs at many levels. miRNAs have important roles in regulating gene expression during innate and adaptive immune responses (9, 10). However, the impact of miRNAs during monocytic cell differentiation and activation is relatively understudied. To investigate this, a better understanding of miRNA expression regulation during these processes is needed.
Biogenesis of miRNAs is a multistep process (11). In the cell nucleus, a stem loop structure within a primary miRNA (pri-miRNA) transcript is recognized and cleaved by the exonuclease Drosha. After export to the cytoplasm, cleavage of the terminal loop by a complex containing the exonuclease Dicer results in a double-stranded RNA composed of a guide strand and a passenger strand. The guide strand has a long half-life as it is protected within the miRNA-binding cleft of an miRNA-induced silencing complex, whereas the passenger strand is excluded from the miRNA-induced silencing complex and degraded rapidly. Watson-Crick base pairing between the guide strand of the miRNA-induced silencing complex and its targeted mRNAs diminishes gene expression primarily by mRNA destabilization (12). Furthermore, the miRNA-induced silencing complex acts to inhibit protein translation at multiple steps (13).
We recently monitored miRNA expression in MDMs treated with a variety of polarizing conditions including IFNγ plus LPS (M1-polarized), IL-4 (M2a-polarized), immune complexes plus LPS (M2b-polarized), and TGFβ (M2c-polarized). There were consistent changes in several miRNA passenger strands, but surprisingly there were few miRNA guide strands whose abundance changed significantly in these conditions (14). With the exception of miR-193b, the amplitude of change in the abundance of individual guide strands was less than 2-fold (14). In contrast to the small changes in miRNA expression described in our previous study, several studies have described robust induction of a subset of miRNAs in human monocytes responding to LPS and other inflammatory treatments (15–19). These observations led us to the important discovery that all activation-associated miRNAs tested had higher basal expression levels in MDMs than in monocytes (14). Therefore, these miRNAs accumulated at some point during monocyte-to-macrophage differentiation.
In the current study, we characterized the expression regulation of the miRNAs that were induced by macrophage-activating conditions (14) during monocyte-to-macrophage differentiation under a variety of environmental conditions. We hypothesized that the low basal expression of these miRNAs in monocytes would permit monocytes to adopt unique miRNA expression profiles in response to varied environmental conditions during monocyte-to-macrophage differentiation. Supporting this hypothesis, the accumulation rate of these miRNAs in monocytic cells was modified by IFNβ, IFNγ, IL-4, and LPS. We also show that co-culture of monocytes with non-monocytic peripheral blood mononuclear cells (PBMCs) during differentiation, a standard method of generating MDMs ex vivo, profoundly altered the expression of many miRNAs and numerous mRNA markers of macrophage polarization. These findings suggest that, by maintaining low expression levels of activation-associated miRNAs such as miR-155 and miR-193b, monocytes can have appropriately tailored responses to environmental stimuli such as cytokines, cell-cell interactions, and pathogen-derived molecules as they differentiate to macrophages in peripheral tissues.
EXPERIMENTAL PROCEDURES
Human Subjects
Human subject protocols were approved by Institutional Review Boards of the University of Iowa and the Iowa City Veterans Affairs Medical Center. Peripheral blood samples were obtained anonymously in the form of leukocyte reduction system cones from the DeGowin Blood Bank at the University of Iowa.
Cell Culture
Monocytic cells were isolated from blood samples using two standard protocols. Each method started with PBMCs isolated using Ficoll-Paque Plus (GE Healthcare) density gradients.
In the first method, monocytes were isolated on the day of blood donation directly from PBMCs using CD14 MicroBeads (Miltenyi Biotec). The purified monocytes were then cultured in tissue culture plates at 1 × 106 cells/ml in RP-10 (RPMI 1640 medium (Invitrogen) with FBS (10%, v/v; Invitrogen) and l-glutamine (2 mm; Invitrogen)) supplemented with M-CSF (50 ng/ml). In some experiments, monocytes (1 × 106 cells/ml) were co-cultured with negatively selected, non-monocytic PBMCs from the CD14 MicroBead-mediated sort (5 × 106 cells/ml) in RP-10 supplemented with M-CSF (50 ng/ml).
The second method, described in our previous study (14), selects MDMs from total PBMC cultures based on adherence. Briefly, PBMCs were cultured at 37 °C with 5% CO2 in Petri dishes at a density of 5 × 106 cells/ml in RP-10 supplemented with M-CSF (5 ng/ml; eBioscience). After 4 days of culture, Petri plates were washed extensively with Hanks' balanced salt solution lacking divalent cations (Invitrogen) to remove nonadherent cells. The adherent MDMs were trypsinized and pelleted. Upon resuspension at 1 × 106 cells/ml in RP-10 with M-CSF (5 ng/ml), the MDMs were incubated at 37 °C in Teflon jars overnight. MDMs were subsequently seeded in tissue culture plates and incubated for 8 h at 37 °C with 5% CO2 prior to study.
Monocyte Activation
Activation of purified monocytes was achieved by supplementing RP-10 with phenol-extracted Escherichia coli 055:B5 LPS (Sigma-Aldrich), IL-4 (Peprotech), IFNγ (Peprotech), or IFNβ (Peprotech) at the indicated concentrations.
Reverse Transcription-Quantitative PCR (RT-qPCR)
Total cellular RNA was purified from monocytes and MDMs using TRIzol (Invitrogen). For TaqMan MicroRNA Assays, miRNA-specific reverse transcription reactions were performed with MegaScript RT (Applied Biosystems). For TaqMan Gene Expression Assays (Applied Biosystems), random hexamer-primed reverse transcription reactions were performed with SuperScript III RT (Invitrogen). PCR with TaqMan Gene Expression Assays used TaqMan Universal PCR Master Mix (Applied Biosystems). Measurements of β-actin and TATA box-binding protein were used as endogenous controls for gene expression assays, whereas RNU48 was used as an endogenous control for miRNA assays. PCR was performed on an 7900HT Fast Real-Time PCR System (Applied Biosystems). Cycle threshold (Ct) values were calculated, and quality control was performed using SDS v2.4 (Applied Biosystems). Changes in expression were calculated using the ΔΔCt method and converted to log2 scale using Excel 2007 (Microsoft).
TaqMan Low Density Array (TLDA) Human miRNA Assays (Applied Biosystems), a gift from Dr. Martha Monick (University of Iowa), were used to profile miRNA abundance. Briefly, cDNA was generated using the TaqMan Reverse Transcription kit and Megaplex Primer Pool A v2.0. Preamplification was performed using TaqMan PreAmp Master Mix and Megaplex PreAmp Primer Pool A v2.0. Finally, TLDA cards were loaded with preamplified reactions, and PCR was performed on a 7900HT Fast Real-Time PCR System. Changes in miRNA expression levels were calculated (ΔΔCt method) using SDS v2.3 and SDS RQ Manager v1.2 software in tandem. RNU48 was used as an endogenous control. miRNA expression in MDMs differentiated either in the presence or absence of non-monocytic PBMCs was compared with that in undifferentiated monocytes.
Integrated fluidic circuit-based RT-qPCR was performed using the Bio-Mark System for Genetic Analysis (Fluidigm). First, RNA was reverse transcribed using random hexamer-primed reactions with SuperScript III RT. Next, preamplification of the RT product was performed using a pool of 48 Individual TaqMan Gene Expression Assays with TaqMan PreAmp MasterMix according to recommendations by Fluidigm. Finally, gene expression was monitored by real time PCR on 48.48 Dynamic Array integrated fluidic circuits. Ct values were calculated, and quality control was performed using Fluidigm Real-Time PCR Analysis v3.1.3 software (Fluidigm). Measurements of ACTB, B2M, and TBP protein were used as endogenous controls. Changes in expression were calculated using the ΔΔCt method and converted to log2 scale using Excel 2007. Unsupervised hierarchal clustering of transcripts that had regulated expression (>4-fold in one or more of the samples relative to monocytes harvested immediately after MicroBead-based purification) was performed using Partek Genomic Suite v6.5 (Partek Inc.). Data sets for both the TLDA assays measuring miRNA abundance and the integrated fluidic circuit-based RT-PCR experiments are available in the Gene Expression Omnibus as a SuperSeries under accession number GSE60550.
Immunoblotting
In some experiments, protein lysates were collected at the indicated times from cultures of purified monocytes or from adherent MDMs in SDS-PAGE buffer. In other experiments, stocks of control conditioned medium were obtained on day 5 from cultures of CD14 MicroBead-purified monocytes grown at 1 × 106 cells/ml in RP-10 supplemented with M-CSF (50 ng/ml). Conditioned medium was also collected on day 5 from PBMC cultures grown at 5 × 106 cells/ml in RP-10 supplemented with M-CSF (5 ng/ml). CD14 MicroBead-purified monocytes were cultured at 1 × 106 cells/ml in RP-10 with M-CSF (50 ng/ml) for 5 days in 24-well plates to obtain MDMs. Cell culture medium was removed from the MDMs and immediately replaced with the two conditioned medium types. Lysates were collected in SDS-PAGE buffer at the indicated times.
After separation of protein samples on 10% acrylamide Tris-glycine gels and transfer to nitrocellulose, membranes were stained using Ponceau S to confirm equivalent protein loading for each sample. The membranes were then probed with antibodies specific for Dicer (Abcam), GAPDH (Abcam), phosphotyrosine 701 (Tyr(P)-701) STAT1 (Abcam), total STAT1 (Abcam), IκBα (Cell Signaling Technology), and β-tubulin (Developmental Studies Hybridoma Bank, University of Iowa) as indicated. HRP-conjugated secondary antibodies were detected using an LAS-4000 imaging system (Fujifilm) after adding Pierce ECL Western blotting substrate (Thermo Scientific).
Statistical Analyses
Paired, two-tailed t tests or linear mixed model analyses for a randomized block design were used to compare ΔCt values between treatments and controls. For linear mixed model analyses, Dunnett's post hoc tests were performed as indicated. p values were calculated using GraphPad Prism 5 (GraphPad Software, Inc.).
RESULTS
LPS and Interferon Treatments Modified the Accumulation Rate of Several Activation-associated miRNAs in ex Vivo Monocyte Cultures
We reported previously that expression of several miRNAs is regulated in response to macrophage-activating conditions. We also observed that these miRNAs were expressed at lower abundance in monocytes relative to MDMs (14), leading us to hypothesize that the expression of these miRNAs occurred during the process of differentiation in addition to activation. We began our current study of these polarization-associated miRNAs by investigating the timing of expression level changes during the monocyte-to-macrophage differentiation process and whether the accumulation rates were modified in response to activating conditions. In untreated monocytes, the abundance of all seven miRNAs tested increased during the first 24 h of ex vivo culture (Fig. 1). The amplitude of miR-125a-5p increase in untreated monocytes was large (5.77 on a log2 scale, the equivalent of 55-fold change), whereas the increase was small for miR-146a, miR-155, and miR-222 during this period.
FIGURE 1.
The accumulation of some miRNAs during 24 h of monocyte ex vivo culture was modified by LPS, IFNβ and IFNγ. RT-qPCR was used to compare miRNA abundance in freshly isolated monocytes versus monocytes maintained in culture for 24 h with or without LPS (10 ng/ml) and/or interferons (each at 20 ng/ml). Graphs represent the mean ± S.E. -fold change at 24 h post-treatment (n = 3) relative to monocytes collected immediately after cell sorting. Dotted horizontal lines across each graph mark the expression change in the untreated monocytes at the 24-h time point. Solid horizontal bars indicate statistical significance (p < 0.05) using Tukey's post-test of mixed model analysis. Error bars represent S.E.
The data showed that expression of each of the miRNAs tested is uniquely modified by three biological agents involved in induction of inflammatory activation patterns (Fig. 1). As reported by us and others, LPS treatment augmented the accumulation of miR-146a and miR-155 in human monocytic cells (14, 15, 18). In contrast, LPS treatment repressed miR-27a to levels lower than those observed in monocytes prior to ex vivo culture (Fig. 1). Our prior report showed that IFNγ could antagonize LPS-mediated induction of miR-125a-5p, miR-146a, and miR-222 but not miR-155 in phorbol 12-myristate 13-acetate-differentiated THP-1 cells (14). In primary human monocytes, Toll-like receptor and IFN signaling pathways also modified the regulation of these miRNAs (Fig. 1). We included the type 1 IFN, IFNβ, in our analysis to contrast with results seen with the type 2 IFN, IFNγ. Treatment with either IFN significantly antagonized the accumulation of not only miR-27a but also miR-125a-5p and miR-222. Furthermore, each IFN partially antagonized LPS-mediated induction of miR-146a; a 19-fold increase (4.3 on log2 scale) in miR-146a abundance in response to LPS was diminished to 11-fold (3.5 on log2 scale) in response to the LPS/IFN combinational treatments.
IFNγ and IFNβ treatments were not always similar in their ability to modify miRNA expression as exemplified by their effects on miR-27a and miR-29b. Although each IFN treatment antagonized miR-27a, the repression in response to IFNβ treatment was significantly greater than that in response to IFNγ treatment. Expression of miR-29b was also significantly lower in response to IFNβ treatment than to IFNγ treatment.
IL-4 Treatment Modified the Accumulation Rate of a Subset of Activation-associated miRNAs in ex Vivo Monocyte Cultures
We monitored miRNA expression levels in monocytes responding to IL-4 treatment. Accumulation of both miR-193b and miR-222 was augmented in monocytes treated with IL-4 (Fig. 2). It is important to note that miR-222 is one of the most abundant miRNAs in monocytes according to both microarray data (15) and RT-PCR Ct values (data not shown), so the apparently modest increase in this miRNA may have a larger impact on monocyte biology than the small -fold change would imply.
FIGURE 2.

Several miRNAs had altered accumulation rates in human monocytes responding to IL-4 stimulation. RT-qPCR was used to compare miRNA abundance in freshly isolated monocytes versus monocytes maintained in culture for 24 h with or without IL-4 (20 ng/ml). Graphs represent the mean ± S.E. -fold change at 24 h post-treatment (n = 3) relative to monocytes collected immediately after cell sorting. Asterisks indicate statistical significance (p < 0.05) between treated and untreated monocytes at the 24-h time point using a paired, two-tailed t test. Error bars represent S.E.
IL-4 treatment may have reduced the rate at which several miRNAs accumulated relative to untreated cells. However, among these miRNAs, only miR-125a-5p reached statistical significance (Fig. 2).
Diverse Rates of Developmentally Induced miRNA Accumulation Were Observed during ex Vivo Culture of Purified Monocytes
The above results indicated that accumulation of all seven miRNAs examined began early in the monocyte-to-macrophage differentiation process. The kinetics of accumulation was monitored in purified monocytes over the course of 5 days of differentiation toward MDMs. Each miRNA accumulated in cultured monocytes with a unique rate and amplitude (Fig. 3). Whereas the increase in miR-27a, miR-29b, and miR-125a-5p abundance occurred mostly within the 1st day, the abundance of miR-146a and miR-193b did not reach maximal expression levels until day 5. The accumulation of miR-155 reached statistical significance by day 5 but was small in amplitude at each time point. The induction of miR-222 was transient, reaching a 2-fold increase (1.02 on log2 scale) after 1 day of ex vivo culture but declining thereafter (p < 0.0001 for the increase on day 1 when data from all six donors described in Figs. 1 and 3 were compiled).
FIGURE 3.
miRNAs accumulated with varying kinetics during MDM differentiation. RT-qPCR was used to measure miRNA abundance in purified monocytes cultured ex vivo for the indicated number of days. miRNA abundance was also measured in MDMs isolated from PBMC cultures based on adherence. Graphs represent the mean ± S.E. -fold change (n = 3) compared with monocyte samples collected immediately after cell sorting. Asterisks indicate statistical significance (p < 0.05) compared with monocyte samples collected immediately after sorting using Dunnett's post-test of mixed model analysis. Horizontal bars indicate statistical significance (p < 0.05) comparing both day 5 samples using a paired, two-tailed t test. Error bars represent S.E.
Accumulation of miR-155 and miR-193b Was Augmented in Monocytes Co-cultured with Non-monocytic PBMCs
Not only did LPS, interferons, and IL-4 influence the rate of miRNA change during differentiation, but the presence of other cells in the environment also exhibited a major impact on miRNA expression. We compared miRNAs during MDM differentiation in two alternate environments. The first method, used in experiments described up to this point, utilized culture of monocytes purified from PBMCs using CD14 MicroBeads on the day of blood donation. The second method allowed differentiation of monocytes into MDMs in cultures of unsorted PBMCs for 4 days followed by an additional day of culture in the absence of non-monocytic PBMCs. We used the second method to generate MDMs in our previous study to document large differences in expression of some miRNAs between monocytes and MDMs (14). Unexpectedly, the abundance of miRNAs in MDMs generated by these two methods was substantially different. With the exception of miR-27a, the accumulation of the miRNAs under study was equal to or higher in MDMs co-incubated with non-monocytic PBMCs than in MDMs generated from purified monocytes cultured in the absence of non-monocytic PBMCs (Fig. 3). The difference was statistically significant for both miR-155 and miR-193b and nearly significant for miR-29b (p = 0.13), miR-146a (p = 0.12), and miR-222 (p = 0.06).
Dicer Protein and mRNA Expression Levels Were Constant during Monocyte-to-Macrophage Differentiation
Every miRNA that we evaluated in this study had either progressive or transient increased expression levels as purified monocytes differentiated to MDMs. To investigate the mechanism, we first considered the possibility that there was a global enhancement of miRNA processing during MDM differentiation. The rationale for this hypothesis was supported by a report that Dicer and other RNAi-associated proteins accumulate following phorbol 12-myristate 13-acetate-induced differentiation of human monocytic cells and primary monocytes (20). However, we did not detect any increase in expression of Dicer protein (Fig. 4A) or mRNA (Fig. 4B) during MDM differentiation.
FIGURE 4.

Dicer expression remained constant during monocyte-to-macrophage differentiation. A, immunoblots of Dicer and GAPDH were performed using lysates from CD14 MicroBead-purified monocytes and from adherent cells of PBMC cultures after the indicated number of days in culture. B, RT-qPCR was used to measure Dicer transcript abundance in samples collected from monocytes cultured for the indicated number of days and in samples from MDMs isolated after culturing PBMCs for 5 days. Graphs represent the mean ± S.E. -fold change (n = 4) compared with monocyte samples collected immediately after cell sorting. No statistically significant (p < 0.05) results were identified using Dunnett's post-test of mixed model analysis. Error bars represent S.E.
Pri-miRNA Expression Patterns Correlated with Mature miRNA Accumulation during MDM Differentiation
Because the increase in miRNA abundance was not associated with corresponding increases in Dicer levels, we hypothesized that the changes in mature miRNA abundance occurred upstream of miRNA processing at the level of pri-miRNA abundance.
To test this hypothesis, we monitored the expression of the four pri-miRNAs whose mature forms had the largest expression changes during monocyte-to-macrophage differentiation of the seven miRNAs we evaluated. Unfortunately, we were unable to follow the kinetics of pri-miR-193b expression because its abundance remained too low at all of the time points to obtain reliable readings. Among the three pri-miRNAs (pri-miR-125a, pri-miR-146a, and pri-miR-193b) we could measure, each had unique kinetics of expression (Fig. 5). Pri-miR-125a transiently accumulated to ∼50-fold (5.62 on log2 scale) during the 1st day of ex vivo culture before being stably maintained at ∼20-fold through day 5 of culture. Pri-miR-146a increased expression ∼40-fold above baseline monocyte expression and maintained this level throughout days 1–5 of culture. Although the kinetics of pri-miR-125a-5p and pri-miR-146a (Fig. 5) were not identical to the kinetics of the mature forms of these two miRNAs (Fig. 3), it is important to factor RNA stability into the interpretation. Mature miRNAs have long half-lives, whereas pri-miRNAs are rapidly cleaved by the microprocessor complex (21). Because of the difference in stability, the transient induction of pri-miR-125a (Fig. 5) was consistent with the plateau in mature miR-125a-5p abundance (Fig. 3). The immediate plateau in pri-miR-146a abundance (Fig. 5) was consistent with the progressive accumulation of a more stable mature miR-146a (Fig. 3).
FIGURE 5.

Primary miRNA expression correlated with mature miRNA accumulation during MDM differentiation. RT-qPCR was used to measure transcript abundance of the indicated primary miRNAs in samples collected from monocytes cultured for the indicated number of days and in samples from MDMs isolated after culturing PBMCs for 5 days. Graphs represent the mean ± S.E. -fold change (n = 3–4) compared with monocyte samples collected immediately after cell sorting. Asterisks indicate statistical significance (p < 0.05) compared with monocyte samples collected immediately after sorting using Dunnett's post-test of mixed model analysis. Error bars represent S.E.
The profile of pri-miR-155 (Fig. 5) was similar to that of mature miR-155 (Fig. 3). Specifically, we detected modest increases in both forms of miR-155 in cultures of purified monocytes during differentiation to MDMs and a markedly greater increase in MDMs derived from total PBMC cultures than from purified monocytes. In contrast, differences in MDM differentiation conditions minimally altered the expression of the primary and mature forms of miR-125a-5p and miR-146a (Figs. 3 and 5). Overall, these observations support a model in which the accumulation of developmentally up-regulated miRNAs is mechanistically due to changes in expression of the corresponding pri-miRNA. In the case of miR-155, this up-regulated pri-miRNA abundance seems to occur at least in response to signals from non-monocytes (pursued below).
Culturing Monocytes with Non-monocytic PBMCs Increased the Expression Levels of miR-155 and miR-193b
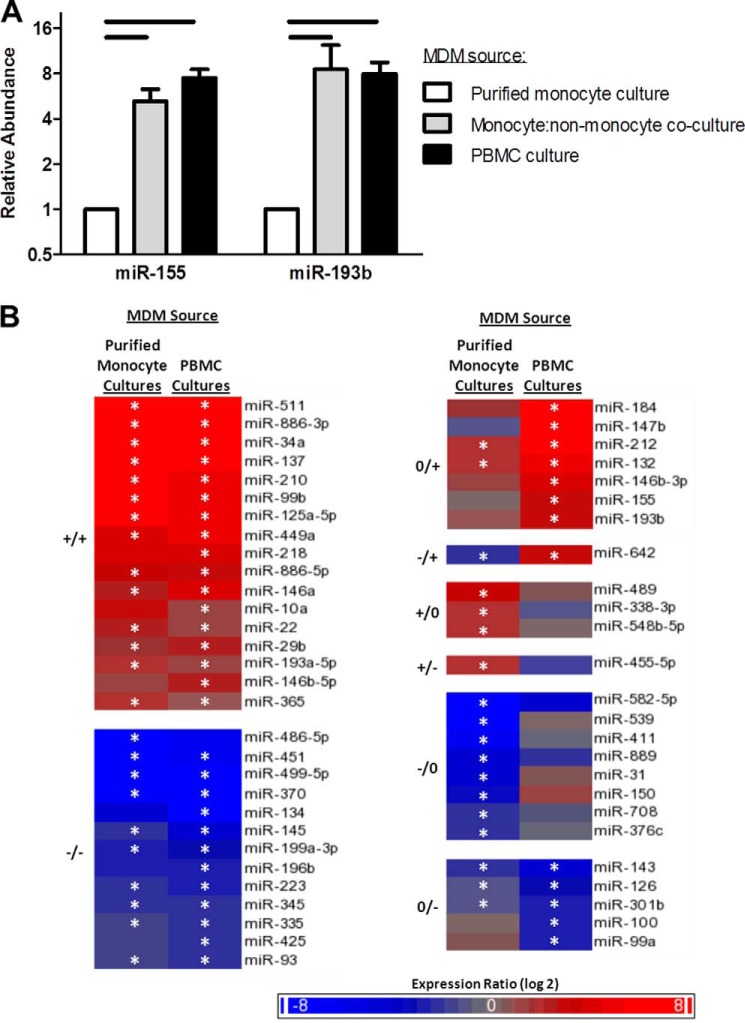
Differences between the two MDM preparation methods included exposure of purified monocytic cells to CD14 MicroBeads in the first method and co-incubation of monocytes with non-monocytic PBMCs for 4 days in the second method. We hypothesized that co-culture of monocytes with non-monocytic PBMCs during MDM differentiation, rather than CD14 MicroBead-mediated sorting, explained the differences in miR-155 and miR-193b accumulation. To test this, monocytes were positively selected from PBMCs using CD14 MicroBeads and either cultured alone or co-cultured with negatively selected, non-monocytic PBMCs from the CD14 MicroBead-mediated sort. A monocyte-to-non-monocyte ratio of 1:5 was chosen for the co-culture condition because monocytes isolated by CD14 MicroBeads typically constitute 15–20% of the total PBMC population. After 5 days, the accumulation of both miR-155 and miR-193b was enhanced in MDMs from the co-culture conditions compared with no co-culture, and the level was similar to the accumulation of these miRNAs in MDMs isolated from unfractionated PBMC cultures (Fig. 6A).
FIGURE 6.
The expression levels of many miRNAs differed between MDMs cultured in either the presence or absence of non-monocytic PBMCS. A, RT-qPCR was used to measure miR-155 and miR-193b abundance in MDMs generated using three different methods: culture of purified monocytes, co-culture of purified monocytes and non-monocytic PBMCs, and unsorted PBMC cultures. In all three methods, MDM purification was based on selecting adherent cells. Graphs represent the mean expression (n = 4) of the indicated miRNA relative to the MDMs differentiated as purified monocyte cultures. Horizontal bars indicate statistical significance (p < 0.05) using a paired, two-tailed t test. B, miRNA expression profiles were determined using TaqMan Low Density Array assays in samples from CD14 MicroBead-purified monocytes collected immediately after sorting and from MDMs differentiated in the presence of non-monocytic PBMCs. The heat maps indicate the average miRNA expression level change in the indicated MDM type relative to the expression level in purified monocytes at the time of sorting (n = 3). Expression changes were determined to be increased (+), decreased (−), or unchanged (0) in each of the two types of MDMs, resulting in eight expression pattern categories. Asterisks indicate statistical significance (p < 0.05) in MDMs compared with monocyte samples collected immediately after sorting using a paired, two-tailed t test. Error bars represent S.E.
Expression Profiling of miRNAs Identified Changes That Were Either Dependent on or Independent of Differences in Culturing Conditions during Monocyte-to-macrophage Differentiation
The presence of non-monocytic cells had a large impact on miR-155 and miR-193b expression in MDMs. We profiled miRNA expression using TaqMan TLDA assays to identify additional miRNAs with expression levels that were affected by differences in culturing conditions during monocyte-to-macrophage differentiation.
We first noted that five of seven miRNAs we evaluated in this report (miR-29b, miR-125a-5p, miR-146a, miR-155, and miR-193b) were among the 29 miRNAs up-regulated more than 4-fold in the TLDA analysis (Fig. 6B). We then compared our results with a previous study that profiled miRNA expression in primary human CD14 MicroBead-purified monocytes differentiated to MDMs in the presence of GM-CSF (19). The most highly up-regulated miRNA in both MDM types in both the current study and the MDMs in the previous study (19) was miR-511. Additional miRNAs (miR-34a, miR-99b, miR-132, miR-146b-5p, miR-210, miR-212, and miR-449a) were also up-regulated in both the current (Fig. 6B) and the previous studies (19). In the case of miR-132 and miR-212, the regulation was enhanced by more than 4-fold when non-monocytic PBMCs were present during monocyte-to-macrophage differentiation, leading us to categorize this miRNA as being preferentially up-regulated in the MDMs cultured in the presence of non-monocytic PBMCs (Fig. 6B). Because miR-132 and miR-212 are expressed as a bicistronic pri-miRNA, the similar expression pattern of these miRNAs suggests that they were regulated at a preprocessing step.
Both Tserel et al. (19) and Wang et al. (22) described miR-150 as an miRNA with repressed expression during monocyte-to-macrophage differentiation. We found that, although this miRNA was repressed during differentiation of purified monocyte cultures, the presence of non-monocytic PBMCs prevented the loss of expression for this miRNA in MDMs (Fig. 6B). Fig. 6B, right column, describes 24 additional miRNAs that are differentially regulated in MDMs cultured in the presence and absence of non-monocytic PBMCs. Considering data resulting from our work and these publications, there seems to be a core set of miRNAs that are regulated as a result of monocyte-to-macrophage differentiation. The miRNAs that are regulated uniquely in the different MDM culturing conditions may highlight a set of miRNAs that are responsive to exogenous stimuli.
Culturing Monocytes with Non-monocytic PBMCs Altered the Expression of Monocyte-to-macrophage Differentiation and Macrophage Polarization Markers
The differences in miRNA expression between MDMs derived from the two culturing conditions could represent either an alteration in the monocyte-to-macrophage differentiation program or an activated phenotype for the MDMs differentiated in the presence of non-monocytic cells. To distinguish between these possibilities, we monitored changes in the expression of 48 transcripts as purified monocytes differentiated to MDMs and in MDMs differentiated in the presence of non-monocytic PBMCs to characterize these MDMs. The panel of transcripts was selected from published data sets of a profiling study that characterized monocyte-to-macrophage differentiation and macrophage responses to activating conditions (DataSet Records GDS2429 and GDS2430) (23).
The most abundantly changed mRNAs are indicated in the heat map (Fig. 7A). These 25 transcripts had average changes of more that 4-fold in one or more of the five sample types relative to freshly isolated monocytes. Genes reported to be altered during monocyte-to-macrophage differentiation (23) are indicated with color-coded gene names signifying whether the transcript was up-regulated or down-regulated in the previous study (Fig. 7A). Unsupervised hierarchal clustering analysis showed that the gene expression profiles in our panel underwent many changes by the day 1 time point, continued to change through day 3, and then stabilized as CD14 MicroBead-purified monocytes differentiated to macrophages (Fig. 7A). This is consistent with microarray results from Martinez et al. (23) showing that gene expression patterns of purified monocytes change dramatically during the first 3 days of culture and become relatively stable thereafter.
FIGURE 7.
Analysis of monocytic cell differentiation and polarization marker abundance revealed a unique signature in MDMs cultured in the presence of non-monocytic cells. A, a heat map summarizes results from integrated fluidic circuit-based RT-qPCR that measured the abundance of putative monocyte-to-macrophage differentiation and polarization markers. RNA was collected from CD14 MicroBead-purified monocytes or from MDMs of PBMC cultures after the indicated number of days in culture. Average changes in marker abundance relative to monocyte samples collected immediately after cell sorting are shown (n = 3–4). Genes and treatments are arranged according to results from unsupervised hierarchal clustering. To facilitate comparison with the study by Martinez et al. (23), putative differentiation marker gene names from this report were color-coded (red for up-regulation, blue for down-regulation, and black for unaltered expression) based on the microarray-based gene expression measurements from purified monocytes after ex vivo culture for 3 days. B, immunoblots were performed to detect the indicated proteins using lysates from CD14 MicroBead-purified monocytes and from adherent cells (MDMs) of PBMC cultures after the indicated number of days in culture. C, prior to blocking, the nitrocellulose membranes used for immunoblots in B were stained with Ponceau S. A representative membrane is shown (L, ladder). D, immunoblots were performed to detect the indicated proteins. Lysates were collected from MDMs derived from CD14 MicroBead-purified monocytes following the replacement of cell culture medium with conditioned medium either from CD14 MicroBead-purified monocytes as a control or from unsorted PBMCs.
The gene expression pattern in MDMs from cultures of purified monocytes on day 5 differed drastically from time-matched samples from MDMs differentiated in the presence of non-monocytic PBMCs (Fig. 7A). Notably, the expression patterns of MDMs differentiated within cultures of unsorted PBMCs were very similar to the expression pattern of MDMs differentiated from purified monocytes co-cultured with non-monocytic PBMCs (Fig. 7A). This latter observation indicates that the CD14 MicroBead-mediated monocyte purification procedure does not explain the difference in gene expression patterns between MDMs derived from purified monocyte cultures and MDMs derived from unsorted PBMC cultures. Because some of the most dramatic differences in transcript expression between MDM types were markers of polarization rather than differentiation, we conclude that the MDMs differentiated in the presence of non-monocytic cells ended up at a different state of activation than those differentiated as cultures of purified monocytes. An interaction occurs during monocyte co-incubation with non-monocytic PBMCs, drastically altering the abundance of both miRNAs and mRNAs in MDMs.
NF-κB and STAT1 Pathways Were Activated within MDMs Responding to Secreted Factors in PBMC-conditioned Medium
Several of the mRNAs with altered expression in MDMs due to the presence of non-monocytic cells during differentiation suggested that pathways associated with inflammation such as NF-κB and STAT1 were activated. For example, the IFN-responsive genes ITGB7 and ISG20 were more abundant in MDMs cultured in the presence of non-monocytic PBMCs consistent with STAT1 pathway activation. We tested whether STAT1 and NF-κB pathways were activated in MDMs co-cultured with non-monocytic PBMCs using two experimental approaches.
First, immunoblot analyses were performed to assess activation of STAT1 and NF-κB pathways during the monocyte-to-macrophage differentiation process in the presence or absence of non-monocytic PBMCs (Fig. 7B). Total STAT1 levels remained constant and unphosphorylated over time in the purified monocyte cultures, showing that STAT1 pathways were not activated under these conditions. In contrast, we observed an increased abundance of total STAT1 and a phosphorylated form of STAT1 in MDMs differentiated in the presence of non-monocytic PBMCs, demonstrating that this pathway was activated by this culturing method (Fig. 7B). Moreover, IκBα was present throughout the monocyte-to-macrophage differentiation process in cultures of purified monocytes but absent in MDMs differentiated in the presence of non-monocytic cells (Fig. 7B). We hesitate to conclude that the absence of IκBα indicated that NF-κB was activated under the latter condition because the loading control, β-tubulin, was difficult to detect in this sample (Fig. 7B). However, Ponceau S staining (Fig. 7C) suggested that equal amounts of protein were loaded in the samples evaluated in Fig. 7B.
Next, we specifically assessed whether secreted factors present in the PBMC cultures were capable of activating MDM STAT1 and NF-κB pathways. Medium in wells of MDMs derived from culturing CD14 MicroBead-purified monocytes for 5 days was replaced with either PBMC-conditioned medium or purified monocyte-conditioned medium as a control. Immunoblots showed that STAT1 was phosphorylated and IκBα was degraded during the first 15 min of culturing the MDMs with PBMC-conditioned medium but not with control medium (Fig. 7D). This suggests that both STAT1 and NF-κB pathways were activated by factors in the PBMC-conditioned medium. The return of IκBα expression by 1 h further demonstrated that NF-κB was activated in response to the PBMC-conditioned medium because IκBα is an NF-κB-induced gene.
Low Basal Expression Levels of miR-155 and Macrophage Polarization Markers in MDMs Derived from Purified Monocyte Cultures Poise These Genes for “Large” Induction in Response to LPS Treatment
A cluster of genes in Fig. 7A (CXCL10, CCL5, TGM2, and CXCL9) had expression profiles that were similar to miR-155 and miR-193b (Figs. 3 and 6). Specifically, the expression of these genes changed minimally during differentiation of purified monocytes, but they were expressed at high levels in MDMs differentiated in the presence of non-monocytic PBMCs. We hypothesized that the differences in basal abundance of these transcripts in the different MDM populations would inversely correlate with the amplitude of expression level changes following treatment with a macrophage-activating factor such as LPS similar to miR-155 and miR-193b.
We tested our hypothesis by monitoring transcript induction in LPS-treated MDMs differentiated in the presence or absence of non-monocytic PBMCs. In MDMs differentiated without non-monocytic PBMCs, all four transcripts (Fig. 8A) and miR-155 (Fig. 8B) were highly up-regulated following LPS treatment but only modestly altered in LPS-treated MDMs co-cultured in the presence of non-monocytic PBMCs. In contrast to miR-155, another LPS-responsive miRNA, miR-146a, only modestly accumulated in MDMs with no difference in amplitude of response when comparing MDMs differentiated in the presence or absence of PBMCs (Fig. 8B). The similar amplitude of miR-146a induction in response to LPS treatment was consistent with our hypothesis because miR-146a accumulation was similar in the MDMs from different culturing methods (Fig. 3). As expected, miR-125a-5p and miR-193b showed neither a strong response to LPS treatment nor consistent differences in expression between MDMs differentiated in the presence or absence of non-monocytic PBMCs (Fig. 8B).
FIGURE 8.
The amplitude of LPS-induced changes in transcripts or miRNAs in MDMs was inversely related to the abundance of these RNAs prior to LPS treatment. RT-qPCR was used to measure LPS-induced changes in expression of the indicated mRNA (A) or miRNA (B). MDMs were generated using three different methods and treated with LPS (10 ng/ml) for 24 h. Graphs represent the mean ± S.E. changes in RNA abundance (n = 4) for the indicated mRNAs and miRNAs after LPS treatment on day 5 of MDM differentiation. Horizontal bars indicate statistical significance (p < 0.05) using a paired, two-tailed t test. Error bars represent S.E.
Prior to LPS treatment, miR-155 basal levels were lower in MDMs derived from cultures of purified monocytes compared with MDMs that were differentiated in the presence of non-monocytic PBMCs (Fig. 9). This confirmed results described in Fig. 6 with an independent set of MDMs. After LPS treatment, miR-155 levels were indistinguishable between MDMs regardless of the culture method used during differentiation (Fig. 9A). Similar trends in ΔCt value profiles were observed for CCL5, CXCL9, CXCL10, and TGM2 before and after LPS treatment (Fig. 9B). By interpreting gene expression using ΔCt values, we conclude that basal abundance, rather than final abundance, accounts for the difference in amplitude of transcript and miRNA expression level changes in response to LPS between MDMs differentiated in the presence or absence of non-monocytic PBMCs. Thus, the surprisingly small changes in miRNA abundance we recently reported for MDMs differentiated in response to various activation conditions (14) were a result of some activation-associated miRNAs such as miR-155 accumulating robustly in response to activation of monocytic cells co-cultured with non-monocytic PBMCs, whereas other activation-associated miRNAs such as miR-146a likely accumulate as part of a general monocyte-to-macrophage differentiation program.
FIGURE 9.
In MDMs differentiated in the presence or absence of non-monocytic PBMCs, differences in miR-155 and polarization marker expression exist before, but not after, LPS treatment. RT-PCR was used to monitor miRNA (A) and mRNA (B) expression levels in MDMs. Samples were collected on day 5, the day of LPS treatment (“Before LPS”), and on day 6 after treatment with LPS (100 ng/ml) for 24 h (“After LPS”). Error bars represent S.E.
DISCUSSION
Mounting evidence describes many roles for miRNAs in regulating important macrophage functions (9, 10, 24, 25). Most studies evaluating the role of miRNAs in macrophages have focused on miRNAs that have regulated expression either during monocyte-to-macrophage differentiation or in response to treatment with activating stimuli. Our previous report describing miRNA expression regulation in human macrophages yielded the unexpected observation that the measured -fold changes in expression of miRNAs in response to strong polarizing conditions were small in amplitude despite the fact that these were biologically relevant changes (14). An important observation from the prior study was that polarization-associated miRNAs had low expression in monocytes relative to MDMs. This raised the possibility that the basal levels of these activation-associated miRNAs were actually preinduced during the process of differentiation from monocyte to macrophage and that we were observing the smaller -fold change in response to additional stimuli that cause polarization. In the current study, we characterized the accumulation of several miRNAs during differentiation of human monocytes ex vivo. These miRNAs were selected because their abundance is also regulated during macrophage polarization (14). We evaluated miRNA expression regulation in monocytic cells responding to variations in the external environment. First, we determined the kinetics of miRNA expression during monocyte-to-macrophage differentiation in the presence and absence of well defined activating stimuli. Second, we evaluated miRNA expression in monocytes that were allowed to differentiate to MDMs ex vivo in the presence or absence of non-monocytic PBMCs. We found that the abundance of these miRNAs was low in monocytes prior to differentiation and that their accumulation was dynamic in response to variations in environmental stimuli. These modifications of miRNA abundance were regulated at the level of pri-miRNA expression.
The first set of experiments showed that the rate of miRNA accumulation during differentiation was modified in response to cytokines (IFNβ, IFNγ, and IL-4) and a Toll-like receptor agonist (LPS). We had previously reported that IFNγ treatment could antagonize the LPS-mediated induction of some miRNAs in phorbol 12-myristate 13-acetate-differentiated THP-1 cells (14). We reassessed the cross-talk in primary human monocytes between signaling pathways downstream of LPS and IFNγ and expanded the analysis to include the type 1 interferon, IFNβ. Two well studied miRNAs, miR-146a and miR-155, were induced by LPS in monocytes, but only miR-146a was antagonized by the addition of either type of interferon. Substantial evidence suggests that miR-146a acts as a negative regulator of inflammation by targeting TRAF6 and IRAK1 (18, 26, 27). Although sometimes described as anti-inflammatory, miR-155 has more commonly been described to act as a positive regulator of inflammation by targeting SOCS1 and SHIP1 (for a review, see Ref. 9). In monocytes exposed to inflammatory conditions, the simultaneous antagonism of miR-146a and augmentation of miR-155 accumulation by interferon treatments may function to extend the duration of the inflammatory gene expression program by these cells.
Treatment of monocytes with IFNγ and IFNβ regulated miRNA expression in a similar fashion with the exceptions of miR-27a and miR-29b, which were both expressed at lower levels in the IFNβ-treated samples. Interestingly, each of these miRNAs has been implicated in interferon responses. miR-27a enhances IFNβ signaling (28) and is targeted for degradation by a number of viruses (29–31). In contrast, miR-29b antagonizes IFNγ production in multiple hematopoietic cell types, although a consensus has not been reached on whether miR-29b acts by directly targeting IFNγ transcripts or by repressing the expression of transcription factors involved in IFNγ transcription (32, 33).
Our final set of experiments focused on understanding miRNA and mRNA expression differences in MDMs that were cultured in the presence or absence of non-monocytic PBMCs. We considered the possibility that the MDMs derived from the alternate methods were at different stages of differentiation after 5 days of ex vivo culture. In agreement with Martinez et al. (23), we observed stabilization in the gene expression profiles from cultures of purified monocytes starting at day 3. We interpret this observation to indicate that the differentiation process may be mostly complete by this time point. The gene expression profiles obtained from MDMs cultured in the presence of non-monocytic PBMCs were substantially different from those seen in purified monocyte cultures at any time point. Collectively, this set of observations argues against the non-monocytic PBMCs blocking monocyte-to-macrophage differentiation. Instead, it is possible that MDMs may each be differentiated toward uniquely polarized states. In support of this interpretation, we documented that the presence of non-monocytic PBMCs during monocyte-to-macrophage differentiation activates STAT1 and NF-κB signaling pathways and that the factors involved in activation were present in PBMC-conditioned medium.
Our initial experiments showed that treatment with IFNs, presumably through STAT1 activation, did not alter miR-155 accumulation, enhanced miR-193b accumulation, repressed miR-125a-5p accumulation, and partially antagonized LPS-mediated induction of miR-146a. Thus, the discovery that both STAT1 and NF-κB pathways were active in MDMs differentiated in the presence of non-monocytic cells correlates well with the preferential up-regulation of miR-155 and miR-193b, but not miR-146a and miR-125a-5p, in these MDMs relative to MDMs differentiated in the absence of PBMCs. TLDA-based RT-PCR detected five additional miRNAs that were preferentially up-regulated in MDMs differentiated in the presence of non-monocytic PBMCs and independently confirmed this regulation pattern for miR-155 and miR-193b. Notably, inflammatory stimuli have been reported to induce miR-146b and miR-147 in murine macrophages as well as the miR-132/miR-212 cluster in human macrophages, consistent with our conclusion that MDMs differentiated with non-monocytic PBMCs appear to be activated (34–36).
Our study highlights many contrasting characteristics between MDMs differentiated in the presence or absence of non-monocytic PBMCs. However, it remains unknown which MDM culturing method best models human MDMs in vivo. Obtaining MDMs from cultures of pure monocytes isolated using CD14 MicroBeads is advantageous from a reductionist point of view because the culturing conditions are more defined. Conversely, differentiation of MDMs in the presence of non-monocytic PBMCs may provide environmental cues through secreted factors, cell-cell interactions, cell density, or some other intercellular interaction that could more closely mimic in vivo environments.
In this work, we evaluated miRNAs that are regulated both in response to activating conditions and as part of monocyte-to-macrophage differentiation. Because miRNAs have long half-lives, increasing the abundance of activation-associated miRNAs may be a more tractable and rapid regulatory mechanism than reducing their levels as cells respond to new environmental cues. We propose that by expressing low basal levels of key miRNAs circulating monocytes are poised to induce miRNAs in response to diverse stimuli as they differentiate to macrophages in peripheral tissues.
This work was supported, in whole or in part, by National Institutes of Health Grants AI080801, AI045540, and AI07511 (to M. E. W.) and T35HL007485 (a training grant to R. L. E.). This work was also supported by Grants 1IK2BX001627 (to J. W. G.), 1I01BX001983 (to M. E. W.), and 5I01BX000536 (to M. E. W.) from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development.
- MDM
- monocyte-derived macrophage
- Ct
- cycle threshold
- miRNA or miR
- microRNA
- PBMC
- peripheral blood mononuclear cell
- qPCR
- quantitative PCR
- pri-miRNA
- primary miRNA
- M-CSF
- macrophage colony-stimulating factor
- TLDA
- TaqMan Low Density Array.
REFERENCES
- 1. Hashimoto D., Chow A., Noizat C., Teo P., Beasley M. B., Leboeuf M., Becker C. D., See P., Price J., Lucas D., Greter M., Mortha A., Boyer S. W., Forsberg E. C., Tanaka M., van Rooijen N., García-Sastre A., Stanley E. R., Ginhoux F., Frenette P. S., Merad M. (2013) Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38, 792–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schulz C., Gomez Perdiguero E., Chorro L., Szabo-Rogers H., Cagnard N., Kierdorf K., Prinz M., Wu B., Jacobsen S. E., Pollard J. W., Frampton J., Liu K. J., Geissmann F. (2012) A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90 [DOI] [PubMed] [Google Scholar]
- 3. Yona S., Kim K. W., Wolf Y., Mildner A., Varol D., Breker M., Strauss-Ayali D., Viukov S., Guilliams M., Misharin A., Hume D. A., Perlman H., Malissen B., Zelzer E., Jung S. (2013) Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barth M. W., Hendrzak J. A., Melnicoff M. J., Morahan P. S. (1995) Review of the macrophage disappearance reaction. J. Leukoc. Biol. 57, 361–367 [DOI] [PubMed] [Google Scholar]
- 5. Davies L. C., Rosas M., Smith P. J., Fraser D. J., Jones S. A., Taylor P. R. (2011) A quantifiable proliferative burst of tissue macrophages restores homeostatic macrophage populations after acute inflammation. Eur. J. Immunol. 41, 2155–2164 [DOI] [PubMed] [Google Scholar]
- 6. Liddiard K., Rosas M., Davies L. C., Jones S. A., Taylor P. R. (2011) Macrophage heterogeneity and acute inflammation. Eur. J. Immunol. 41, 2503–2508 [DOI] [PubMed] [Google Scholar]
- 7. Epelman S., Lavine K. J., Beaudin A. E., Sojka D. K., Carrero J. A., Calderon B., Brija T., Gautier E. L., Ivanov S., Satpathy A. T., Schilling J. D., Schwendener R., Sergin I., Razani B., Forsberg E. C., Yokoyama W. M., Unanue E. R., Colonna M., Randolph G. J., Mann D. L. (2014) Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40, 91–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wynn T. A., Chawla A., Pollard J. W. (2013) Macrophage biology in development, homeostasis and disease. Nature 496, 445–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu G., Abraham E. (2013) MicroRNAs in immune response and macrophage polarization. Arterioscler. Thromb. Vasc. Biol. 33, 170–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O'Connell R. M., Rao D. S., Chaudhuri A. A., Baltimore D. (2010) Physiological and pathological roles for microRNAs in the immune system. Nat. Rev. Immunol. 10, 111–122 [DOI] [PubMed] [Google Scholar]
- 11. Bartel D. P. (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 [DOI] [PubMed] [Google Scholar]
- 12. Guo H., Ingolia N. T., Weissman J. S., Bartel D. P. (2010) Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466, 835–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Filipowicz W., Bhattacharyya S. N., Sonenberg N. (2008) Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat. Rev. Genet. 9, 102–114 [DOI] [PubMed] [Google Scholar]
- 14. Graff J. W., Dickson A. M., Clay G., McCaffrey A. P., Wilson M. E. (2012) Identifying functional microRNAs in macrophages with polarized phenotypes. J. Biol. Chem. 287, 21816–21825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bazzoni F., Rossato M., Fabbri M., Gaudiosi D., Mirolo M., Mori L., Tamassia N., Mantovani A., Cassatella M. A., Locati M. (2009) Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc. Natl. Acad. Sci. U.S.A. 106, 5282–5287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen T., Huang Z., Wang L., Wang Y., Wu F., Meng S., Wang C. (2009) MicroRNA-125a-5p partly regulates the inflammatory response, lipid uptake, and ORP9 expression in oxLDL-stimulated monocyte/macrophages. Cardiovasc. Res. 83, 131–139 [DOI] [PubMed] [Google Scholar]
- 17. Forrest A. R., Kanamori-Katayama M., Tomaru Y., Lassmann T., Ninomiya N., Takahashi Y., de Hoon M. J., Kubosaki A., Kaiho A., Suzuki M., Yasuda J., Kawai J., Hayashizaki Y., Hume D. A., Suzuki H. (2010) Induction of microRNAs, mir-155, mir-222, mir-424 and mir-503, promotes monocytic differentiation through combinatorial regulation. Leukemia 24, 460–466 [DOI] [PubMed] [Google Scholar]
- 18. Taganov K. D., Boldin M. P., Chang K. J., Baltimore D. (2006) NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. U.S.A. 103, 12481–12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tserel L., Runnel T., Kisand K., Pihlap M., Bakhoff L., Kolde R., Peterson H., Vilo J., Peterson P., Rebane A. (2011) MicroRNA expression profiles of human blood monocyte-derived dendritic cells and macrophages reveal miR-511 as putative positive regulator of Toll-like receptor 4. J. Biol. Chem. 286, 26487–26495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coley W., Van Duyne R., Carpio L., Guendel I., Kehn-Hall K., Chevalier S., Narayanan A., Luu T., Lee N., Klase Z., Kashanchi F. (2010) Absence of DICER in monocytes and its regulation by HIV-1. J. Biol. Chem. 285, 31930–31943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gantier M. P., McCoy C. E., Rusinova I., Saulep D., Wang D., Xu D., Irving A. T., Behlke M. A., Hertzog P. J., Mackay F., Williams B. R. (2011) Analysis of microRNA turnover in mammalian cells following Dicer1 ablation. Nucleic Acids Res. 39, 5692–5703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang X., Ye L., Hou W., Zhou Y., Wang Y. J., Metzger D. S., Ho W. Z. (2009) Cellular microRNA expression correlates with susceptibility of monocytes/macrophages to HIV-1 infection. Blood 113, 671–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martinez F. O., Gordon S., Locati M., Mantovani A. (2006) Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 177, 7303–7311 [DOI] [PubMed] [Google Scholar]
- 24. Baltimore D., Boldin M. P., O'Connell R. M., Rao D. S., Taganov K. D. (2008) MicroRNAs: new regulators of immune cell development and function. Nat. Immunol. 9, 839–845 [DOI] [PubMed] [Google Scholar]
- 25. O'Neill L. A., Sheedy F. J., McCoy C. E. (2011) MicroRNAs: the fine-tuners of Toll-like receptor signalling. Nat. Rev. Immunol. 11, 163–175 [DOI] [PubMed] [Google Scholar]
- 26. Lu L. F., Boldin M. P., Chaudhry A., Lin L. L., Taganov K. D., Hanada T., Yoshimura A., Baltimore D., Rudensky A. Y. (2010) Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell 142, 914–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nahid M. A., Pauley K. M., Satoh M., Chan E. K. (2009) miR-146a is critical for endotoxin-induced tolerance: implication in innate immunity. J. Biol. Chem. 284, 34590–34599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shirasaki T., Honda M., Shimakami T., Horii R., Yamashita T., Sakai Y., Sakai A., Okada H., Watanabe R., Murakami S., Yi M., Lemon S. M., Kaneko S. (2013) MicroRNA-27a regulates lipid metabolism and inhibits hepatitis C virus replication in human hepatoma cells. J. Virol. 87, 5270–5286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Buck A. H., Perot J., Chisholm M. A., Kumar D. S., Tuddenham L., Cognat V., Marcinowski L., Dölken L., Pfeffer S. (2010) Post-transcriptional regulation of miR-27 in murine cytomegalovirus infection. RNA 16, 307–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cazalla D., Yario T., Steitz J. A. (2010) Down-regulation of a host microRNA by a herpesvirus saimiri noncoding RNA. Science 328, 1563–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marcinowski L., Tanguy M., Krmpotic A., Rädle B., Lisnić V. J., Tuddenham L., Chane-Woon-Ming B., Ruzsics Z., Erhard F., Benkartek C., Babic M., Zimmer R., Trgovcich J., Koszinowski U. H., Jonjic S., Pfeffer S., Dölken L. (2012) Degradation of cellular miR-27 by a novel, highly abundant viral transcript is important for efficient virus replication in vivo. PLoS Pathog. 8, e1002510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ma F., Xu S., Liu X., Zhang Q., Xu X., Liu M., Hua M., Li N., Yao H., Cao X. (2011) The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-gamma. Nat. Immunol. 12, 861–869 [DOI] [PubMed] [Google Scholar]
- 33. Steiner D. F., Thomas M. F., Hu J. K., Yang Z., Babiarz J. E., Allen C. D., Matloubian M., Blelloch R., Ansel K. M. (2011) MicroRNA-29 regulates T-box transcription factors and interferon-γ production in helper T cells. Immunity 35, 169–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu G., Friggeri A., Yang Y., Park Y. J., Tsuruta Y., Abraham E. (2009) miR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses. Proc. Natl. Acad. Sci. U.S.A. 106, 15819–15824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nahid M. A., Yao B., Dominguez-Gutierrez P. R., Kesavalu L., Satoh M., Chan E. K. (2013) Regulation of TLR2-mediated tolerance and cross-tolerance through IRAK4 modulation by miR-132 and miR-212. J. Immunol. 190, 1250–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang Z., Filgueiras L. R., Wang S., Serezani A. P., Peters-Golden M., Jancar S., Serezani C. H. (2014) Leukotriene B4 enhances the generation of proinflammatory microRNAs to promote MyD88-dependent macrophage activation. J. Immunol. 192, 2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]