Abstract
Background and Purpose
Social Isolation (SI) increases stroke incidence and delays post-stroke recovery. Women may be at greater risk from the negative consequences of SI, but few studies have examined both sexes in experimental models, and none have evaluated the effects of isolation initiated after stroke. The effects of post-stroke SI in males and females were examined and the role of mitochondrial P53 was evaluated.
Methods
C57Bl6 mice were pair-housed (male and ovariectomized female) for 2 weeks, subjected to stroke and then assigned to a housing condition (isolated or pair-housed (PH)). The effects of housing on infarct volume and recovery were examined. Changes in Bcl-2 and mitochondrial p53 were assessed by western blot. A mitochondrial p53 inhibitor (PFT-μ) was given to mice of both sexes.
Results
Compared to PH mice, post-stroke SI significantly increased infarct size in both sexes; SI mice also had worse neurological deficits. The detrimental effects of SI paralleled increases in mitochondrial p53 levels. Pharmacological inhibition of mitochondrial p53 using PFT-μ abolished the detrimental effects of SI and reduced cell death.
Conclusions
Post-stroke SI results in increased ischemic injury in both sexes. The effect of housing on infarct was more pronounced in females. Targeting the mitochondrial P53 pathway could minimize the detrimental effects of isolation after stroke.
Introduction
Social isolation enhances morbidity and mortality from a multitude of health conditions, including stroke1. It is increasingly recognized that sex differences exist in the etiology, presentation, management, and outcome from stroke2,3. Large gaps still remain in our understanding of the mechanism underlying these sex disparities2,4. Early reports suggested that women were less likely than men to receive appropriate care after an acute stroke, but these gender gaps are closing5. Despite equivalent care, women continue to have poorer functional outcomes compared to age matched males5. Importantly, women are 3.5 times more likely than men to be widowed and living alone at the time of their stroke and it this lack of social support and the higher prevalence of depression could be the important contributing factors to the poorer recovery in women2,4. Attesting to the importance of social factors on stroke outcome, studies have successfully modeled these detrimental effects in animals6–9. However most of these experimental studies have been performed exclusively in males that were isolated before stroke. No studies have examined the effects of post-stroke isolation in females.
The tumor protein p53, is known to be activated in response to stress and ischemic insults10–12. Recent studies have demonstrated that mitochondrial association of p53 is an important regulator of mitochondrial membrane pore opening, leading to the release of proapoptotic signaling molecules including cytochrome c and apoptosis-inducing factor (AIF)10,11,13 which are known to exhibit sex differences14 . We hypothesized that sex differences in SI enhanced stroke injury are mediated by mitochondrial p53.
Methods
Experimental Animals and Housing
All animal protocols were approved by the Institutional Animal Care and Use Committee at The University of Connecticut Health Center and were performed in accordance with NIH guidelines and STAIR criteria15. Female mice were ovariectomized (Ovx) as in6 ten days before pre-screening for baseline laterality deficits and locomotor activity. Mice were then pair housed (male with Ovx female) for 14 days before subjecting them to a 90 min right middle cerebral artery occlusion (MCAO) followed by reperfusion as described previously6,7. Mice were randomly assigned using SPSS software to either individual housing immediately after stroke or continued pair housing (PH) with their original partner. Animal numbers were calculated with the predicted mean based on prior studies performed in males and common SD assuming a 2-sided α-level of 0.05, power of 0.8, and homogeneous variances for the samples to be compared.
P53 inhibitor Treatment
Pifithrin-μ (PFT-μ) (Calbiochem, CA) was dissolved in dimethylsulfoxide (DMSO) and diluted to 4% in saline (2mg/kg) and a final volume of 100μl/10g body weight of drug or vehicle (4% DMSO in saline) was injected intraperitoneally to randomized mice at 3h, 24h & 48h after stroke. PFT-μ is a selective and specific mitochondrial inhibitor as determined previously in vivo and in vitro. Binding studies have shown that PFT-μ binds to p53. The dose used was determined by pilot experiments and from the literature10; a low dose was selected to avoid non-selective infarct reduction in all groups with a higher dose. This dose did not completely abolish p53 levels to sham levels but reduced it to levels comparable with PH stroke mice.
Neurological deficit Scores (NDS), Infarct analysis and survival rates
Following 72 hours of reperfusion, the NDS were recorded by a blinded investigator as in6,7. Infarcts were quantified from 2,3,5-triphenyltetrazolium chloride (TTC) stained coronal sections as detailed previously6,7. Mice that died during reperfusion were included in mortality rates, and if paired, the partner was not used for analysis.
Open Field analysis
Spontaneous locomotor activity was performed during the light phase of the circadian cycle, between 9:00 am and 12:00 pm under normal fluorescent room lights. For testing, mice were acclimatized to the room conditions and were individually placed in the open field chamber (15” × 15”) equipped with 16 infrared beam emitting LEDs on each side for a duration of 20 minutes. The total number of beam breaks was automatically collected by a computer-operated PAS Open Field system (San Diego Instruments, San Diego, CA)6. The open field chambers were cleaned after each individual test session using 70 % ethanol. All animals were assessed by a blinded investigator.
Western Blots
An additional cohort of mice was euthanized at twenty-four hours after stroke for protein analysis. Brains were homogenized and a portion of lysate was used for whole cell lysate analysis. The mitochondrial fraction was obtained as described previously6. Protein levels were assessed for mitochondrial P53 (1:200; abcam) and Bcl-2(1:500; Cell Signaling) using actin and COX IV (1:2000; abcam) as loading controls. Densitometry was performed with ImageJ software.
Statistics
Data are presented as mean±SEM. except for NDS, which was presented as median (interquartile range). IBM SPSS Ver.20 was used to perform Student’s t-test or ANOVA with Tukey post-hoc correction to determine significance and interactions. P<0.05 was considered statistically significant. Investigators performing infarct size analysis and NDS were blinded to treatment conditions.
Results
Infarct Analysis
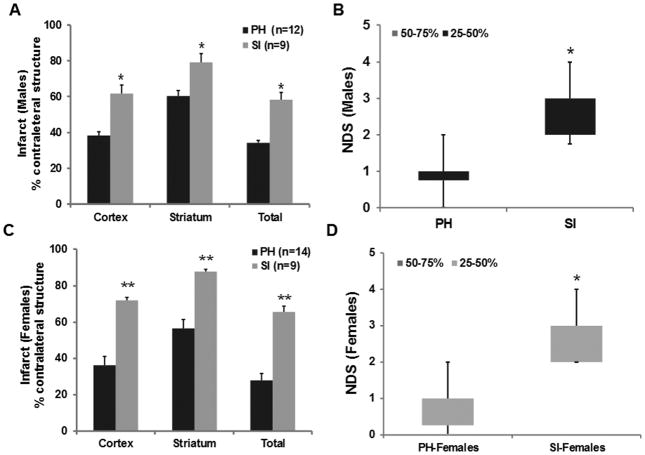
Post-stroke SI significantly exacerbated infarct size compared to pair housed (PH) animals both in males (Figure 1A; P<0.05; t-test) and females, (Figure 1B; P<0.001; t-test). Equivalent blood flow reduction was seen by laser Doppler in both sexes and in both housing conditions. A significant three-way interaction between social isolation, infarct size and sex was also found F(1,44)=4.2, p<0.05, suggesting that the detrimental effect of housing on infarct is more pronounced in ovariectomized females.
Figure 1.
SI mice had significantly increased Infarct size after stroke in males (A) and in females (C). Values are Mean±SEM. At 72h after stroke NDS were higher in SI mice suggesting poorer recovery compared to PH mice in males (B) and in females (D), data presented as box-and-whisker plot. *P<0.05, **P<0.01 indicates statistically significant differences.
NDS and Mortality
The detrimental effect of SI was also reflected in the NDS. SI males had significant worsening of their NDS (Figure 1C; P<0.05); as did females (Figure 1D; P<0.05) compared to PH. A higher mortality was also seen in SI male (29%) and female (36%) mice compared to PH male (7%) and female (0%) mice.
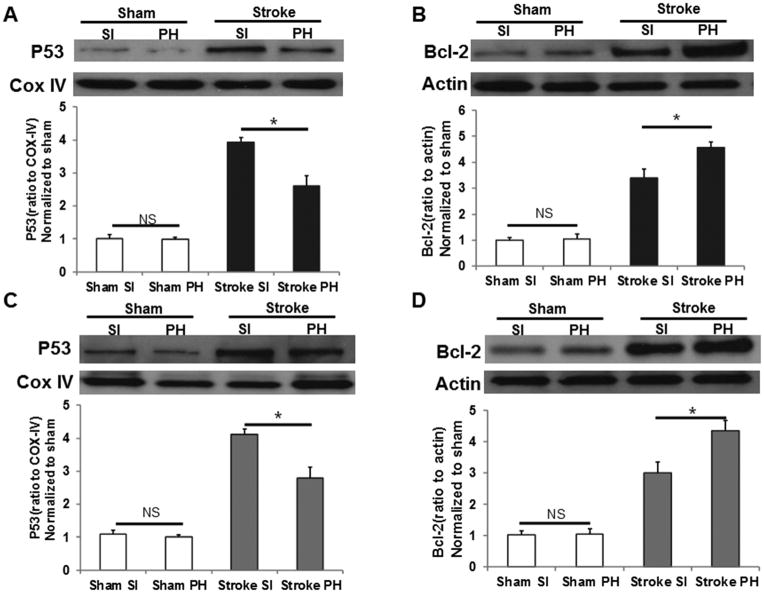
Protein analysis
ANOVA for mitochondrial P53 protein levels revealed a significant effect of stroke in males, (Figure 2B; p<0.05) and females (Figure 2D) both by stroke p<0.05, and by housing p<0.05. Bcl-2 levels were significantly elevated after stroke in both housing conditions, but this increase was less in SI mice (Figure 2B&2D; p<0.05); and in SI females, p<0.05, compared to PH mice. Moreover, a significant stroke X housing interaction was seen in both sexes (p<0.05), suggesting that stroke-induced increases in p53 and Bcl-2 expression was significantly altered in SI mice vs. PH mice.
Figure 2.
Western blot images and quantitative analysis showing increased mitochondrial p53 levels 24h after stroke (COX-IV as loading control). The increase is significantly higher in both males (A) and females (C) after stroke and in SI mice compared to stroke PH mice (n=6/grp). No significant differences were seen in sham groups (n=4/grp). Bcl-2 levels are significantly lower after stroke in SI group compared to PH group (n=6/grp) both in males (B) and females (D). *indicates P<0.05.
Pifithrin-μ treatment reverses SI effects
PFT-μ (2mg/kg) abolished the detrimental effects of SI on infarct (Figure 3A) but had no neuroprotective effect in PH mice. Two-way ANOVA yielded a significant effect of housing, F(1,42)=17.1, p<0.05, and a significant effect of drug, F(1,42)=18.7, p<0.05, and a significant interaction between housing and drug, F(1,42)=10.9, p=0.002 in males(Figure 3A) and also in females(Figure 3B). The beneficial effect of P53 inhibition was also reflected in the restoration of spontaneous locomotor activity after stroke in SI males. Two-way ANOVA yielded a significant effect of housing, F(1,42)=4.49, p<0.05, and a significant effect of drug, F(1,42)=4.38, p<0.05, and a significant interaction between housing and drug, F(1,42)=5.88, p<0.05. These findings indicate that drug abolished the detrimental effects of isolation.
Figure 3.
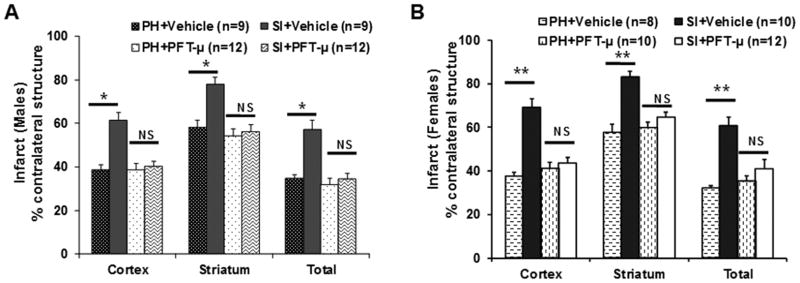
Mice were housed SI or PH immediately after a 90 min MCAO and treated with either vehicle or PFT-μ (2mg/kg) at 3h, 24h and 48h after surgery. Infarct analysis at 72h after stroke demonstrates a significant protective effect of drug in the SI cohort but drug has no significant effect in PH cohort, in males (A) and in females (B). Values are presented as Mean±SEM; *p<0.05, **p<0.001.
Discussion
In this study, we found that post-stroke SI significantly worsens stroke damage in both sexes, and this effect can be abolished by inhibition of mitochondrial P53 activation. SI prior to stroke worsens outcomes in experimental models, as well as in clinical populations, in both sexes2,6,8,9. However, much less is known regarding the effects of SI initiated after injury. As most patients who are isolated do not come to medical attention until after the injury occurs, the ability to manipulate post-stroke housing environments has broader translational significance to functional recovery. Stroke is a sexually dimorphic disease. Several molecular pathways have been identified as important contributors to cell death after ischemic injury and these pathways, although not completely distinct, are differentially regulated by sex. Females are more sensitive to caspase-dependent cell death, whereas caspase-independent or poly[ADP-ribose] polymerase-1 (PARP-1) mediated cell death predominates in males4,14. Both of these cell death signaling pathways converge on mitochondrial permeability transition pore dysfunction10,11. This work suggests that mitochondrial stress underlies some of the detrimental effects of SI. Other mechanisms may also contribute such enhancement of inflammation or late effects on cerebral blood flow.
In this work we found that SI significantly increased cell death after ischemic stroke in both sexes, although females were more susceptible to the detrimental effects of isolation. Although statistically significant, this interaction between isolation, infarct, and sex requires further study to determine its physiological relevance. Both sexes responded to a p53 inhibitor; with a reversal in isolation-induced brain injury. Future studies are needed to specifically examine post stroke depressive phenotypes and cognitive deficits after SI in both sexes, using chronic endpoints and aged animals. As these deficits often lead to nursing home placement in stroke survivors, especially elderly women, the impact of social factors on stroke recovery deserves investigation.
Conclusion
Our findings suggest that mitochondrial association of p53 is an important underlying mechanism for SI enhanced ischemic injury in both sexes. These findings demonstrate that post-stroke SI has detrimental effects on ischemic injury. Consistent with clinical studies, females appear to have a greater susceptibility to the negative effects of isolation.
Acknowledgments
Sources of Funding: This work was supported by National Institutes of Health R01 NS050505 and NS055215 (to L.D.M.) and American Heart Association grant 11POST7430045 (to V.R.V).
Footnotes
Disclosures:
None
References
- 1.Steptoe A, Shankar A, Demakakos P, Wardle J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci USA. 2013;110:5797–5801. doi: 10.1073/pnas.1219686110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kapral MK, Fang J, Hill MD, Silver F, Richards J, Jaigobin C, et al. Sex differences in st7roke care and outcomes: Results from the registry of the canadian stroke network. Stroke. 2005;36:809–814. doi: 10.1161/01.STR.0000157662.09551.e5. [DOI] [PubMed] [Google Scholar]
- 3.Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, et al. Guidelines for the prevention of stroke in women: A statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2014;45:1545–1588. doi: 10.1161/01.str.0000442009.06663.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turtzo LC, McCullough LD. Sex differences in stroke. Cerebrovascular diseases. 2008;26:462–474. doi: 10.1159/000155983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gattringer T, Ferrari J, Knoflach M, Seyfang L, Horner S, Niederkorn K, et al. Sex-related differences of acute stroke unit care: Results from the austrian stroke unit registry. Stroke. 2014;45:1632–8. doi: 10.1161/STROKEAHA.114.004897. [DOI] [PubMed] [Google Scholar]
- 6.Venna VR, Weston G, Benashski SE, Tarabishy S, Liu F, Li J, et al. Nf-kappab contributes to the detrimental effects of social isolation after experimental stroke. Acta neuropathologica. 2012;124:425–438. doi: 10.1007/s00401-012-0990-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venna VR, Xu Y, Doran SJ, Patrizz A, McCullough LD. Social interaction plays a critical role in neurogenesis and recovery after stroke. Translational psychiatry. 2014;4:e351. doi: 10.1038/tp.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karelina K, Norman GJ, Zhang N, Morris JS, Peng H, DeVries AC. Social isolation alters neuroinflammatory response to stroke. Proc Natl Acad Sci USA. 2009;106:5895–5900. doi: 10.1073/pnas.0810737106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stuller KA, Jarrett B, DeVries AC. Stress and social isolation increase vulnerability to stroke. Exp Neurol. 2012;233:33–39. doi: 10.1016/j.expneurol.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Nijboer CH, Heijnen CJ, van der Kooij MA, Zijlstra J, van Velthoven CT, Culmsee C, et al. Targeting the p53 pathway to protect the neonatal ischemic brain. Annals of neurology. 2011;70:255–264. doi: 10.1002/ana.22413. [DOI] [PubMed] [Google Scholar]
- 11.Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM. P53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell. 2012;149:1536–1548. doi: 10.1016/j.cell.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elkholi R, Chipuk JE. How do i kill thee? Let me count the ways: P53 regulates parp-1 dependent necrosis. BioEssays. 2014;36:46–51. doi: 10.1002/bies.201300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Speidel D. Transcription-independent p53 apoptosis: An alternative route to death. Trends in cell biology. 2010;20:14–24. doi: 10.1016/j.tcb.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 14.McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (adp-ribose) polymerase-1 in cerebral ischemia: Male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- 15.Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]