Abstract
Long-term and high-dose treatment with metformin is known to be associated with vitamin B12 deficiency in patients with type 2 diabetes. We investigated whether the prevalence of B12 deficiency was different in patients treated with different combination of hypoglycemic agents with metformin during the same time period. A total of 394 patients with type 2 diabetes treated with metformin and sulfonylurea (S+M group, n = 299) or metformin and insulin (I+M group, n = 95) were consecutively recruited. The vitamin B12 and folate levels were quantified using the chemiluminescent enzyme immunoassay. Vitamin B12 deficiency was defined as vitamin B12≤300 pg/mL without folate deficiency (folate>4 ng/mL). The mean age of and duration of diabetes in the subjects were 59.4±10.5 years and 12.2±6.7 years, respectively. The mean vitamin B12 level of the total population was 638.0±279.6 pg/mL. The mean serum B12 levels were significantly lower in the S+M group compared with the I+M group (600.0±266.5 vs. 757.7±287.6 pg/mL, P<0.001). The prevalence of vitamin B12 deficiency in the metformin-treated patients was significantly higher in the S+M group compared with the I+M group (17.4% vs. 4.2%, P = 0.001). After adjustment for various factors, such as age, sex, diabetic duration, duration or daily dose of metformin, diabetic complications, and presence of anemia, sulfonylurea use was a significant independent risk factor for B12 deficiency (OR = 4.74, 95% CI 1.41–15.99, P = 0.012). In conclusion, our study demonstrated that patients with type 2 diabetes who were treated with metformin combined with sulfonylurea require clinical attention for vitamin B12 deficiency and regular monitoring of their vitamin B12 levels.
Introduction
Metformin, which belongs to the biguanide class, is one of the most generally used oral hypoglycemic agents. It has been used for more than 50 years and was approved by the US Food and Drug Administration (FDA) in 1994 [1]. Currently, many clinical practice guidelines for patients with type 2 diabetes, including the American Diabetes Association (ADA), the European Association for the Study of Diabetes (EASD), and the Korean Diabetes Association (KDA), recommend that metformin treatment should begin at the time of diagnosis of diabetes with lifestyle modification in the absence of contraindications [2]–[4]. Moreover, metformin is preferentially selected for combination therapy with sulfonylurea or insulin to achieve the glycemic target goal. Thus, the use of metformin has steadily increased worldwide. According to data using the IMS Health National Disease and Therapeutic Index of the U.S., more than 50% of treatment visits before 2012 were associated with metformin [5].
Long-term metformin treatment is a known pharmacological cause of vitamin B12 deficiency, as was evident within the first 10–12 years [6]–[10] after it started to be used, and is associated with decreased serum folate concentrations [11], [12]. In addition, metformin treatment may be an iatrogenic cause for the exacerbation of peripheral neuropathy in patients with type 2 diabetes who exhibit depressed vitamin B12 levels and elevated fasting total homocysteine and methylmalonic acid (MMA) levels [11]. This is clinically important because patients with diabetes often suffer from neurological symptoms, such as numbness, paresthesia, and impaired vibration sensation and proprioception. These clinical symptoms can easily be considered symptoms of diabetic peripheral neuropathy, and anticonvulsants or tricyclic antidepressants (TCAs) are frequently used as drugs to control these uncomfortable symptoms. However, the symptoms of vitamin B12 deficiency can be easily misunderstood as diabetic peripheral neuropathy, which can lead to unnecessary drug use and misdiagnosis as other demyelinating nerve diseases.
We previously reported a high prevalence of vitamin B12 deficiency in patients with type 2 diabetes treated with metformin, particularly in subjects with a longer duration and higher daily dose of metformin use [13]. However, most patients with diabetes are not treated with metformin monotherapy for glycemic control, and there have been few studies on the effect of combinations of hypoglycemic agents with metformin on vitamin B12 deficiency. Thus, we focused on the effect of combinations of metformin with either sulfonylurea or insulin, which are frequently used hypoglycemic agents, on vitamin B12 deficiency during the same time period.
Thus, the aim of this study was to investigate the effect of sulfonylurea or insulin on vitamin B12 deficiency when used as a combinatorial therapy with metformin for patients with type 2 diabetes.
Methods
Ethics Statement
This study was performed according to the Declaration of Helsinki and approved by the Institutional Review Board of the Catholic University Medical Centre. Written informed consent was obtained from all of the participants.
Study Design and Subjects
Between January 2012 and April 2014, patients with type 2 diabetes, aged 25 to 80 years, who had taken metformin plus sulfonylurea (S+M group) or metformin plus insulin (I+M group) for at least six months were recruited consecutively at the university-affiliated diabetes center of St. Vincent's Hospital in South Korea. To exclude any effects of different treatment periods of sulfonylurea or insulin on the vitamin B12 level, we included only those patients who used either insulin plus metformin or sulfonylurea plus metformin during the same time period. A maximum difference of three months of treatment for each combination was allowed for inclusion in this study.
Patients with newly diagnosed type 2 diabetes, type 1 diabetes, pernicious anemia, or decreased renal function (serum creatinine level>1.7 mg/dL for males or >1.5 mg/dL for females) and pregnant patients were excluded. Patients with a previous history of vitamin B12 injections, gastrectomy, colectomy, inflammatory bowel disease, or chronic heavy alcohol drinker and vegetarians were also excluded. Patients with severe medical illnesses, such as severe infection, sepsis, malignancies, liver cirrhosis, heart failure or renal failure, were also excluded from this study.
The medication history of the enrolled subjects was evaluated using a dietary supplement questionnaire, and the medication history included over-the-counter multivitamins, calcium supplements, histamine-2 receptor blockers (H2 blockers) and proton pump inhibitors (PPIs). To analyze the effects of sulfonylurea dosage on vitamin B12 deficiency, we classified the patients into 4 groups according to the dosages of their sulfonylurea regimens as follows: Group 1: glimepiride 1 mg, gliclazide 80 mg, gliclazide MR 30 mg, and glibenclamide 5 mg (n = 40); Group 2: glimepiride 2 mg, gliclazide 160 mg, gliclazide MR 60 mg, and glibenclamide 10 mg (n = 116); Group 3: glimepiride 3 mg, gliclazide 240 mg, gliclazide MR 90 mg, and glibenclamide 15 mg (n = 56); and Group 4: glimepiride 4 mg, gliclazide 320 mg, gliclazide MR 120 mg, and glibenclamide 20 mg (n = 87)[14], [15].
The patients were defined “alcohol” drinkers if their average consumption was 1 to 2 drinks per day (>1 g alcohol/day after converting the average frequency and amount of alcoholic beverages consumed into units of pure alcohol (in grams) consumed per day[16]. The patients were also divided into current smokers, ex-smokers or non-smokers according to their smoking habits. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or any use of antihypertensive medications [17].
Assessments and Outcome Measures
The primary outcome was biochemical vitamin B12 deficiency, which was determined by the serum vitamin B12 concentrations. The serum vitamin B12 and folate levels were quantified using a chemiluminescent enzyme immunoassay (Immulite 2000; Siemens, Berlin, Germany). We defined biochemical vitamin B12 deficiency as serum levels ≤300 pg/mL without folate deficiency [18], [19]. In the absence of recent anorexia or fasting, a serum folate concentration <2 ng/mL was diagnostic of folate deficiency. Anemia was defined as Hb <13 g/dL for males and <12 g/dL for females based on the WHO guidelines [20]. The blood glucose level was measured using an automated enzymatic method, and the HbA1c level was determined through high-performance liquid chromatography (HPLC-723 G8, Tosoh, Tokyo, Japan). The total cholesterol, triglyceride, and HDL-cholesterol levels were measured enzymatically using an automatic analyzer (Hitachi 736-40, Hitachi, Tokyo, Japan). The measurement of microalbuminuria was performed using immunoturbidimetry (Hitachi 7600-110) through random spot urine collection, and the albumin-to-creatinine ratio (ACR) was calculated. Diabetic nephropathy was defined as ACR≥30 µg/mg creatinine [2]. Diabetic retinopathy was assessed from retinal photographs at baseline, and the findings were reviewed by a board-certified ophthalmologist and classified by the absence or presence of diabetic retinopathy. Diabetic neuropathic symptoms were defined by the presence of typical symptoms, such as pain, burning or aching, prickling sensations, hypoesthesia or numbness in both of the lower legs or feet, through a questionnaire [21]–[23].
Statistical analyses
We used SAS version 9.2 (SAS Institute, Inc., Cary, NC, USA) for statistical analyses. The clinical characteristics and parameters are expressed as the means ± standard deviation (SD) or numbers (percentages). Pearson's Chi-square tests were used to test the differences in the proportion of categorical variables, and independent t-tests were used to evaluate the differences between the means of two continuous variables. One-way ANOVAs were used to evaluate the differences in the means of the continuous variables between the sulfonylurea groups. Pearson correlation analyses were performed to examine the linear relationship between the serum vitamin B12 levels and the duration of metformin use. The variables that were found to be statistically significant in the univariable analysis or reported to affect the vitamin B12 levels were included in the multivariable analysis. A multivariable logistic regression analysis was performed to assess the independent association of each factor with vitamin B12 deficiency. P values <0.05 were considered to be statistically significant.
Results
After exclusion during the study period, 394 eligible patients were enrolled and completed the evaluation. The clinical characteristics of the patients are shown in Table 1. Of the 394 patients with type 2 diabetes, the mean age and duration of diabetes were 59.4±10.5 years and 12.2±6.7 years, respectively. In this study, 45.4% of the patients were males, 51.0% had hypertension, and the mean HbA1c level was 7.8±1.5% at baseline. The mean vitamin B12 level was 638.0±279.6 pg/mL, and 56 patients (14.2%) showed vitamin B12 deficiency (Table 1).
Table 1. Baseline clinical characteristics of the subjects.
| Characteristic | Total subjects (n = 394) |
| Age (years) | 59.4±10.5 |
| Men, n (%) | 179 (45.4) |
| Diabetic duration (years) | 12.2±6.7 |
| Alcohol (yes, %) | 94 (23.9) |
| Smoking (yes, %) | 69 (17.5) |
| BMI (kg/m2) | 24.8±3.2 |
| Hypertension (yes, %) | 201 (51.0) |
| Diabetic retinopathy (yes, %) | 109 (27.7) |
| Diabetic neuropathic symptom (yes, %) | 86 (21.8) |
| Diabetic nephropathy (yes, %) | 132 (33.5) |
| Duration of metformin use (months) | 82.6±49.4 |
| Daily dose of metformin (mg) | 1305.5±449.4 |
| Hypertensive medication (yes, %) | 242 (61.4) |
| Use of statin (yes, %) | 212 (53.8) |
| Over-the-counter multivitamin(yes, %) | 46 (11.7) |
| Calcium supplement (yes, %) | 18 (4.6) |
| H2 blocker or PPI (yes, %) | 30 (7.6) |
| FBS (mg/dL) | 145.5±53.9 |
| Creatinine (mg/dL) | 0.8±0.2 |
| TC (mg/dL) | 166.9±37.4 |
| TG (mg/dL) | 131.5±115.9 |
| HDL-cholesterol (mg/dL) | 49.0±23.3 |
| LDL-cholesterol (mg/dL) | 95.9±40.7 |
| HbA1c (%) | 7.8±1.5 |
| Vitamin B12 deficiency, n (%) | 56 (14.2) |
| Vitamin B12 (pg/mL) | 638.0±279.6 |
| Serum folate (ng/mL) | 9.9±5.7 |
| Anemia*(yes, %) | 61 (15.5) |
| Hemoglobin (g/dL) | 13.9±5.3 |
| MCV (fL) | 89.0±5.0 |
| MCH (fL) | 30.7±1.8 |
| MCHC (fL) | 34.2±1.3 |
The data are shown as the means (SD) or n (%). *Hb<13 g/dL for men and <12 g/dL for women (WHO guidelines); BMI, body mass index; H2 blocker, histamine 2 receptor blocker; PPI, proton pump inhibitor; FBS, fasting blood sugar; TC, total cholesterol; TG, triglyceride; MCV, mean corpuscular volume; fL, femtoliter.
Among the total population, 299 patients were treated with metformin combined with sulfonylurea (S+M group), and 95 patients were treated with metformin and insulin (I+M group). Compared with the S+M group, the subjects in the I+M group showed a longer diabetic duration, higher BMI, higher prevalence of diabetic retinopathy and nephropathy, and higher HbA1c level (Table 2). Remarkably, the mean vitamin B12 level was significantly lower in the S+M group compared with the I+M group (600.0±266.5 vs. 757.7±287.6 pg/mL, P<0.001). In addition, the prevalence of vitamin B12 deficiency was significantly higher in the S+M group than in the I+M group (17.4 vs. 4.2%, P<0.005). However, the duration of metformin, the daily dose of metformin, and the prevalence of diabetic neuropathic symptoms and anemia were not significantly different between the two groups (Table 2).
Table 2. Characteristics of the subjects according to combination treatment with metformin.
| Characteristic | S+M (n = 299) | I+M (n = 95) | P value |
| Age (years) | 59.7±10.3 | 58.2±10.8 | .223 |
| Men, (%) | 143 (47.8) | 36 (37.9) | .091 |
| Diabetic duration (years) | 11.2±6.3 | 15.5±7.2 | <.001 |
| Alcohol (yes, %) | 80 (26.8) | 14 (14.7) | .017 |
| Smoking (yes, %) | 53 (17.7) | 16 (16.8) | .741 |
| BMI (kg/m2) | 24.5±3.2 | 25.5±3.2 | .008 |
| Hypertension (yes, %) | 152 (50.8) | 49 (51.6) | .900 |
| Diabetic retinopathy (yes, %) | 67 (22.4) | 42 (44.2) | <.001 |
| Diabetic neuropathic symptom (yes, %) | 64 (21.4) | 22 (23.2) | .422 |
| Diabetic nephropathy (yes, %) | 81 (27.1) | 51 (53.7) | <.001 |
| Duration of metformin use (months) | 80.5±47.0 | 89.1±56.1 | .180 |
| Daily dose of metformin (mg) | 1276.1±451.2 | 1397.9±432.9 | .175 |
| Use of statin (yes, %) | 154 (51.5) | 58 (61.1) | .104 |
| Over-the-counter multivitamin(yes, %) | 34 (11.4) | 12 (12.6) | .740 |
| Calcium supplement (yes, %) | 14 (4.7) | 4 (4.2) | .848 |
| H2 blocker or PPI (yes, %) | 20 (6.7) | 10 (10.5) | .220 |
| FBS (mg/dL) | 144.6±52.3 | 148.3±58.9 | .582 |
| Creatinine (mg/dL) | 0.8±0.2 | 0.8±0.3 | .288 |
| TC (mg/dL) | 168.1±37.0 | 163.3±38.3 | .275 |
| TG (mg/dL) | 132.4±127.6 | 128.7±67.0 | .789 |
| HDL-cholesterol (mg/dL) | 50.1±24.3 | 45.5±19.3 | .057 |
| LDL-cholesterol (mg/dL) | 97.5±42.0 | 91.0±36.3 | .183 |
| HbA1c (%) | 7.6±1.3 | 8.7±1.8 | <.001 |
| Vitamin B12 deficiency, n (%) | 52 (17.4) | 4 (4.2) | .001 |
| Vitamin B12 (pg/mL) | 600.0±266.5 | 757.7±287.6 | <.001 |
| Serum folate (ng/mL) | 9.8±5.6 | 10.3±6.0 | .444 |
| Anemia*(yes, %) | 42 (14.0) | 19 (20.0) | .176 |
| Hemoglobin (g/dL) | 14.1±6.0 | 13.4±1.8 | .318 |
| MCV (fL) | 89.1±5.1 | 89.0±4.4 | .949 |
| MCH (fL) | 30.7±1.7 | 30.6±1.8 | .551 |
| MCHC (fL) | 34.2±1.3 | 34.2±1.2 | .893 |
The data are presented as the means (SD) or n (%).*Hb<13 g/dL for men and <12 g/dL for women (WHO guidelines); BMI, body mass index; H2 blocker, histamine 2 receptor blocker; PPI, proton pump inhibitor; FBS, fasting blood sugar; TC, total cholesterol; TG, triglyceride; MCV, mean corpuscular volume; fL, femtoliter.
S+M: Metformin + Sulfonylurea; I+M: Metformin + Insulin.
The mean vitamin B12 levels of the normal B12 level group and the vitamin B12 deficiency group were 704.5±244.3 pg/mL and 237.0±46.0 pg/mL, respectively (P<0.005). Compared with the subjects without vitamin B12 deficiency, the patients with vitamin B12 deficiency were older in age, used more sulfonylurea, and had lower levels of HbA1c and hemoglobin (Table 3). In addition, the patients with vitamin B12 deficiency demonstrated a higher frequency of anemia but not megaloblastic type anemia than the normal vitamin B12 level group (26.8% vs. 13.6%, P = 0.017).
Table 3. Characteristics of the two selected groups according to vitamin B12 deficiency.
| Characteristic | Vitamin B12 deficiency (−) | Vitamin B12 deficiency (+) | P value |
| n | 338 | 56 | |
| Age (years) | 58.7±10.4 | 63.3±10.3 | .003 |
| Men, (%) | 156 (46.2) | 23 (41.1) | .563 |
| Diabetic duration (years) | 12.2±6.9 | 12.7±5.5 | .548 |
| Alcohol (yes, %) | 80 (23.7) | 14 (25.0) | .866 |
| Smoking (yes, %) | 60 (17.8) | 9 (16.1) | .826 |
| BMI (kg/m2) | 24.8±3.3 | 24.4±2.9 | .330 |
| Hypertension (yes, %) | 170 (50.3) | 31 (55.4) | .564 |
| Sulfonylurea use (yes, %) | 244 (72.2) | 52 (92.9) | <.001 |
| Insulin use (yes, %) | 91 (26.9) | 4 (7.1) | .001 |
| Diabetic retinopathy (yes, %) | 97 (28.7) | 12 (21.4) | .332 |
| Diabetic neuropathic symptom (yes, %) | 70 (20.7) | 16 (28.6) | .221 |
| Diabetic Nephropathy (yes, %) | 117 (34.6) | 15 (26.8) | .287 |
| Duration of metformin use (months) | 79.2±49.5 | 102.6±44.5 | .001 |
| Daily dose of metformin (mg) | 1252.8±425.9 | 1623.2±459.9 | <.001 |
| Hypertensive medication (yes, %) | 205 (60.7) | 37 (66.1) | .463 |
| Use of statin (yes, %) | 179 (53.0) | 33 (58.9) | .470 |
| Over-the-counter multivitamin(yes, %) | 38 (11.2) | 8 (14.3) | .502 |
| Calcium supplement (yes, %) | 16 (4.7) | 2 (3.6) | 1.000 |
| H2 blocker or PPI (yes, %) | 24 (7.1) | 6 (10.7) | .410 |
| FBS (mg/dL) | 146l5±55l4 | 139.0±43.2 | .248 |
| Creatinine (mg/dL) | 0.8±0.2 | 0.8±0.2 | .685 |
| TC (mg/dL) | 168.0±37.7 | 160.5±34.8 | .145 |
| TG (mg/dL) | 131.2±121.9 | 133.1±69.6 | .869 |
| HDL-cholesterol (mg/dL) | 49.6±24.1 | 45.8±17.4 | .160 |
| LDL-cholesterol (mg/dL) | 96.6±41.6 | 92.0±35.1 | .379 |
| HbA1c (%) | 7.9±1.6 | 7.4±1.4 | .006 |
| Vitamin B12 (pg/mL) | 704.5±244.3 | 237.0±46.0 | <.001 |
| Serum folate (ng/mL) | 9.9±5.7 | 9.7±5.6 | .747 |
| Anemia*(yes, %) | 46 (13.6) | 15 (26.8) | .017 |
| Hemoglobin (g/dL) | 14.1±5.7 | 13.1±1.5 | .010 |
| MCV (fL) | 89.1±5.1 | 89.0±4.2 | .800 |
| MCH (fL) | 30.8±1.8 | 30.4±1.7 | .150 |
| MCHC (fL) | 34.3±1.2 | 33.9±1.7 | .106 |
The data are presented as the means (SD) or n (%).*Hb<13 g/dL for men and <12 g/dL for women (WHO guidelines); BMI, body mass index; H2 blocker, histamine 2 receptor blocker; PPI, proton pump inhibitor; FBS, fasting blood sugar; TC, total cholesterol; TG, triglyceride; MCV, mean corpuscular volume; fL, femtoliter.
Of the total subjects involved in the present study, 61 (15.5%) had anemia, and 14 (4.7%) of the patients also had vitamin B12 deficiency. Of the patients with B12 deficiency, 15 (26.8%) patients were anemic, but 46 (13.6%) of the patients without B12 deficiency were anemic (P = 0.017, Table 3). Only four of the total subjects (1.02%) had a mean corpuscular volume (MCV)>100 fL, and only two patients had anemia (Hb 12.4 g/dL in males, Hb 10.4 g/dL in females).
The duration of treatment and daily dose of metformin were significantly higher in the patients with vitamin B12 deficiency compared with the patients without vitamin B12 deficiency (Table 3). However, the presence of diabetic complications and the duration of diabetes were not different between the two groups.
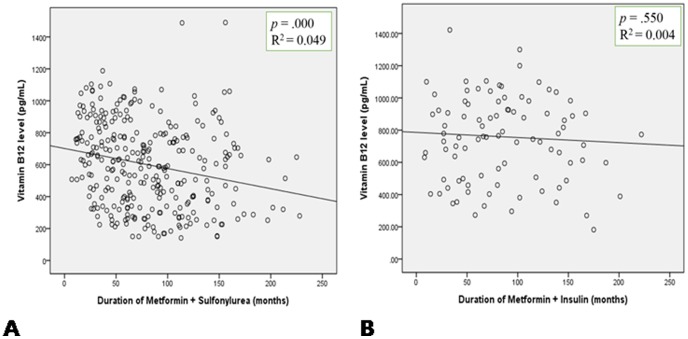
The correlation between the serum vitamin B12 level and the duration of metformin use was evaluated. The vitamin B12 levels showed a significant negative correlation with the duration of metformin treatment in the S+M group (R2 = 0.049, P<0.001, Fig. 1A) but not in the I+M group (R2 = 0.004, P = 0.550, Fig. 1B).
Figure 1. Correlation between the vitamin B12 levels and the duration of metformin treatment in two different groups.
A: S+M group, B: I+M group. R2: coefficient of determination.
After adjusting for confounding factors, such as age, sex, diabetic duration, duration and daily dose of metformin treatment, the presence of hypertension or anemia, the HbA1c level, and diabetic complications, the multivariable logistic regression analysis revealed that sulfonylurea use was significantly associated with vitamin B12 deficiency in patients with type 2 diabetes (OR = 4.74, 95% CI 1.41–15.99, P = 0.012, Table 4).
Table 4. Logistic regression analysis for potential risk factors of Vitamin B12 deficiency among patients with type 2 diabetes.
| Risk factor | OR (95% CI) | P value |
| Male gender | 0.63 (0.27–1.48) | .284 |
| Age (per year) | 1.05 (1.01–1.09) | .028 |
| Sulfonylurea use | 4.74 (1.41–15.99) | .012 |
| Diabetic duration (per year) | 0.93 (0.86–1.00) | .056 |
| Duration of metformin use (year) | .009 | |
| <5 years | 1 | |
| 5 to 9 years | 4.72 (1.82–12.27) | .001 |
| ≥10 years | 6.74 (1.94–23.50) | .003 |
| Daily dose of metformin (mg) | <.001 | |
| <1000 mg/day | 1 | |
| 1000 to 1999 mg/day | 3.64 (0.43–30.66) | .234 |
| ≥2000 mg | 31.15 (3.44–282.20) | .002 |
| Use of H2 blocker or Proton pump inhibitor | 1.23 (0.39–3.89) | .731 |
| Over-the-counter multivitamin use | 1.39 (0.51–3.77) | .516 |
| Calcium use | 0.38 (0.07–2.22) | .282 |
| HbA1c (%) | 0.74 (0.56–0.99) | .044 |
| Anemia | 2.03 (0.87–4.71) | .101 |
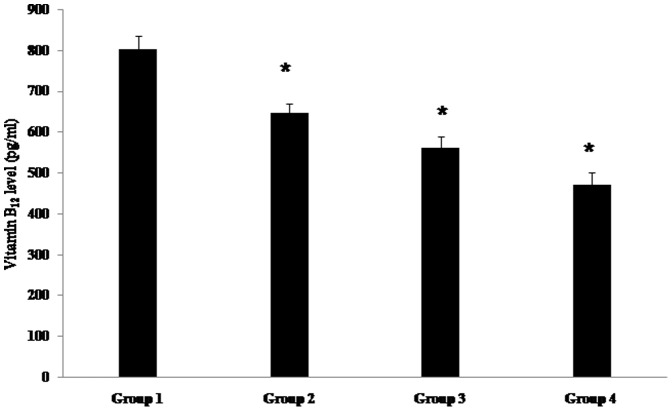
To investigate the effects of the dosage of sulfonylurea on vitamin B12 deficiency, we performed further analyses according to the mean daily dosages of sulfonylurea. There were no significant differences between the clinical variables of the patients in each of the four groups at the beginning of the study with the exception of diabetic duration and HbA1c level (Table 5). When we classified the patients into four groups according to daily dosages of sulfonylurea, we found that the vitamin B12 level was significantly lower in the higher dosage group (Group 4) than in Group 1, P<0.001; Fig. 2, Table 5). The prevalence of vitamin B12 deficiency also increased with increasing sulfonylurea dosage. Compared to Group 1, the percentage of vitamin B12 deficiency was approximately 15-fold higher in the patients of Group 4 (2.5% vs. 39.1%, P<0.001, Table 5). Even after adjusting for age, sex, diabetic duration, hypertension, alcohol drinker, duration and daily dose of metformin, hemoglobin level, HbA1c level, and presence of anemia in the multivariable logistic regression, this analysis revealed that the highest dosage of sulfonylurea was significantly associated with the presence of vitamin B12 deficiency in patients with type 2 diabetes (OR 20.3, 95% CI 2.39–171.9, P = 0.006, Table 6).
Table 5. Clinical characteristics according to daily dosage of sulfonylurea.
| Group 1 (n = 40) | Group 2 (n = 116) | Group 3 (n = 56) | Group 4 (n = 87) | P value | |
| Age | 59.8±10.5 | 58.9±10.2 | 60.6±11.9 | 60.0±9.8 | 0.768 |
| Men, (%) | 21 (52.5) | 57 (49.1) | 25 (44.6) | 40 (46.0) | 0.855 |
| Diabetic duration (years) | 9.23±5.2 | 10.6±6.0 | 12.9±6.0 | 11.8±6.0 | 0.017 |
| Alcohol (yes, %) | 8 (20.0) | 40 (34.5) | 10 (17.9) | 20 (23.0) | 0.064 |
| BMI (kg/m2) | 24.1±3.1 | 24.7±3.4 | 24.5±2.9 | 24.5±3.1 | 0.729 |
| Hypertension (yes, %) | 26 (65.0) | 52 (44.8) | 31 (55.4) | 37 (42.5) | 0.093 |
| Duration of metformin use (months) | 71.1±45.9 | 73.4±47.0 | 92.2±45.7 | 86.7±46.8 | 0.026 |
| Daily dose of metformin (mg) | 906.3±342.9 | 1181.0±395.3 | 1369.6±394.0 | 1512.6±453.7 | <0.001 |
| FBS (mg/dL) | 134.1±25.0 | 138.8±40.8 | 148.5±57.3 | 154.9±68.0 | 0.081 |
| Creatinine (mg/dL) | 0.79±0.2 | 0.80±0.2 | 0.78±0.2 | 0.83±0.2 | 0.651 |
| HbA1c (%) | 7.03±0.6 | 7.47±1.2 | 7.61±1.2 | 7.90±1.7 | 0.006 |
| Vitamin B12 (pg/mL) | 801.8±214.2 | 647.3±238.9 | 561.0±216.9 | 471.3±281.4 | <0.001 |
| Vitamin B12 deficiency (yes, %) | 1 (2.5) | 9 (7.8) | 8 (14.3) | 34 (39.1) | <0.001 |
| Serum folate (ng/mL) | 11.8±6.3 | 9.36±5.2 | 9.78±5.8 | 9.40±5.5 | 0.120 |
| Anemia (yes, %)* | 3 (7.5) | 12 (10.3) | 12 (21.4) | 15 (17.2) | 0.055 |
| Hemoglobin (g/dL) | 16.7±1.61 | 13.9±1.4 | 13.5±1.6 | 13.5±1.6 | 0.032 |
The data are presented as the means (SD) or n (%).*Hb<13 g/dL for men and <12 g/dL for women (WHO guidelines); BMI, body mass index; FBS, fasting blood sugar.
Group 1; glimepiride 1 mg, gliclazide 80 mg, gliclazide MR 30 mg, glibenclamide 5 mg, Group 2; glimepiride 2 mg, gliclazide 160 mg, gliclazide MR 60 mg, glibenclamide 10 mg, Group 3; glimepiride 3 mg, gliclazide 240 mg, gliclazide MR 90 mg, glibenclamide 15 mg, Group 4; glimepiride 4 mg, gliclazide 320 mg, gliclazide MR 120 mg, glibenclamide 20 mg.
Figure 2. The mean value of vitamin B12 according to daily sulfonylurea dosage (* P<0.05 vs. Group 1).
Group 1: glimepiride 1 mg, gliclazide 80 mg, gliclazide MR 30 mg, and glibenclamide 5 mg (n = 40); Group 2: glimepiride 2 mg, gliclazide 160 mg, gliclazide MR 60 mg, and glibenclamide 10 mg (n = 116); Group 3: glimepiride 3 mg, gliclazide 240 mg, gliclazide MR 90 mg, and glibenclamide 15 mg (n = 56); and Group 4: glimepiride 4 mg, gliclazide 320 mg, gliclazide MR 120 mg, and glibenclamide 20 mg (n = 87).
Table 6. Relationship between daily dosage of sulfonylurea regimen and vitamin B12 deficiency.
| Odds ratio (95% CI) | ||||
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Group 1 | 1.0 | 1.0 | 1.0 | 1.0 |
| Group 2 | 3.46 (0.42–28.5) | 4.15 (0.49–34.7) | 3.43 (0.40–29.3) | 2.68 (0.31–23.3) |
| Group 3 | 6.13 (0.73–51.8) | 7.57 (0.88–65.4) | 4.67 (0.52–42.2) | 3.96 (0.43–36.6) |
| Group 4 | 27.6 (3.57–213.0) | 39.5 (4.9–317.0) | 23.4 (2.79–195.0) | 20.3 (2.39–171.9) |
| P value | <0.001 | <0.001 | <0.001 | <0.001 |
Multivariable logistic regression analysis models were adjusted as follows: model 1: sex, age; model 2: model 1 + diabetes duration, hypertension, HbA1c; model 3: model 2 + duration of metformin, daily dose of metformin; model 4: model 3 + alcohol, hemoglobin, presence of anemia.
Group 1; glimepiride 1 mg, gliclazide 80 mg, gliclazide MR 30 mg, glibenclamide 5 mg, Group 2; glimepiride 2 mg, gliclazide 160 mg, gliclazide MR 60 mg, glibenclamide 10 mg, Group 3; glimepiride 3 mg, gliclazide 240 mg, gliclazide MR 90 mg, glibenclamide 15 mg, Group 4; glimepiride 4 mg, gliclazide 320 mg, gliclazide MR 120 mg, glibenclamide 20 mg.
Discussion
In this study, we found a more significant association between the combined treatment of sulfonylurea plus metformin and a higher prevalence of vitamin B12 deficiency in patients with type 2 diabetes compared with that found for the insulin plus metformin combination therapy. We also demonstrated that vitamin B12 deficiency was significantly correlated with the duration of metformin use. After adjusting for confounding factors, the sulfonylurea combination was significantly associated with metformin-related vitamin B12 deficiency compared with the insulin combination during the same time period. This study provides the first analysis of metformin-associated vitamin B12 deficiency in response to combined hypoglycemic medications.
Metformin treatment with lifestyle modifications is generally recommended as the first-line treatment for type 2 diabetes [2]–[4]. If the glycemic control status does not reach the target range, additional hypoglycemic medications should be added to the metformin therapy based on the patients' clinical characteristics. With a steady increase in the number of patients with diabetes, a longer life expectancy of diabetic patients, and the clinical benefits of intensive glycemic control, oral hypoglycemic agents or insulin are consequently expected to be used more often in combination with metformin.
Vitamin B12 deficiency is one of the clinically important side effects of metformin treatment. Approximately 6–10% and up to 30% of patients receiving metformin for diabetes treatment have been reported to experience reduced vitamin B12 absorption [23]–[27], and the serum vitamin B12 levels were decreased in 14% to 30% of metformin-treated patients [11], [28], [29]. A recent study from the National Health and Nutrition Examination Survey (NHANES) revealed that 5.8% of patients with diabetes using metformin presented with vitamin B12 deficiency (serum vitamin B12 level ≤200 pg/dL) compared with 2.4% of patients with diabetes not using metformin and 3.3% of patients without diabetes [25].
Patients with metformin-induced vitamin B12 deficiency exhibit some neurological symptoms, such as paresthesias, impaired vibration sensation and proprioception, which are a potential result of neurological damage. These symptoms may be mistaken as diabetic peripheral neuropathy [7].A previous study reported an association between low vitamin B12 levels and poor nerve conduction velocities with poorer responses to light touch via monofilament detection [30]. This may lead patients to use unnecessary anticonvulsants or tricyclic antidepressants [7], [31], [32]. Because vitamin B12-associated neuropathy is a reversible and treatable condition, the early detection and treatment of vitamin B12 deficiency is clinically important in patients with diabetes using metformin [32], [33].
Based on our results, 14.2% of the subjects showed vitamin B12 deficiency. The patients treated with metformin plus sulfonylurea (S+M group) showed a significantly higher prevalence of vitamin B12 deficiency compared with those treated with metformin plus insulin (I+M group) for the same time period. However, the prevalence of diabetic neuropathic symptoms or anemia was not different between the two groups. In addition, the patients with vitamin B12 deficiency were older in age and used metformin for a longer duration and at a higher daily dose; in addition, more patients with vitamin B12 deficiency used sulfonylurea, and approximately a two-fold higher number of patients with vitamin B12 deficiency had anemia compared with the patients without vitamin B12 deficiency. The diabetic duration and the presence of diabetic microvascular complications did not affect vitamin B12 deficiency.
Classically, vitamin B12 deficiency is related to megaloblastic anemia (MCV>100 fL) [6], [18]. However, the observed mean MCV level in our subjects with vitamin B12 deficiency was not greater than 100 fL, and the prevalence of megaloblastic anemia was approximately 0.5% (n = 2). No difference was found in the mean MCV between the groups with and without vitamin B12 deficiency. Thus, the anemia was most likely caused from chronic illness. Previous reports have indicated that up to 30% of vitamin B12-responsive disorders have normal MCVs [33]–[37]. Moreover, the masking of the macrocytic expression of megaloblastic anemia by coexisting thalassaemia, iron deficiency and chronic illness has been widely reported [37], [38]. Thus, investigating the red cell distribution width and the reticulocyte index and the careful examination of the blood using a peripheral blood smear may be helpful for distinguishing vitamin B12 deficiency-related anemia from anemia due to other causes [37], [38].
In our study population, 86 participants (21.8%) had typical diabetic neuropathic symptoms. The mean diabetic duration and the duration of metformin treatment were 12.2 years and 82.6 months, respectively. Remarkably, the presence of diabetic neuropathic symptoms was not different between the groups with and without vitamin B12 deficiency. Although the duration of use and the daily dose of metformin were correlated with vitamin B12 deficiency, a long-standing diabetic duration with long-term metformin use may contribute to this lack of difference in the presence of neuropathic symptoms. In addition, diabetic neuropathy, as suggested by typical symptoms, did not reflect the severity of the diabetic neuropathy despite the presence of vitamin B12 deficiency.
There is no known mechanism of metformin-induced vitamin B12 deficiency. Some hypotheses include bacterial overgrowth in the small intestine, which has been attributed to diabetes mellitus, changes in small bowel motility, changes in bacterial flora, competitive inhibition or inactivation of vitamin B12 absorption, or an effect of calcium on cell membranes [39]–[41]. Moreover, none of the studies explain the effect of sulfonylurea on vitamin B12 metabolism in metformin-induced vitamin B12 deficiency. In the present study, we clearly demonstrated that the mean blood vitamin B12 level significantly decreased and that the prevalence of vitamin B12 deficiency significantly increased with the daily dosage of sulfonylurea in patients with type 2 diabetes. Remarkably, this finding remained significantly after adjustments for the daily dosage and duration of metformin treatment among the patients taking the maximal sulfonylurea dosage. Therefore, we suggest that sulfonylurea, in contrast to insulin, might affect intestinal vitamin B12 absorption or metabolism when combined with metformin particularly in patients taking the maximal dosage for extended periods.
There are some limitations to this study. First, this hospital-based cross-sectional study included a small number of patients with relatively long-standing diabetes. In addition, the assessment of typical diabetic neuropathic symptoms may make it difficult to observe the effect of vitamin B12deficiency on diabetic neuropathy. Second, we did not measure the MMA, homocysteine or iron levels. These laboratory examinations could not be performed routinely on an outpatient basis. Finally, this cohort study consisted entirely of an Asian population. Thus, additional studies are required to apply this finding to other ethnic groups.
In conclusion, our data suggested the need for regular vitamin B12 monitoring in patients with type 2 diabetes, particularly patients receiving higher daily dosage of sulfonylurea plus metformin treatment for a long time period, even in the absence of hematological abnormalities. Further evaluation is needed to clarify the pathological mechanisms and clinical recommendations for B12 supplementation for patients with prolonged metformin therapy in the future.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. Bouchoucha M, Uzzan B, Cohen R (2011) Metformin and digestive disorders. Diabetes Metab 37: 90–96. [DOI] [PubMed] [Google Scholar]
- 2. American Diabetes Association (2014) Standards of medical care in diabetes–2014. Diabetes Care 37 Suppl 1 S14–80. [DOI] [PubMed] [Google Scholar]
- 3. Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, et al. (2009) Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 32: 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ko SH, Kim SR, Kim DJ, Oh SJ, Lee HJ, et al. (2011) 2011 clinical practice guidelines for type 2 diabetes in Korea. Committee of ClinicalPractice Guidelines, Korean Diabetes Association. Diabetes Metab J 35: 431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Turner LW, Nartey D, Stafford RS, Singh S, Alexander GC (2014) Ambulatory treatment of type 2 diabetes in the U.S., 1997–2012. Diabetes Care 37: 985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andres E, Noel E, Goichot B (2002) Metformin-associated vitamin B12 deficiency. Arch Intern Med 162: 2251–2252. [DOI] [PubMed] [Google Scholar]
- 7. Bell DS (2010) Metformin-induced vitamin B12 deficiency presenting as a peripheral neuropathy. South Med J 103: 265–267. [DOI] [PubMed] [Google Scholar]
- 8. Filioussi K, Bonovas S, Katsaros T (2003) Should we screen diabetic patients using biguanides for megaloblastic anaemia? Aust Fam Physician 32: 383–384. [PubMed] [Google Scholar]
- 9. Liu KW, Dai LK, Jean W (2006) Metformin-related vitamin B12 deficiency. Age Ageing 35: 200–201. [DOI] [PubMed] [Google Scholar]
- 10. de Jager J, Kooy A, Lehert P, Wulffele MG, van der Kolk J, et al. (2010) Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial. BMJ 340: c2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wile DJ, Toth C (2010) Association of metformin, elevated homocysteine, and methylmalonic acid levels and clinically worsened diabetic peripheral neuropathy. Diabetes Care 33: 156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sahin M, Tutuncu NB, Ertugrul D, Tanaci N, Guvener ND (2007) Effects of metformin or rosiglitazone on serum concentrations of homocysteine, folate, and vitamin B12 in patients with type 2 diabetes mellitus. J Diabetes Complications 21: 118–123. [DOI] [PubMed] [Google Scholar]
- 13. Ko SH, Ko SH, Ahn YB, Song KH, Han KD, et al. (2014) Association of Vitamin B12 Deficiency and Metformin Use in Patients with Type 2 Diabetes. J Korean Med Sci 29: 965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schernthaner G, Grimaldi A, Di Mario U, Drzewoski J, Kempler P, et al. (2004) GUIDE study: double-blind comparison of once-daily gliclazide MR and glimepiride in type 2 diabetic patients. Eur J Clin Invest 34: 535–542. [DOI] [PubMed] [Google Scholar]
- 15.Kimberley Standard Drug List. Available: http://www.ksdl.kamsc.org.au/dtp/switching_ sulphonylureas.html. Accessed 2014 Aug 10.
- 16. Agarwal DP (2002) Cardioprotective effects of light-moderate consumption of alcohol: a review of putative mechanisms. Alcohol Alcoholism 37: 409–415. [DOI] [PubMed] [Google Scholar]
- 17. Ko SH, Kwon HS, Kim DJ, Kim JH, Kim NH, et al. (2014) Higher prevalence and awareness, but lower control rate of hypertension in patients with diabetes than general population: the fifth Korean National Health and Nutrition Examination Survey in 2011. Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Diabetes Metab J. 38: 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mazokopakis EE, Starakis IK (2012) Recommendations for diagnosis and management of metformin-induced vitamin B12 (Cbl) deficiency. Diabetes Res Clin Pract 97: 359–367. [DOI] [PubMed] [Google Scholar]
- 19. Long AN, Atwell CL, Yoo W, Solomon SS (2012) Vitamin B(12) deficiency associated with concomitant metformin and proton pump inhibitor use. Diabetes Care 35: e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO/UNICEF/UNU (2001) Iron Deficiency Anemia: Assessment, Prevention, and Control. World Health Organization: 1–130.
- 21. Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, et al. (2004) Nephropathy in diabetes. Diabetes Care 27 Suppl 1 S79–83. [DOI] [PubMed] [Google Scholar]
- 22. Galer BS, Gianas A, Jensen MP (2000) Painful diabetic polyneuropathy: epidemiology, pain description, and quality of life. Diabetes research and clinical practice 47: 123–128. [DOI] [PubMed] [Google Scholar]
- 23. Meijer JW, Smit AJ, Sonderen EV, Groothoff JW, Eisma WH, et al. (2002) Symptom scoring systems to diagnose distal polyneuropathy in diabetes: the Diabetic Neuropathy Symptom score. Diabet Med 19: 962–965. [DOI] [PubMed] [Google Scholar]
- 24. Adams JF, Clark JS, Ireland JT, Kesson CM, Watson WS (1983) Malabsorption of vitamin B12 and intrinsic factor secretion during biguanide therapy. Diabetologia 24: 16–18. [DOI] [PubMed] [Google Scholar]
- 25. Pflipsen MC, Oh RC, Saguil A, Seehusen DA, Seaquist D, et al. (2009) The prevalence of vitamin B(12) deficiency in patients with type 2 diabetes: a cross-sectional study. J Am Board Fam Med 22: 528–534. [DOI] [PubMed] [Google Scholar]
- 26. Reinstatler L, Qi YP, Williamson RS, Garn JV, Oakley GP Jr (2012) Association of biochemical B12 deficiency with metformin therapy and vitamin B12 supplements: the National Health and Nutrition Examination Survey, 1999–2006. Diabetes Care 35: 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sparre Hermann L, Nilsson B, Wettre S (2004) Vitamin B12 status of patients treated with metformin: a cross-sectional cohort study. The British Journal of Diabetes & Vascular Disease 4: 401–406. [Google Scholar]
- 28. Tomkin GH, Hadden DR, Weaver JA, Montgomery DA (1971) Vitamin-B12 status of patients on long-term metformin therapy. Br Med J 2: 685–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. DeFronzo RA, Goodman AM (1995) Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med 333: 541–549. [DOI] [PubMed] [Google Scholar]
- 30. Leishear K, Boudreau RM, Studenski SA, Ferrucci L, Rosano C, et al. (2012) Relationship between vitamin B12 and sensory and motor peripheral nerve function in older adults. J Am Geriatr Soc 60: 1057–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naha K, Dasari S, Vivek G, Prabhu M (2012) Vitamin B(12) deficiency: an unusual cause for recurrent generalised seizures with pancytopaenia. BMJ Case Rep 2012. [DOI] [PMC free article] [PubMed]
- 32. Hin H, Clarke R, Sherliker P, Atoyebi W, Emmens K, et al. (2006) Clinical relevance of low serum vitamin B12 concentrations in older people: the Banbury B12 study. Age Ageing 35: 416–422. [DOI] [PubMed] [Google Scholar]
- 33. Kibirige D, Mwebaze R (2013) Vitamin B12 deficiency among patients with diabetes mellitus: is routine screening and supplementation justified? J Diabetes Metab Disord 12: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carmel R (1988) Pernicious anemia. The expected findings of very low serum cobalamin levels, anemia, and macrocytosis are often lacking. Arch Intern Med 148: 1712–1714. [DOI] [PubMed] [Google Scholar]
- 35. Lindenbaum J, Healton EB, Savage DG, Brust JC, Garrett TJ, et al. (1988) Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med 318: 1720–1728. [DOI] [PubMed] [Google Scholar]
- 36. Stabler SP, Allen RH, Savage DG, Lindenbaum J (1990) Clinical spectrum and diagnosis of cobalamin deficiency. Blood 76: 871–881. [PubMed] [Google Scholar]
- 37. Wickramasinghe SN (2006) Diagnosis of megaloblastic anaemias. Blood Rev 20: 299–318. [DOI] [PubMed] [Google Scholar]
- 38. Chan CW, Liu SY, Kho CS, Lau KH, Liang YS, et al. (2007) Diagnostic clues to megaloblastic anaemia without macrocytosis. Int J Lab Hematol 29: 163–171. [DOI] [PubMed] [Google Scholar]
- 39. Wulffele MG, Kooy A, Lehert P, Bets D, Ogterop JC, et al. (2003) Effects of short-term treatment with metformin on serum concentrations of homocysteine, folate and vitamin B12 in type 2 diabetes mellitus: a randomized, placebo-controlled trial. J Intern Med 254: 455–463. [DOI] [PubMed] [Google Scholar]
- 40. Ting RZ, Szeto CC, Chan MH, Ma KK, Chow KM (2006) Risk factors of vitamin B12 deficiency in patients receiving metformin. Arch Intern Med 166: 1975–1979. [DOI] [PubMed] [Google Scholar]
- 41. Bauman WA, Shaw S, Jayatilleke E, Spungen AM, Herbert V (2000) Increased intake of calcium reverses vitamin B12 malabsorption induced by metformin. Diabetes Care 23: 1227–1231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.