Background: NTPDase1 hydrolyzes ATP, which is the agonist of the P2X1 receptor.
Results: NTPDase1 is expressed in vas deferens smooth muscles and regulates its contraction and spermatozoa concentration in the semen.
Conclusion: NTPDase1 controls male fertility via the regulation of P2X1 activation.
Significance: This mechanism may unveil a cause of infertility in males and open a new therapeutic area.
Keywords: ATP, ATPase, Mouse, Nucleotide, Reproduction, NTPDase1/CD39, P2X1, Male Fertility, Smooth Muscle Cells, Vas Deferens
Abstract
In this work, we report that Entpd1−/− mice, deficient for the ectonucleotidase nucleoside triphosphate diphosphohydrolase-1 (NTPDase1), produce smaller litters (27% reduction) compared with wild-type C57BL6 animals. This deficit is linked to reduced in vivo oocyte fertilization by Entpd1−/− males (61 ± 11% versus 88 ± 7% for Entpd1+/+). Normal epididymal sperm count, spermatozoa morphology, capacitation, and motility and reduced ejaculated sperm number (2.4 ± 0.5 versus 3.7 ± 0.4 million for Entpd1+/+) pointed to vas deferens dysfunction. NTPDase1 was localized by immunofluorescence in the tunica muscularis of the vas deferens. Its absence resulted in a major ATP hydrolysis deficiency, as observed in situ by histochemistry and in primary smooth muscle cell cultures. In vitro, Entpd1−/− vas deferens displayed an exacerbated contraction to ATP, a diminished response to its non-hydrolysable analog αβMeATP, and a reduced contraction to electrical field stimulation, suggesting altered P2X1 receptor function with a propensity to desensitize. This functional alteration was accompanied by a 3-fold decrease in P2X1 protein expression in Entpd1−/− vas deferens with no variation in mRNA levels. Accordingly, exogenous nucleotidase activity was required to fully preserve P2X1 receptor activation by ATP in vitro. Our study demonstrates that NTPDase1 is required to maintain normal P2X1 receptor functionality in the vas deferens and that its absence leads to impaired peristalsis, reduced spermatozoa concentration in the semen, and, eventually, reduced fertility. This suggests that alteration of NTPDase1 activity affects ejaculation efficacy and male fertility. This work may contribute to unveil a cause of infertility and open new therapeutic potentials.
Introduction
Extracellular nucleotides such as ATP take part in a wide range of physiological and pathological processes in virtually every tissue, acting through membrane-bound purinergic (P2) receptors (1). In the genitourinary apparatus, ATP has long been known to be coreleased with noradrenaline (NA)7 from sympathetic nerve terminals and varicosities. The function of the vas deferens is to drive spermatic fluid from the epididymis to the prostatic urethra during ejaculation. The smooth muscles in the wall of the vas deferens contract reflexively, propelling the sperm forward (peristalsis). The contraction, in response to electrical field stimulation, has been shown to be biphasic. ATP, released from sympathetic nerves, stimulates the initial fast phase of the contraction, with coreleased NA causing a slower and more sustained contraction by acting through G protein-coupled adrenoceptors (2–5). The existence of a specific ion-gated P2X receptor responsible for vas deferens contraction in response to ATP was proposed in 1987 (6). The receptor was cloned and identified as P2X1 (7). Binding of ATP on postjunctional P2X1 receptors leads to Ca2+ influx and, consequently, smooth muscle cell (SMC) contraction and semen peristalsis (8). Underlying the essential contribution of this receptor in ejaculation process, invalidation of P2X1 receptor gene dramatically impairs male fertility. Indeed, P2rx1−/− mice display an 85% decrease in male fertility because of defective neurogenic vas deferens contraction and sperm reduction in the ejaculate (9).
The concentration of ATP and other nucleotides at the cell surface is tightly regulated by ectonucleotidases (10, 11) Members of the ectonucleoside triphosphate diphosphohydrolase (E-NTPDase, EC 3.6.1.5) family appear to be particularly important in the termination of nucleotide signaling in a variety of cells. Following the generation of NTPDase1-deficient mice in 1999 (12), we observed a reduction of pups per litter with Entpd1−/− breeders. At the time there was no information that could link NTPDase1 to such a phenotype. NTPDase1 is known as the major ectonucleotidase at the surface of the vascular endothelial cells and leukocytes (13), where it plays important functions in the regulation of thrombosis and inflammation (11). More recently, we reported that NTPDase1 is also the dominant ectonucleotidase in vascular SMCs (14). Interestingly, smooth muscle layers from the vas deferens also exhibit high ectonucleotidase activity of an unknown nature (15).
The goal of this study was to identify the cause of the reduced fertility observed in Entpd1−/− mice. The designed experiments led us to identify a deficiency in male ejaculation. More specifically, in this work, we demonstrate an important role of NTPDase1 in vas deferens peristalsis via the regulation of P2X1 receptor activity.
EXPERIMENTAL PROCEDURES
Materials
All reagents were obtained from Sigma-Aldrich (Oakville, ON, Canada) unless stated otherwise.
Animals
Experiments were approved by the Institutional Ethical Committee for Experimental Animals. NTPDase1-deficient (Entpd1−/−) mice were provided by Dr. S. C. Robson (BIDMC, HMS, Boston, MA) (12). Entpd1−/− mice, originally from the 129 SVJ × C57BL/6 background, were backcrossed 12 generations onto the C57BL/6 background. Adult males (11–22 weeks old) were used in all experiments.
Computer-assisted Sperm Analysis (CASA) and Acrosomal Reaction
Spermatozoa from 12- to 20-week-old Entpd1+/+ or Entpd1−/− males were collected from the cauda epididymidis and incubated for 10 min at 37 °C in 109 mm NaCl, 25 mm HEPES, 25 mm NaHCO3, 5.6 mm glucose, 5 mm KCl, 1.7 mm CaCl2, 1.2 mm MgCl2, 1 mm pyruvate, and 3 mg/ml BSA (pH 7.4) gassed with 5% CO2. Sperm motility was evaluated using a CASA System (Hamilton Thorne Research, Danvers, MA) as described previously (16). Acrosomal reaction was assessed 30 and 60 min after collection by chlortetracycline fluorescent assay as described previously (17). Spermatozoa were classified according to three different chlortetracycline fluorescence patterns: uncapacitated (F pattern), capacitated (B pattern), and acrosome-reacted (AR pattern). Two slides of each sample were prepared, and 100 spermatozoa/slide were counted.
Oocyte Fertilization
Female mice 9–14 weeks of age were mated overnight with 14- to 22-week-old males. The following morning, females identified for the presence of copulatory plugs were sacrificed, and oocytes were collected from the oviduct, placed in M2 medium, dissociated with 0.3 mg/ml hyaluronidase, and washed three times with M2 medium. Oocyte fertilization was verified by the presence of two pronuclei 0.5 day post-coitum.
Collection of Male Ejaculates and Sperm Count
Male ejaculates were collected from the female reproductive tract after copulation. C57BL/6 female mice 8–16 weeks of age were mated overnight with Entpd1+/+ or Entpd1−/− males. The following morning, females identified for the presence of copulatory plugs were sacrificed. The proximal edge of the uterus was cut with a scalpel blade, and the sperm content was recovered with a micropipette. The total volume of semen was measured, and an aliquot was diluted in warm PBS (37 °C), followed by 10-min incubation to allow spermatozoa to swim out from the dense uterine mass. A second dilution was made in PBS containing 4% paraformaldehyde to fix spermatozoa. Following counterstaining with trypan blue, spermatozoa were counted with a hemocytometer.
Enzyme Histochemistry and Immunofluorescence
ATP hydrolysis in situ was evaluated using the lead orthophosphate precipitation method as described before (18). Cryosections of mouse vas deferens were incubated with either ATP or ADP (200 μm) as a substrate. Because NTPDases require divalent cations for their activity, in control experiments, CaCl2 and MnCl2 were replaced with 10 mm EDTA. Free phosphate resulting from nucleotide hydrolysis was visualized as a brown deposit by incubating the sections in an aqueous solution of 1% v/v (NH4)2S. Sections were counterstained with hematoxylin. Immunofluorescence experiments were performed using guinea pig anti-mouse NTPDase1 (mN1–2c) (19) and rabbit anti-mouse NTPDase2 (mN2–36l) (20) antisera, followed by detection with Alexa Fluor 594- and 488-conjugated goat anti-guinea pig and anti-rabbit antibodies, respectively. Sections were mounted using antifading Mowiol and analyzed with an Olympus BX51 microscope. For the quantification of fluorescence intensities, sections were analyzed with a spinning disk confocal system (WaveFX, Quorum Technologies), and fluorescence was quantified using NIH ImageJ software (http://rsbweb.nih.gov/ij).
Vas Deferens Isometric Contraction
Mice were sacrificed by CO2 inhalation, and their vas deferens were excised and placed in ice-cold physiological salt solution containing 130 mm NaCl, 3.7 mm KCl, 1.6 mm CaCl2, 1.2 mm KH2PO4, 1.2 mm MgSO4, 14.9 mm NaHCO3, and 5 mm HEPES implemented with 5.5 glucose. Segments from the prostatic portion of the vas deferens (2 mm) were mounted on a wire myograph (DMT, Aarhus, Denmark). The vas deferens were bathed in physiological salt solution maintained at 37 °C (pH 7.4) (pO2, 160 mm Hg; pCO2, 37 mmHg). The tension was adjusted to 7.5 mN. Isometric changes in vas deferens tone were collected by a Biopac data acquisition system (MP 100, Biopac, La Jolla, CA) and recorded and analyzed by Acqknowledge software (Biopac). For electrically evoked contractions, segments of 4 mm were mounted on two tungsten wires bathed in Krebs-Ringer buffer at 25 °C. Repeated electric field stimulations (10-s duration, 20 Hz, 45 V, 0.5-ms pulse) were applied at 1-min intervals in the presence or absence of prazosin (1 μm) and preincubated for 20 min.
Western Blot Analysis
Mice were euthanized by cervical dislocation. Excised vas deferens were immediately frozen on dry ice and kept at −80 °C until use. Protein homogenates were separated by SDS-PAGE, transferred to a PVDF membrane, and blotted with the following antibodies: anti-flotillin 1 (catalog no. 610821, BD Biosciences), P2X1 and P2X4 (catalog nos. APR-001 and APR-002, Alomone Labs Ltd., Jerusalem, Israel), mN1–2c guinea pig anti-mouse NTPDase1 antisera (19), and mN2–36l rabbit anti-mouse NTPDase2 antisera (20). Quantification of the protein bands was performed by densitometry using ImageJ software (NIH) and normalized to flotillin 1 band intensity.
ATP Release
Vas deferens SMCs of Entpd1+/+ or Entpd1−/− mice were prepared from collagenase elastase-digested vas deferens as described previously (21). Cells were grown in 24-well plates, washed twice, and incubated at different time points in Hanks' balanced salt solution supplemented with 1.25 mm CaCl2, 0.5 mm MgCl2, and 5 mm HEPES. Supernatants were collected at different time points, centrifuged for 1 min at 100 × g, and heated for 1 min at 95 °C. ATP content was measured using a firefly luciferase-based bioluminescent kit (Sigma-Aldrich) and a Tecan Infinite200 microplate luminometer (Tecan Group Ltd., Morrisville, NC).
NTPDase Activity Assays
Vas deferens SMCs of Entpd1+/+ or Entpd1−/− mice were prepared from collagenase elastase-digested vas deferens as described previously (21). Cells grown in 24-well plates were incubated with ATP (10–1000 μm) for 15 min at 37 °C in a medium containing 5 mm CaCl2 and 145 mm NaCl in Tris/HCl buffer (pH 7.4). Supernatants were centrifuged to remove detached cells, and ATP hydrolysis was evaluated by measuring the Pi released using the malachite green technique (22). For more precision, these assays were performed with 50 and 100% confluent wild-type and knockout mouse cells, respectively. ATP (100 μm) hydrolysis by intact vas deferens SMCs was also determined using a firefly luciferase-based bioluminescent kit and a Luminoskan Ascent microplate luminometer (Thermo Electron Corp., Miliford, MA).
Effect of Nucleotidase Activity in ATP-induced Calcium Influx
Human embryonic kidney 293 cells stably transfected with the human P2X1 receptor (HEK293-hP2X1) were maintained in minimal essential medium with Earle salts (with GlutaMAX I) supplemented with 10% fetal bovine serum and 1% nonessential amino acids (Invitrogen) at 37 °C in a humidified atmosphere of 5% CO2 and 95% O2. For calcium experiments, cells were cultured in 96-well plates (80–90% confluence) and loaded with the calcium probe Fluo-3/AM (2 μm, Invitrogen) in 150 mm NaCl, 2.5 mm KCl, 10 mm HEPES, 2.5 mm CaCl2, 1.0 mm MgCl2, 1% mm BSA, 0.02% mm pluronic acid (pH 7.3), for 40 min in the dark in the presence of 0.16 IU/ml apyrase grade VII. Cells were washed with the same buffer, and ATP (0, 0.1, 0.3 or 1 μm) was added to evaluate the desensitization elicited. Five minutes later, apyrase (0.01, 0.03, 0.1, 0.3, 1, 3, or 10 IU/ml) was added for 10 min at room temperature. Calcium influx in response to ATP (0.3 μm) or αβMeATP (3 μm) was then measured (apyrase still present) using a FlexStation® II scanning fluorometer (Molecular Devices Ltd., Wokingham, UK). In a control experiment, the P2X1 antagonist NF449 (1 μm) was added for 10 min before ATP stimulation. Softmax Pro (Molecular Devices) software was used for data analysis.
Statistical Analysis
Raw paired data were compared using paired Student's t test. When appropriate, we performed two-way analysis of variance followed by multiple comparisons test. Values were considered statistically significant with p < 0.05.
RESULTS
Reduced Fertility of Entpd1−/− Mice because of Deficiency in Ejaculation Efficacy
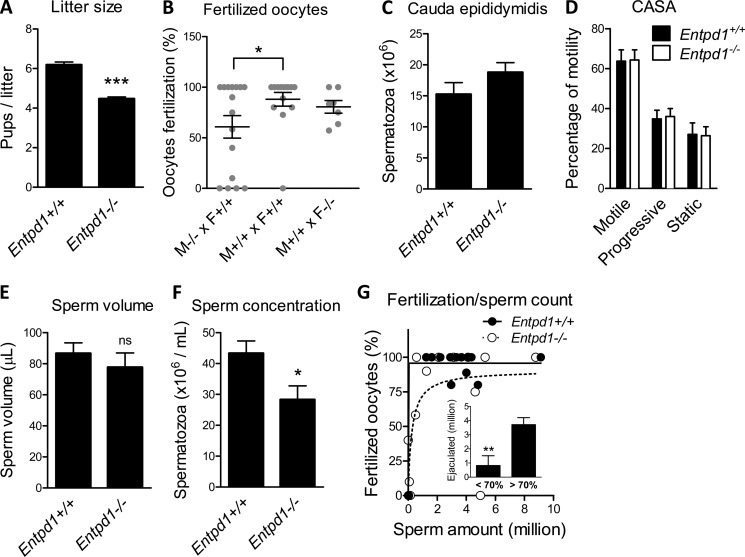
Compilation of births over 7 years involving 117 males and 166 females in the Entpd1−/− strain and 75 males and 109 females in the wild-type strain evidenced a small litter size in Entpd1−/− mouse breeder pairs compared with wild-type couples (4.47 ± 0.10, n = 425 versus 6.18 ± 0.15, n = 252; p < 0.001) (Fig. 1A), which produced a litter in the expected range for this strain (23). No difference in sexual behavior was observed in mutant mice, and the lag time after mating to obtain the first offspring was not different (35.8 ± 1.3 days, n = 112 versus 34.5 ± 1.2 days, n = 195 for Entpd1+/+ and Entpd1−/−, respectively). To identify the cause of this decline in fertility in the Entpd1−/− strain, we evaluated in utero oocyte fertilization. A reduction in fertilized oocytes was observed when comparing wild-type couples (88.1 ± 6.7%) to Entpd1−/− males crossed with wild-type females (60.8 ± 11.1%) but not when Entpd1−/− females were crossed with wild-type males (80.5 ± 6.3%), suggesting that the impaired fertility observed in Fig. 1A was at least mostly related to Entpd1−/− males (Fig. 1B). The amount of spermatozoa recovered in the cauda epididymidis was not significantly different (15.3 ± 1.9, n = 8 and 18.8 ± 1.5, n = 8 for Entpd1+/+ and Entpd1−/− males, respectively, Fig. 1C). The morphology of Entpd1−/− epididymal spermatozoa visualized by eosin-nigrosin staining was also normal (data not shown), as well as their motility, as assessed by CASA (Fig. 1D). Chlortetracycline assays showed no difference between Entpd1−/− and Entpd1+/+ spermatozoa patterns, namely uncapacited (F pattern), capacited (B pattern), and acrosome-reacted (pattern AR), as evaluated 30 and 60 min after collection (data not shown). These data suggest that the absence of NTPDase1 does not influence sperm capacitation processes. Hence, these data revealed no obvious abnormality in sperm morphology or function. The ejaculation volume (86.7 ± 6.7 μl, n = 34 in WT versus 77.7 ± 9.3 μl, n = 34 in Entpd1−/− mice) (Fig. 1E) was unaffected in mutant mice, and post-coitum vaginal coagulum formation was normal, suggesting normal coagulating gland function. The major difference was revealed to be in the number and concentration of spermatozoa recovered in the uterus, which were reduced significantly with Entpd1−/− males (43.3 ± 4.0 million/ml, n = 34 versus 28.3 ± 4.5 million/ml, n = 32 p < 0.05) (Fig. 1F). Importantly, reduced oocyte fertilization by Entpd1−/− males (fertilization efficacy <70%) was associated with a lower amount of sperm collected from the mouse uterus (Fig. 1G). These results indicate that the reduction in Entpd1−/− male fertility results from a reduced sperm emission, possibly because of a vas deferens contractile dysfunction.
FIGURE 1.
Analysis of male reproductive parameters in Entpd1−/− mice. A, the Entpd1−/− mouse strain generates small litters compared with wild-type couples. ***, p < 0.0001. B, in utero oocyte fertilization is reduced with Entpd1−/− males. *, p < 0.05. C, CASA evaluation of total motility (any movement), progressive motility (forward movement), and static spermatozoa did not evidence abnormalities in Entpd1−/− males. Epididymal sperm count (D) and semen ejaculation volumes (E) are normal in Entpd1−/− males. ns, not significant. F, reduced sperm concentration in Entpd1−/− male ejaculate. *, p < 0.05. G, reduced oocyte fertilization by Entpd1−/− males correlates with a lower amount of sperm emission. The inset shows that the sperm count is reduced significantly. **, p < 0.001 in semen retrieved from females with <70% fertilization efficacy.
Major Contribution of NTPDase1 to Vas Deferens Smooth Muscle Nucleotidase Activity
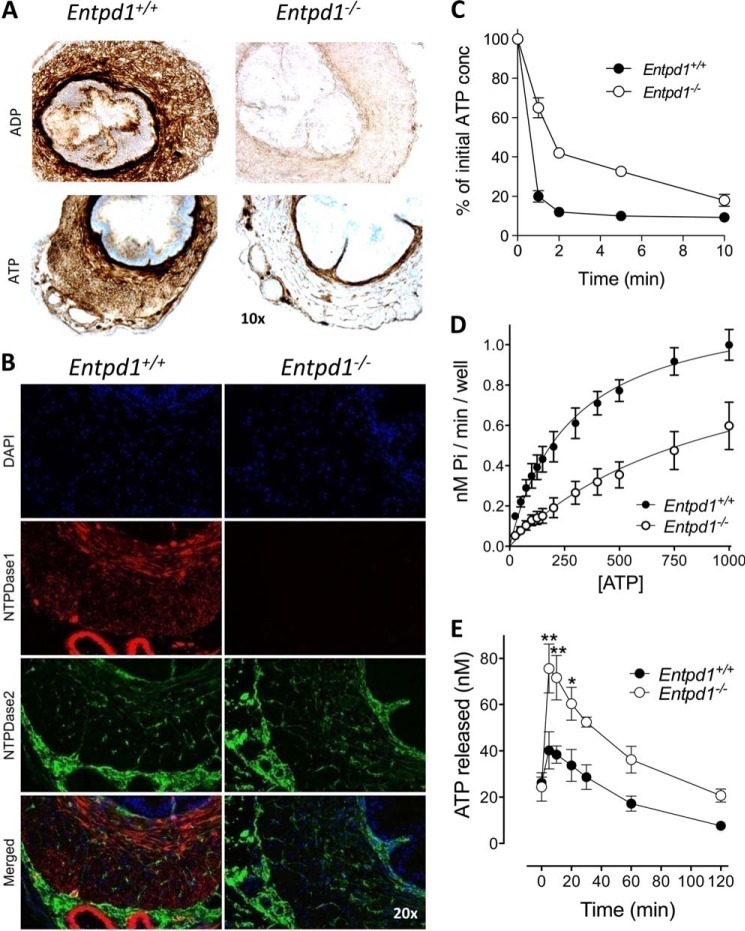
We next investigated whether NTPDase1 contributes to the ectonucleotidase activity observed previously in this tissue (15). We compared the global activity with the two major substrates of NTPDase1, ATP and ADP, by enzyme histochemistry. These experiments revealed a strong activity in wild-type vas deferens, which was reduced dramatically in Entpd1−/− tissue sections (Fig. 2A). This activity localized mainly to the tunica muscularis and correlated with the expression of NTPDase1, as determined by immunofluorescence (Fig. 2B, red). In contrast, the ATPase activity in Entpd1−/− tissues was detected exclusively in connective tissues and nerve-like structures within smooth muscle layers and in the peripheral adventitia surrounding blood vessels. Immunofluorescent labeling showed that this activity correlated with the expression of NTPDase2 (Fig. 2B, green).
FIGURE 2.
NTPDase1 is the main ectonucleotidase of vas deferens SMCs. A, ATP and ADP hydrolysis detected at the surface of Entpd1+/+ vas deferens SMCs (brown deposit) is reduced dramatically in Entpd1−/− mice. The remaining ATPase activity in Entpd1−/− tissue is located in nerve-like structures and in the adventitial layer surrounding blood vessels. Nuclei were counterstained with hematoxylin. B, NTPDase1 (red) was immunodetected in the muscular layer and NTPDase2 (green) in nerve-like structures and in the adventitial layer surrounding blood vessels. Nuclei were counterstained with DAPI (blue). C, the time course of ATP hydrolysis by primary cultures from Entpd1−/− vas deferens SMCs was reduced markedly compared with wild-type cultures. Data represent mean ± S.E. of the percentage of initial ATP concentration (conc, 100 μm) in the supernatant of vas deferens SMC cultures prepared from three different mice of each genotype. D, determination of the kinetic parameters evidenced reduced velocity of ATP hydrolysis at the surface of Entpd1−/− SMC cultures compared with the wild type. Data represent mean ± S.E. of four to five independent primary cell cultures. E, quantification of endogenous ATP released in the supernatant of Entpd1+/+ and Entpd1−/− SMCs following medium change. Data represent mean ± S.E. of four experiments conducted on independent SMC cultures and compared using two-way analysis of variance. *, p < 0.05; **, p < 0.01.
In agreement with these results, ATP hydrolysis in SMC primary cultures prepared from vas deferens of Entpd1−/− mice was reduced significantly compared with Entpd1+/+ cells (Fig. 2, C and D), particularly at low ATP concentrations. The comparison of estimates of Michaelis-Menten kinetics further documented the decreased capacity of Entpd1−/− cells to hydrolyze low micromolar ATP concentrations with an estimated global Km shifted to higher substrate concentrations (961 and 276 μm for WT cells).
In addition, we measured the release of endogenous ATP in culture medium using a luciferin/luciferase assay. We found that the ATP concentration in the supernatant of “resting” Entpd1+/+ and Entpd1−/− SMCs after 24 h in culture was about 25 nm (data not shown). Five minutes following medium change, a mechanical stress that induces the release of endogenous nucleotides, ATP increased and reached 40 nm and 75 nm in wild-type and Entpd1−/− cultures, respectively (Fig. 2E). Noteworthy, ATP measurement in the bulk medium underestimates local/juxtacellular ATP concentration. This experiment shows that the deficit of hydrolysis of exogenously added nucleotides in Entpd1−/− SMC cultures is also observable with endogenous ATP. Because the Km of NTPDase1 for ATP is ∼12 μm (24), low nanomolar concentrations may not be hydrolyzed by the enzyme. This would explain the fact that resting ATP in SMC cultures seemed to be independent of NTPDase1 expression. Therefore, it is conceivable that NTPDase1 contribution may be visible only when ATP concentration gets closer to the “threshold” micromolar range, which is the range of P2 receptor activation. Taken together, these results reveal the presence of ATPase activity at the surface of vas deferens SMCs in which NTPDase1 plays a critical role, particularly in the low micromolar concentration of ATP with a Km in the range of local concentration of nucleotides and P2 receptor EC50s.
The Absence of NTPDase1 Leads to an Abnormal Contractile Response of the Vas Deferens to Nucleotides
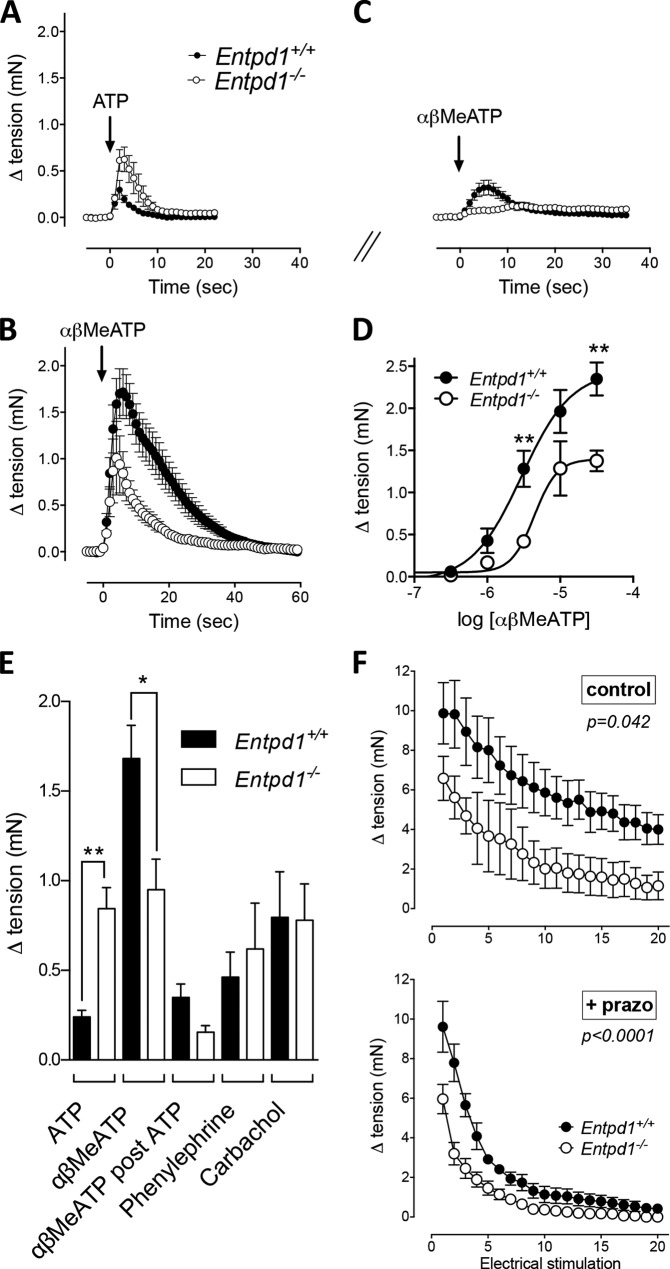
At this point, the most evident hypothesis was that NTPDase1 activity influences P2X1-dependent vas deferens contraction. To test this hypothesis, we evaluated the effect of P2X1 agonist-mediated mouse vas deferens contraction in vitro. The rise in tension induced by ATP (100 μm) was increased 4-fold in Entpd1−/− vas deferens compared with control mice (0.84 ± 0.11 versus 0.24 ± 0.04 mN, respectively; n = 14; p < 0.001) (Fig. 3, A and E). This gain likely reflects the prolonged half-life and availability of ATP in Entpd1−/− tissues. In contrast, the amplitude and the time course of the contraction induced by the P2X1 receptor agonist αβMeATP (10 μm) was reduced in Entpd1−/− vas deferens (Fig. 3, B and E). Concentration-response curves confirmed the reduced potency of αβMeATP in Entpd1−/− vas deferens (Emax = 2.35 ± 0.41 versus 1.37 ± 0.26 mN for Entpd1+/+ and Entpd1−/−, respectively). In contrast to ATP, αβMeATP is resistant to hydrolysis by NTPDase1 (25). The contraction mediated by the latter molecule may consequently reveal the real pool of P2X1 receptors that can be activated independently of the presence or absence of ectonucleotidases. Therefore, the diminished response to this agonist suggests either that P2X1 functionality may be compromised or that Entpd1−/− vas deferens contractibility is abnormal.
FIGURE 3.
NTPDase1 regulates P2X1 activation in the vas deferens. Shown is the time course of contraction induced by exogenous ATP (100 μm) (A), αβMeATP (10 μm) alone (B), or αβMeATP (10 μm) (C) after incubation with 100 μm ATP for 20 min (desensitization). D, concentration-response curve of αβMeATP-induced contraction. **, p < 0.01. E, constrictor responses elicited by phenylephrine and carbachol (10 μm each) were similar in both genotypes. Error bars represent mean ± S.E. of 4–12 independent experiments performed on tissues from four to seven mice and were compared using one-way analysis of variance. *, p < 0.05; **, p < 0.01. F, comparative isometric tension of Entpd1+/+ and Entpd1−/− vas deferens in response to repeated electrical field stimulation (10-s duration, 20 Hz, 45 V, 0.5-ms pulse) every minute. Data represent mean ± S.E. of seven or eight independent preparations. Two-way analysis of variance followed by Fisher's least significant difference test was performed.
The vas deferens is innervated by adrenergic and cholinergic nerve terminals (8). To determine whether Entpd1−/− vas deferens develop normal contraction to non-nucleotide agonists, we compared the effect of the α1 adrenergic receptor agonist phenylephrine and the muscarinic receptor agonist carbachol. The contractile responses to these agonists were equivalent in both genotypes (Fig. 3E), showing that NTPDase1 deletion specifically impairs P2X1-dependent contraction.
To further analyze the P2X1 receptor functional defect in Entpd1−/− mice, vas deferens were challenged with αβMeATP (10 μm) 20 min after a first exposure to ATP (100 μm) (single wash). Although αβMeATP contracted Entpd1+/+, this response was abrogated in Entpd1−/− vas deferens (Fig. 3, A and C). Therefore, depending on the concentration of ATP to which P2X1 is exposed, the absence of NTPDase1 may either allow a greater contractions or a reduced response because of desensitization. Noteworthy is that, in these in vitro experiments, we controlled both the period of exposure and the concentration of the agonist, which cannot be performed in vivo. To assess the effect of endogenous mediators, we evaluated the neurogenic response following electrical field stimulation. Stimulations were repeated every minute to evaluate potential desensitization of the response. Fig. 3F, top panel, shows that Entpd1−/− vas deferens developed reduced tension compared with the wild-type. This difference was highly significant when adrenoceptors were blocked (Fig. 3F, prazosin (prazo), bottom panel). Importantly, the evoked tension between the first approximately five stimulations decreased gradually in wild-type vas deferens and abruptly in mutant vas deferens. These data again suggest that the P2X1 receptor desensitizes quickly in the absence of NTPDase1 and, importantly, that its activation by endogenously released ATP following neurogenic stimulation is impaired.
Ectonucleotidase Activity Is Required for Full P2X1 Activation by ATP
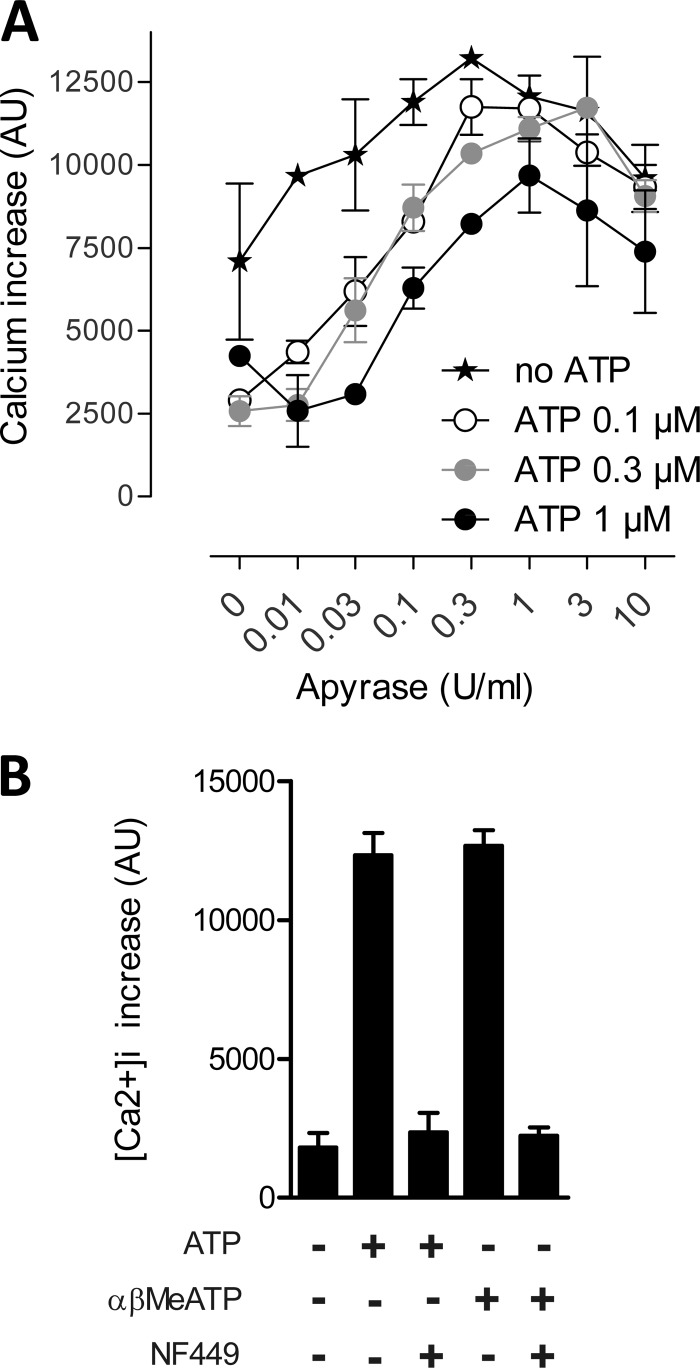
We used HEK293-hP2X1 cells to assess the extent of the interplay between ATP-evoked desensitization, ectonucleotidases, and P2X1 receptor activation in an acute condition. To mimic NTPDase1 activity, we used apyrase, a soluble nucleotidase with similar biochemical properties. A range of apyrase concentrations from 0.01–10 IU/ml was used to influence the ATP-evoked increase in intracellular calcium in HEK293-hP2X1cells. ATP (0.1, 0.3, and 1 μm) pre-exposure (desensitizing) dramatically reduced subsequent ATP (0.3 μm)-induced calcium influx. This effect was reversed with increasing apyrase concentrations up to 1 IU/ml apyrase (Fig. 4A). At concentrations of 3 and 10 IU/ml, apyrase reduced calcium currents. These results indicate that P2X1-mediated calcium influx depends on minimal ATPase activity that is required to preserve P2X1 receptor function. However, apyrase activity >1 IU/ml impaired receptor activation, likely by decreasing the availability of the agonist before receptor activation. Interestingly, similar results were obtained with cells that had not been pre-exposed to ATP, suggesting that desensitization by endogenous ATP release occurs naturally. The selective P2X1 receptor antagonist NF449 (1 μm) abrogated ATP- or αβMeATP-induced calcium influx into cells, confirming the sole involvement of the P2X1 receptor in this response (Fig. 4B).
FIGURE 4.
Influence of ectonucleotidase activity on ATP (0. 3 μm)-evoked calcium influx in HEK293-hP2X1 cells. A, desensitizing ATP (0.1, 0.3, or 1 μm) pre-exposure (5 min) reduced subsequent ATP (0.3 μm)-induced calcium influx. Apyrase (0, 0.01, 0.03, 0.1, 0.3, 1, 3, or 10 IU/ml) protected the response in a concentration-dependent manner. B, no response was induced either by ATP (1 μm) or αβMeATP (3 μm) in the presence of the selective P2X1 receptor antagonist NF449 (1 μm).
Reduced P2X1 Protein in Entpd1−/− Vas Deferens
To evaluate whether the defective contractile response of Entpd1−/− vas deferens to αβMeATP was due to a modification in P2X1 receptor expression, protein and mRNA levels were quantified in Entpd1−/− and Entpd1+/+ vas deferens. Western blot analyses using freshly isolated tissues showed a 3-fold reduction of the P2X1 receptor in Entpd1−/− compared with Entpd1+/+ vas deferens (Fig. 5A). This defect was specific for the P2X1 receptor because the expression of another vas deferens P2X receptor, P2X4, was comparable in both genotypes. No differences in the protein level of NTPDase2 were found, excluding the possibility of functional compensation by this enzyme in Entpd1−/− mice (Fig. 5A). Immunofluorescence experiments corroborated a reduction in P2X1 protein in Entpd1−/− mouse vas deferens cryosections (Fig. 5B). No change in P2X1 mRNA level was detected by quantitative RT-PCR (Fig. 5C), suggesting that the decrease in P2X1 receptor expression is not due to a down-regulation of its gene transcription.
FIGURE 5.
Decreased P2X1 protein level but not mRNA in Entpd1−/− vas deferens. A, Western blot analysis showing that P2X1 immunoreactivity is about 3-fold lower in Entpd1−/− vas deferens, whereas NTPDase2 and P2X4 expression are equivalent in mutant and WT tissues. Error bars show mean ± S.E. of four independent experiments. ***, p < 0.0005. B, immunofluorescence quantification of P2X1 receptor expression in vas deferens (n = 4). *, p < 0.05). C, reverse transcription followed by quantitative PCR analysis shows that P2X1 and P2X4 relative mRNA levels were unchanged between mutant and WT tissues. Expression was normalized to three housekeeping genes (Hprt, Gusb, and Gapdh) and expressed as relative expression = exp (Ct housekeeping − Ct target gene).
DISCUSSION
In this work, we describe that mice deficient for the ecto-ATPase NTPDase1 exhibit reduced male fertility, an abnormal contractile response of the vas deferens to ATP, and reduced P2X1 receptor protein expression. In the male reproductive system, ATP has been proposed to influence the physiology of the reproduction by modulating penile erection, ejaculation, testosterone secretion, and spermatogenesis (26). Our results show that the extracellular nucleotide metabolism, as a consequence, male fertility, as evidenced by a reduction of the average size of pups per litter in Entpd1−/− mice.
Although extracellular nucleotides and nucleosides have been proposed to contribute to sperm maturation and motility (27–29), Entpd1−/− spermatozoa were normal in these aspects and in their number in the epididymis (Fig. 1 and data not shown). The only deficiency we observed in Entpd1−/− mice was a reduced ejaculated sperm count. Together, these data indicate that the impact of NTPDase1 deletion on male fertility was due, in major part, to an impaired vas deferens peristalsis that was corroborated in situ and in vitro. Consequently, this leads to less efficient propulsion of sperm during ejaculation. Noteworthy is that the cauda epididymidis sperm count was increased slightly (p = 0.17) in mutant males, possibly because of an “accumulation” of spermatozoa upstream of the vas deferens. A significant decrease of fertilized oocytes correlated with a low sperm concentration found in the uterus. Accordingly, this decrease of sperm amount directly impacted the efficiency of Entpd1−/− males to fertilize oocytes (Fig. 1G).
We showed here that NTPDase1 deletion leads to an important reduction in the hydrolytic activity at the surface of vas deferens SMCs, especially at low micromolar concentrations of ATP. These data demonstrate that NTPDase1 is the high-affinity enzyme responsible for the main ATPase activity reported previously in this tissue (30) which is also in agreement with the immunolocalization of NTPDase1 in the vas deferens (19). NTPDase1 has been described in virtually all vascular and visceral SMCs (14, 19, 31, 32), making this enzyme the key ectonucleotidase in the regulation of P2 receptor activation on these cells and, consequently, purinergically dependent smooth muscle contraction.
Interestingly, smooth muscle contraction involves different P2 receptors, and differential contribution of NTPDase1 seems to operate according to the nature of the receptor involved (e.g. to their propensity to desensitize). For example, NTPDase1 is the major vascular nucleotidase regulating nucleotide-dependent contraction in vascular (e.g. aortic) and bladder smooth muscle. In both tissues, contraction is mostly dependent on the P2Y6 receptor, which is characterized by its resistance to desensitization (33). As a result, the absence of the enzyme enhances P2Y6 natural agonist UDP bioavailability and receptor activation, which, in turn, unmasks contraction (14, 32).
In the vas deferens, the contraction is dependent on the ATP-activated P2X1 receptor, which, in contrast, displays a high propensity to desensitize in the presence of ATP (34). Rettinger et al. (35) reported that low nanomolar extracellular ATP concentrations are sufficient to obscure P2X1 receptor responses by driving a fraction of the receptor pool into a long lasting refractory state. This occurs with time constants of 300 ms and a slow recovery of 15–20 min (36). In this context, nucleotidase activity is crucial to preserve P2X1 functionality. Indeed, we found that ∼1 IU/ml apyrase, which displays NTPDase1-like activity, preserved the P2X1 receptor from desensitization by endogenous and exogenous nucleotides (Fig. 4). The bell-shaped curve of P2X1-dependent calcium mobilization in the presence of various concentrations of apyrase reveals that an optimal nucleotidase activity is required to prevent the receptor from desensitization, whereas exceeding this optimal amount of activity limits P2X1 activation by fast agonist degradation. In agreement with these results, other studies showed that P2X1 receptor desensitization could be prevented in vitro by soluble potato apyrase (37, 38).
We show here that, in vas deferens, NTPDase1 fulfills P2X1 protection against loss of function. We evidenced that complex modifications of P2X1 responsiveness occur in the absence of NTPDase1 mixing over activation and desensitization. Specifically, Entpd1−/− vas deferens displayed an enhanced contraction to exogenous ATP in vitro (Fig. 3) that reflected prolonged agonist availability because of decreased hydrolysis. These data are in agreement with the observation that the non-hydrolysable inhibitor of NTPDase1, ARL 67156, potentiates ATP-induced guinea pig vas deferens contraction (15). In contrast, contraction in response to the hydrolysis resistant agonist αβMeATP was diminished, suggesting a reduced pool of P2X1 receptors (absent or non-functional) in Entpd1−/− vas deferens. The net effect of these perturbations was a decreased P2X1 receptor-dependent contraction of vas deferens in a physiological setting, i.e. following electrical field stimulation (Fig. 3F), which is closer to the natural activation process of the receptor (neurogenic release of ATP). In agreement, sperm count (not volume), oocyte fertilization (Fig. 1, B and G), and overall male fertility were decreased (Fig. 1). These results indicate that the balance is in favor of a loss of functional P2X1 receptor in vivo.
Ectonucleotidases not only terminate signaling by ATP but also generate adenosine from AMP through the action of ecto-5′-nucleotidase (CD73). Both NTPDase1 (this work) and ecto-5′-nucleotidase (39) are expressed in mouse vas deferens tunica muscularis and potentially generate adenosine from released ATP, and we cannot rule out that a deficit in adenosine generation contributes to the phenotype of Entpd1−/− males. However, some considerations argue in favor of an impaired P2X1 function rather than adenosine generation for these phenotypic observations. For example, real-time generation of adenosine through sequential hydrolysis of ATP by NTPDase1 and ecto-5′-nucleotidase in addition to the time required to activate adenosine receptors (G protein-coupled) seem to barely fit with the rapidity of the contraction/ejaculation process. P2rx1−/− males present an 85% reduced fertility, and, to our knowledge, knockout animals for adenosine receptors expressed in vas deferens (A1 and A2) do not present such a defect. To our knowledge, the absence of the enzyme hydrolyzing AMP to generate adenosine from extracellular ATP and ADP, namely ecto-5′-nucleotidase, has no reported ejaculation defect, and, unlike ATP, adenosine has no proper contractile effect in vas deferens, and the net effect of adenosine on the contraction of vas deferens remains to be established. In vitro adenosine potentiates noradrenaline or ATP-dependent contraction through A1 receptor activation (40) and NA release through presynaptic A2A receptor activation (41). On the other hand, adenosine reduces the contraction through A2B receptors and inhibits NA release through presynaptic A1 (42). Therefore, the contribution of adenosine in vas deferens function still remains to be clarified, and it is not excluded that a chronic reduction in adenosine levels in Entpd1−/− tissues might also have an additional impact on vas deferens function.
Considering P2X1 receptor desensitization, works using chimera P2X1-GFP and protein biotinylation in rat vas deferens proposed that P2X1 desensitization involves the internalization of the receptor in endosomal structures (43, 44). Strikingly, we found that receptor deregulation manifested by in vitro desensitization added with a profound reduction in P2X1 protein amount in the Entpd1−/− vas deferens (Fig. 5). Even if the molecular mechanism involved remains obscure, our data suggest that long lasting ATP exposure does not only lead to acute loss of function but also to cell a surface decrease in P2X1 receptor expression. Importantly, this decrease occurred through non-transcriptional mechanisms because the level of P2X1 mRNA was unchanged in mutant vas deferens. This contrasts with a previously reported P2X1 down-regulation in cultured SMCs, which, in that case, occurs through repression of the transcription (45).
To investigate the potential mechanisms involved in P2X1 receptor desensitization, we followed P2X1 receptor expression by Western blot analysis both in HEK293-transfected cells as well as in primary vas deferens SMCs. The addition of ATP (100 μm) to HEK293-hP2X1 cells did not result in a down-regulation of receptor protein expression (data not shown), as observed in Entpd1−/− vas deferens. We cannot rule out that this apparent discrepancy is due to high expression level of P2X1 receptor as well as differential protein expression in HEK cells that may not allow P2X1 receptor recycling as in primary cells. Vas deferens SMC in culture lost P2X1 receptor expression (data not shown), as reported in vascular SMCs (45). Further investigation would be required to decipher the mechanisms underlying the changes observed in P2X1 expression and subcellular localization when exposed to desensitizing concentrations of ATP.
To summarize, in the absence of NTPDase1 in the vas deferens, the function of P2X1 is perturbed and associated with a decrease in P2X1 protein expression. The net effect is a loss of functional receptors with impaired vas deferens peristalsis. In agreement, the complete deletion of the P2X1 receptor reduces male fertility by 85% because of defective neurogenic vas deferens contraction and sperm reduction in the ejaculate (9). For this reason, it has been proposed recently that the concomitant blockade of α1A-adrenergic G protein-coupled receptors, which is responsible for the residual contraction of vas deferens in P2rx1−/− mice, may constitute a valuable therapeutic approach for male contraception (46). In vivo, sustained P2X1 receptor desensitization could well occur in Entpd1−/−. Indeed, sympathetic nerve terminals (47) continuously release vesicular ATP, and this may lead to ATP buildup in Entpd1−/− mice and, therefore, profoundly desensitize the P2X1 receptor. Therefore, NTPDase1 deletion partially mimics knockout of the P2X1 receptor.
In conclusion, we found that NTPDase1 is essential to maintain P2X1 receptor expression and function in mouse vas deferens. The absence of this enzyme leads to profound receptor desensitization and, as a consequence, impaired contraction, ejaculation, and fertility. These findings point to NTPDase1 as a new regulator of the ejaculatory process, and modifications of the enzyme (mutations or the pathological context affecting NTPDase1 activity) may affect ejaculation efficacy and male fertility.
Acknowledgments
We thank Dr. S. C. Robson (Harvard, MA) for providing Entpd1−/− mice and C. Brèque, C. Maurice, C. Chesseron, and J. L. Bailey from the Centre de Recherche en Biologie de la Reproduction of the Université Laval for technical assistance.
This work was supported by Canadian Institutes of Health Research (CIHR) grants (to J. S.).
- NA
- noradrenaline
- SMC
- smooth muscle cell
- CASA
- computer-assisted sperm analysis.
REFERENCES
- 1. Burnstock G. (2006) Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol. Rev. 58, 58–86 [DOI] [PubMed] [Google Scholar]
- 2. Sneddon P., Burnstock G. (1984) Inhibition of excitatory junction potentials in guinea-pig vas deferens by α, β-methylene-ATP: further evidence for ATP and noradrenaline as cotransmitters. Eur. J. Pharmacol. 100, 85–90 [DOI] [PubMed] [Google Scholar]
- 3. Sneddon P., Westfall D. P. (1984) Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. J. Physiol. 347, 561–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stjarne L., Astrand P. (1984) Discrete events measure single quanta of adenosine 5′-triphosphate secreted from sympathetic nerves of guinea-pig and mouse vas deferens. Neuroscience 13, 21–28 [DOI] [PubMed] [Google Scholar]
- 5. French A. M., Scott N. C. (1983) Evidence to support the hypothesis that ATP is a co-transmitter in rat vas deferens. Experientia 39, 264–266 [DOI] [PubMed] [Google Scholar]
- 6. Benham C. D., Tsien R. W. (1987) A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature 328, 275–278 [DOI] [PubMed] [Google Scholar]
- 7. Valera S., Hussy N., Evans R. J., Adami N., North R. A., Surprenant A., Buell G. (1994) A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature 371, 516–519 [DOI] [PubMed] [Google Scholar]
- 8. Burnstock G., Verkhratsky A. (2010) Vas deferens: a model used to establish sympathetic cotransmission. Trends Pharmacol. Sci. 31, 131–139 [DOI] [PubMed] [Google Scholar]
- 9. Mulryan K., Gitterman D. P., Lewis C. J., Vial C., Leckie B. J., Cobb A. L., Brown J. E., Conley E. C., Buell G., Pritchard C. A., Evans R. J. (2000) Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature 403, 86–89 [DOI] [PubMed] [Google Scholar]
- 10. Yegutkin G. G. (2008) Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim. Biophys. Acta 1783, 673–694 [DOI] [PubMed] [Google Scholar]
- 11. Kukulski F., Lévesque S. A., Sévigny J. (2011) Impact of ectoenzymes on p2 and p1 receptor signaling. Adv. Pharmacol. 61, 263–299 [DOI] [PubMed] [Google Scholar]
- 12. Enjyoji K., Sévigny J., Lin Y., Frenette P. S., Christie P. D., Esch J. S., 2nd, Imai M., Edelberg J. M., Rayburn H., Lech M., Beeler D. L., Csizmadia E., Wagner D. D., Robson S. C., Rosenberg R. D. (1999) Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat. Med. 5, 1010–1017 [DOI] [PubMed] [Google Scholar]
- 13. Koziak K., Sévigny J., Robson S. C., Siegel J. B., Kaczmarek E. (1999) Analysis of CD39/ATP diphosphohydrolase (ATPDase) expression in endothelial cells, platelets and leukocytes. Thromb. Haemost. 82, 1538–1544 [PubMed] [Google Scholar]
- 14. Kauffenstein G., Drouin A., Thorin-Trescases N., Bachelard H., Robaye B., D'Orléans-Juste P., Marceau F., Thorin E., Sévigny J. (2010) NTPDase1 (CD39) controls nucleotide-dependent vasoconstriction in mouse. Cardiovasc. Res. 85, 204–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Westfall T. D., Kennedy C., Sneddon P. (1996) Enhancement of sympathetic purinergic neurotransmission in the guinea-pig isolated vas deferens by the novel ecto-ATPase inhibitor ARL 67156. Br. J. Pharmacol. 117, 867–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olds-Clarke P., Johnson L. R. (1993) t haplotypes in the mouse compromise sperm flagellar function. Dev. Biol. 155, 14–25 [DOI] [PubMed] [Google Scholar]
- 17. Morrier A., Castonguay F., Bailey J. L. (2002) Glycerol addition and conservation of fresh and cryopreserved ram spermatozoa. Can. J. Anim. Sci. 82, 347–356 [Google Scholar]
- 18. Braun N., Sévigny J., Robson S. C., Enjyoji K., Guckelberger O., Hammer K., Di Virgilio F., Zimmermann H. (2000) Assignment of ecto-nucleoside triphosphate diphosphohydrolase-1/cd39 expression to microglia and vasculature of the brain. Eur. J. Neurosci. 12, 4357–4366 [PubMed] [Google Scholar]
- 19. Martín-Satué M., Lavoie E. G., Pelletier J., Fausther M., Csizmadia E., Guckelberger O., Robson S. C., Sévigny J. (2009) Localization of plasma membrane bound NTPDases in the murine reproductive tract. Histochem. Cell Biol. 131, 615–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bartel D. L., Sullivan S. L., Lavoie E. G., Sévigny J., Finger T. E. (2006) Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J. Comp. Neurol. 497, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moon S. K., Thompson L. J., Madamanchi N., Ballinger S., Papaconstantinou J., Horaist C., Runge M. S., Patterson C. (2001) Aging, oxidative responses, and proliferative capacity in cultured mouse aortic smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 280, H2779–H2788 [DOI] [PubMed] [Google Scholar]
- 22. Baykov A. A., Evtushenko O. A., Avaeva S. M. (1988) A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassay. Anal. Biochem. 171, 266–270 [DOI] [PubMed] [Google Scholar]
- 23. Green M. C., Witham B. A. (1991) Handbook on Genetically Standardized JAX Mice, 4th Ed., The Jackson Laboratory, Bar Harbor, ME [Google Scholar]
- 24. Kukulski F., Lévesque S. A., Lavoie E. G., Lecka J., Bigonnesse F., Knowles A. F., Robson S. C., Kirley T. L., Sévigny J. (2005) Comparative hydrolysis of P2 receptor agonists by NTPDases 1, 2, 3 and 8. Purinergic Signal. 1, 193–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Picher M., Sévigny J., D'Orléans-Juste P., Beaudoin A. R. (1996) Hydrolysis of P2-purinoceptor agonists by a purified ectonucleotidase from the bovine aorta, the ATP-diphosphohydrolase. Biochem. Pharmacol. 51, 1453–1460 [DOI] [PubMed] [Google Scholar]
- 26. Burnstock G. (2007) Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 87, 659–797 [DOI] [PubMed] [Google Scholar]
- 27. Banks F. C., Calvert R. C., Burnstock G. (2010) Changing P2X receptor localization on maturing sperm in the epididymides of mice, hamsters, rats, and humans: a preliminary study. Fertil. Steril. 93, 1415–1420 [DOI] [PubMed] [Google Scholar]
- 28. Adeoya-Osiguwa S. A., Fraser L. R. (2002) Capacitation state-dependent changes in adenosine receptors and their regulation of adenylyl cyclase/cAMP. Mol. Reprod. Dev. 63, 245–255 [DOI] [PubMed] [Google Scholar]
- 29. Edwards S. E., Buffone M. G., Knee G. R., Rossato M., Bonanni G., Masiero S., Ferasin S., Gerton G. L., Moss S. B., Williams C. J. (2007) Effects of extracellular adenosine 5′-triphosphate on human sperm motility. Reprod. Sci. 14, 655–666 [DOI] [PubMed] [Google Scholar]
- 30. Harris G. S. (1972) ATPase activity of pharmacological preparations. Eur. J. Pharmacol. 19, 137–139 [DOI] [PubMed] [Google Scholar]
- 31. Lavoie E. G., Gulbransen B. D., Martín-Satué M., Aliagas E., Sharkey K. A., Sévigny J. (2011) Ectonucleotidases in the digestive system: focus on NTPDase3 localization. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G608–G620 [DOI] [PubMed] [Google Scholar]
- 32. Yu W., Sun X., Robson S. C., Hill W. G. (2013) Extracellular UDP enhances P2X-mediated bladder smooth muscle contractility via P2Y(6) activation of the phospholipase C/inositol trisphosphate pathway. FASEB J. 27, 1895–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Robaye B., Boeynaems J. M., Communi D. (1997) Slow desensitization of the human P2Y6 receptor. Eur. J. Pharmacol. 329, 231–236 [PubMed] [Google Scholar]
- 34. North R. A. (2002) Molecular physiology of P2X receptors. Physiol. Rev. 82, 1013–1067 [DOI] [PubMed] [Google Scholar]
- 35. Rettinger J., Schmalzing G. (2004) Desensitization masks nanomolar potency of ATP for the P2X1 receptor. J. Biol. Chem. 279, 6426–6433 [DOI] [PubMed] [Google Scholar]
- 36. Evans R. J., Lewis C., Virginio C., Lundstrom K., Buell G., Surprenant A., North R. A. (1996) Ionic permeability of, and divalent cation effects on, two ATP-gated cation channels (P2X receptors) expressed in mammalian cells. J. Physiol. 497, 413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buell G., Michel A. D., Lewis C., Collo G., Humphrey P. P., Surprenant A. (1996) P2X1 receptor activation in HL60 cells. Blood 87, 2659–2664 [PubMed] [Google Scholar]
- 38. Rolf M. G., Brearley C. A., Mahaut-Smith M. P. (2001) Platelet shape change evoked by selective activation of P2X1 purinoceptors with α,β-methylene ATP. Thromb. Haemost. 85, 303–308 [PubMed] [Google Scholar]
- 39. Martín-Satué M., Lavoie E. G., Fausther M., Lecka J., Aliagas E., Kukulski F., Sévigny J. (2010) High expression and activity of ecto-5′-nucleotidase/CD73 in the male murine reproductive tract. Histochem. Cell Biol. 133, 659–668 [DOI] [PubMed] [Google Scholar]
- 40. Brownhill V. R., Hourani S. M., Kitchen I. (1996) Selective enhancement by an adenosine A1 receptor agonist of agents inducing contraction of the rat vas deferens. Naunyn Schmiedebergs Arch. Pharmacol. 353, 499–504 [DOI] [PubMed] [Google Scholar]
- 41. Queiroz G., Talaia C., Gonçalves J. (2003) Adenosine A2A receptor-mediated facilitation of noradrenaline release involves protein kinase C activation and attenuation of presynaptic inhibitory receptor-mediated effects in the rat vas deferens. J. Neurochem. 85, 740–748 [DOI] [PubMed] [Google Scholar]
- 42. Hardy T. A., Brock J. A. (1999) Effects of A1-adenosine receptor antagonists on purinergic transmission in the guinea-pig vas deferens in vitro. Br. J. Pharmacol. 126, 1761–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dutton J. L., Poronnik P., Li G. H., Holding C. A., Worthington R. A., Vandenberg R. J., Cook D. I., Barden J. A., Bennett M. R. (2000) P2X(1) receptor membrane redistribution and down-regulation visualized by using receptor-coupled green fluorescent protein chimeras. Neuropharmacology 39, 2054–2066 [DOI] [PubMed] [Google Scholar]
- 44. Ennion S. J., Evans R. J. (2001) Agonist-stimulated internalisation of the ligand-gated ion channel P2X(1) in rat vas deferens. FEBS Lett. 489, 154–158 [DOI] [PubMed] [Google Scholar]
- 45. Erlinge D., Hou M., Webb T. E., Barnard E. A., Möller S. (1998) Phenotype changes of the vascular smooth muscle cell regulate P2 receptor expression as measured by quantitative RT-PCR. Biochem. Biophys. Res. Commun. 248, 864–870 [DOI] [PubMed] [Google Scholar]
- 46. White C. W., Choong Y. T., Short J. L., Exintaris B., Malone D. T., Allen A. M., Evans R. J., Ventura S. (2013) Male contraception via simultaneous knockout of α1A-adrenoceptors and P2X1-purinoceptors in mice. Proc. Natl. Acad. Sci. U.S.A. 110, 20825–20830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brain K. L., Jackson V. M., Trout S. J., Cunnane T. C. (2002) Intermittent ATP release from nerve terminals elicits focal smooth muscle Ca2+ transients in mouse vas deferens. J. Physiol. 541, 849–862 [DOI] [PMC free article] [PubMed] [Google Scholar]