Abstract
In insects, the precise timing of moulting and metamorphosis is strictly guided by ecdysteroids that are synthesised from dietary cholesterol in the prothoracic gland (PG). In the past decade, several ecdysteroidogenic enzymes, some of which are encoded by the Halloween genes, have been identified and characterised. Here, we report a novel Halloween gene, noppera-bo (nobo), that encodes a member of the glutathione S-transferase family. nobo was identified as a gene that is predominantly expressed in the PG of the fruit fly Drosophila melanogaster. We generated a nobo knock-out mutant, which displayed embryonic lethality and a naked cuticle structure. These phenotypes are typical for Halloween mutants showing embryonic ecdysteroid deficiency. In addition, the PG-specific nobo knock-down larvae displayed an arrested phenotype and reduced 20-hydroxyecdysone (20E) titres. Importantly, both embryonic and larval phenotypes were rescued by the administration of 20E or cholesterol. We also confirm that PG cells in nobo loss-of-function larvae abnormally accumulate cholesterol. Considering that cholesterol is the most upstream material for ecdysteroid biosynthesis in the PG, our results raise the possibility that nobo plays a crucial role in regulating the behaviour of cholesterol in steroid biosynthesis in insects.
Ecdysteroids, including ecdysone and its derivative 20-hydroxyecdysone (20E), are the principal insect steroid hormones that play pivotal roles in controlling a number of developmental and physiological events, especially moulting and metamorphosis1,2. During postembryonic development, ecdysone is synthesised from dietary sterols, such as cholesterol, via a series of hydroxylation and oxidation steps in an endocrine organ called the prothoracic gland (PG)3,4,5. Ecdysone is secreted in the haemolymph and subsequently converted to the more biologically active hormone 20E in peripheral tissues3.
In the past 15 years, a number of ecdysteroidogenic enzymes have been successfully identified and characterised, especially in the fruit fly, Drosophila melanogaster (See ref. 5 and references therein). Neverland (Nvd) is the [2Fe-2S] Rieske oxygenase and mediates the first step of the ecdysteroid biosynthesis pathway, which is the 7,8-dehydrogenation of cholesterol to 7-dehydrocholesterol (7dC). 7dC is then converted to 5β-ketodiol through the ‘Black Box'. The ‘Black Box' has not yet been biochemically characterised but is thought to be catalysed by at least 3 enzymes, including the cytochrome P450 monooxygenases Spook (Spo), CYP6T3 and the short-chain dehydrogenase/reductase Shroud (Sro). The conversion of 5β-ketodiol to 20E is catalysed by a series of cytochrome P450s: i.e., Phantom (Phm), Disembodied (Dib), Shadow (Sad) and Shade (Shd). In particular, spo, sro, phm, dib, sad and shd are known as Halloween genes, whose complete loss-of-function mutations result in typical embryonic cuticular differentiation defects5,6.
Currently, however, it has not been proved that these identified ecdysteroidogenic enzymes are sufficient to synthesise 20E from dietary sterols. Therefore, it is still possible that uncharacterised enzymes play an essential role in ecdysteroid biosynthesis by catalysing a known or unknown chemical reaction. To uncover an unidentified gene responsible for ecdysteroid biosynthesis, we have been conducting gene expression analyses to identify and characterise genes that are highly expressed in the PG7,8,9,10,11,12,13. Here, we report an additional gene responsible for ecdysteroid biosynthesis in D. melanogaster, designated as noppera-bo (nobo), that encodes a glutathione S-transferase (GST). nobo and its orthologues are conserved in Diptera and Lepidoptera. nobo loss-of-function animals display a number of typical phenotypes caused by a low ecdysteroid titre. Finally, we show that loss-of-function of nobo causes abnormal accumulation of cholesterol in PG cells. We propose that nobo GST proteins are novel, indispensable regulators of ecdysteroid biosynthesis.
Results
The expression of the GST gene CG4688 (noppera-bo) strongly correlates with ecdysteroid biosynthesis
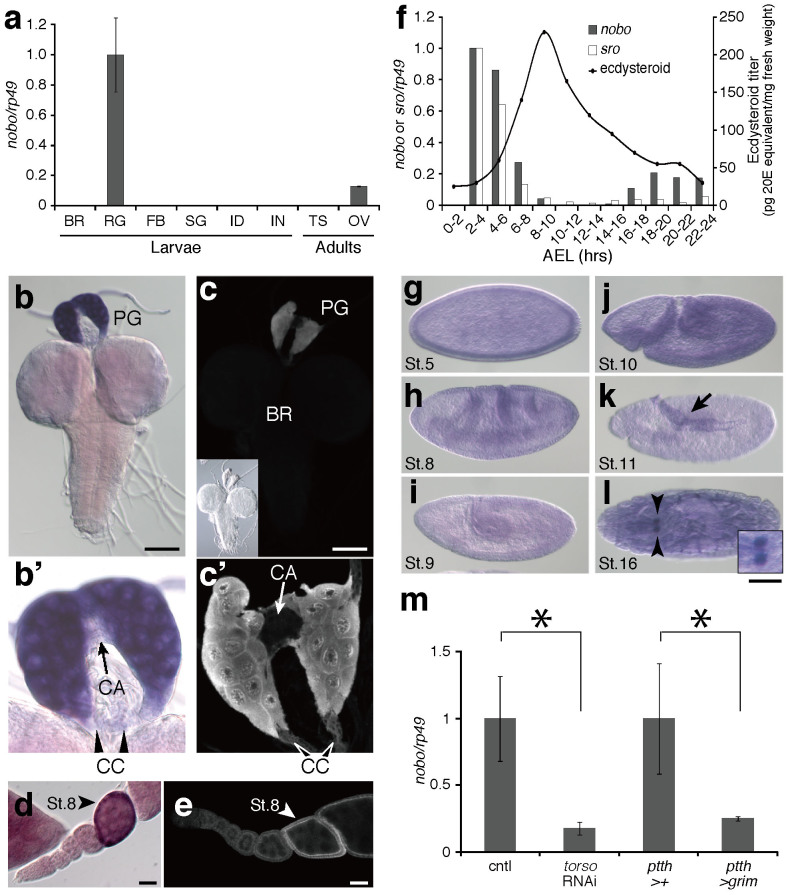
In the course of D. melanogaster microarray analysis to screen for genes predominantly expressed in the ring gland (RG), a composite endocrine organ containing the PG cells (T.S., unpublished data), we focused on a gene called CG4688, also known as GSTe1414. A quantitative reverse-transcription PCR analysis revealed that the spatial expression of CG4688 was restricted to the ring gland (RG), a composite endocrine organ containing the PG cells, during larval development (Fig. 1a). CG4688 expression was also weakly detected in the adult ovary that is known to synthesise ecdysone (Fig. 1a). In situ hybridisation and immunohistochemical analyses also confirmed that CG4688 was predominantly expressed in the PG cells of the third (final) instar larval stages (Fig. 1b,c), as well as in ovarian follicle cells (Fig. 1d,e). The RG contains not only the PG but also other endocrine organs, the corpus allatum (CA) and the corpus cardiacum (CC). The CG4688 transcript was exclusively observed in the PG but not in the CA or CC (Fig. 1b',c').
Figure 1. Expression pattern of nobo.
(a) The expression levels of nobo in several tissues were quantified by qRT-PCR. Total RNA samples were prepared from wandering third instar larvae and adult flies. BR, brain; RG, ring gland; FB, fat body; SG, salivary gland; ID, imaginal discs; IN, intestine; TS, adult testis; OV, adult ovary. Each error bar represents the s.e.m. from three independent samples. The normalised nobo mRNA level in the RG is set as 1. (b-e) Localisation of nobo mRNA and Nobo protein in the PG and the adult ovaries. (b, b') In situ signal with the nobo antisense RNA probe and (c, c') anti-Nobo immunoreactivity were detected in PG cells but not in other regions in the brain-ring gland complex of wandering third instar larvae. An inset of (c) shows a bright-field image of the same specimen. (b') and (c') show higher magnifications of the ring gland in (b) and (c), respectively. The arrow and arrowheads indicate the corpus allatum (CA) and the corpus cardiacum (CC), respectively. (d) nobo mRNA and (e) Nobo protein were strongly detected in the follicle cells of stage 8 (St.8) ovarioles in developing egg chambers. Scale bars: (b, c) 100 µm, (d, e) 25 µm. (f) The temporal expression profile of nobo during embryogenesis. A black line and white bars are schematic representations of the embryonic ecdysteroid titre and sro expression levels, respectively, based on published data15,16. The normalised nobo and sro mRNA levels at 2–4 hours AEL are set as 1. (g–l) in situ RNA hybridisation analysis in embryos with nobo antisense probe. Also see Supplementary fig. S1 for additional data. Lateral (g–k) and ventral (l) views are shown. (k) At stage 11 (St.11), the nobo signal was detected in the amnioserosa (arrow), which is thought to synthesise ecdysteroids. (l) At stage 16 (St.16), the nobo signal was detected in the PG cells (arrowheads). At this stage, a background staining in the epidermis was observed (Also see Supplementary fig. 1g). An inset with a higher magnification of the PG. Scale bar: 100 µm. (m) qRT-PCR analysis of the nobo transcripts in the PGs isolated from 140 ± 6 hour AEL third instar larvae of control, torso RNAi and ptth-expression neuron-ablated (ptth>grim) animals. Each error bar represents the s.e.m. from three independent samples. The nobo expression levels in each control were set as 1. *, P<0.05 with Student's t-test.
In D. melanogaster, the early embryonic expression of the Halloween genes is essential for ecdysteroid biosynthesis prior to the formation of the PG5,6. The embryonic expression pattern of CG4688 correlated well with a change in the embryonic ecdysteroid titre15. While no or little maternal CG4688 mRNA was detected, the maximum level of embryonic CG4688 expression was observed at 2–6 hours after egg laying (AEL) (Fig. 1f), which roughly corresponds to embryonic stages 5–10 (Fig. 1g–j; also see Supplementary fig. S1 for sense probe). CG4688 expression decreased in later embryogenesis (Fig. 1f). It should also be noted that the temporal expression fluctuation of CG4688 is very similar to that of shroud (Fig. 1f), which was described previously16. We also conducted in situ RNA hybridisation analysis to examine nobo expression during embryogenesis. nobo was expressed in the blastoderm embryo with a ventral bias (Fig. 1g and Supplementary fig. S1a). As cellularisation proceeded, nobo expression was observed almost ubiquitously in the epidermal cells (Fig. 1h–j and Supplementary fig. S1b–d). In the germband elongation stage, nobo expression was up-regulated in the amnioserosa (Fig. 1k and Supplementary fig. S1e). At stage 16 and later, nobo expression was detected in the PG cells (Fig. 1l). This expression pattern is reminiscent of some Halloween genes, such as phm and spo7,17,18.
Previous studies have shown that expressions of most ecdysteroidogenic enzyme genes in the PG are positively control by prothoracicotropic hormone (PTTH) and its receptor Torso9,16,19,20,21. We found that CG4688 expression was also significantly reduced in the third instar larvae of ptth neuron-ablated and torso RNA interference (RNAi) animals (Fig. 1m). All of these results demonstrate that CG4688 expression is strongly correlated with ecdysteroid biosynthesis. Hereafter, we refer to CG4688 as noppera-bo (nobo) for a reason explained later.
Noppera-bo is a Halloween gene
Next, we assessed the functional importance of nobo in vivo. Previous studies have shown that six ecdysteroidogenic enzyme genes, spo, sro, phm, dib, sad and shd, belong to the Halloween group of mutants, which were originally identified in Nusslein-Volhard and Wieschaus' large saturated mutant screen and characterised by embryonic lethality and by similar cuticular patterning5,6,22,23. While nobo is located at the 49F12 cytological position of the 2nd chromosome in D. melanogaster, there was no record of any typical Halloween mutants mapped in the vicinity of nobo in the previous screen22.
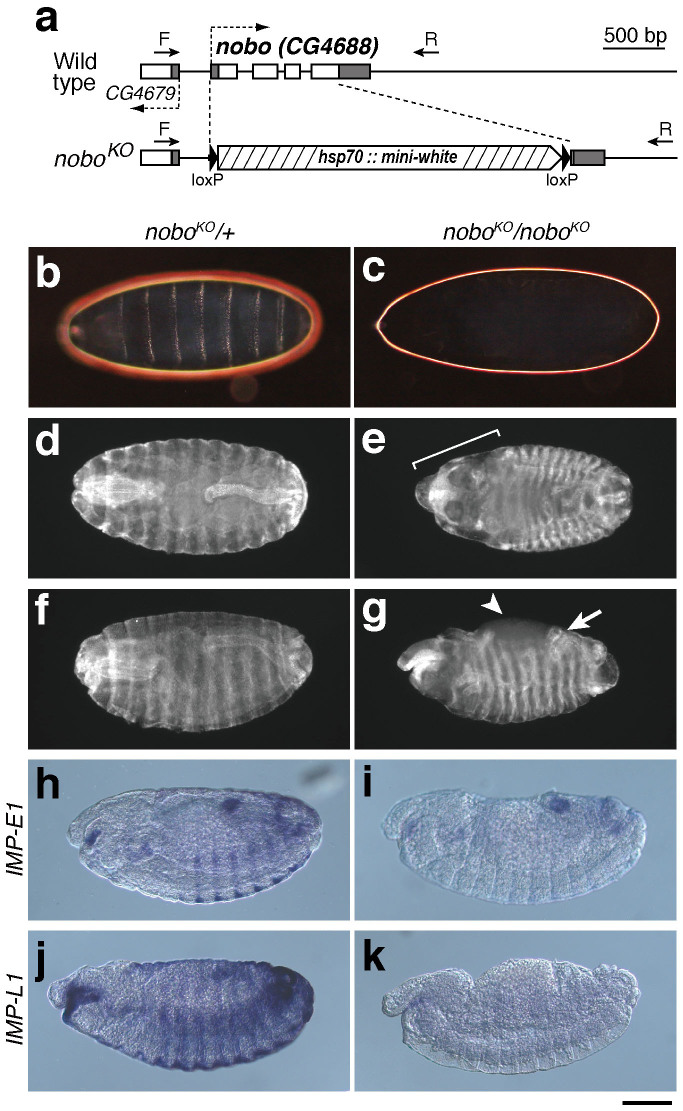
Thus, we created a null allele of nobo by a conventional knock-out technique24,25. In our nobo knock-out allele (noboKO), an almost entire coding sequence region of nobo was replaced with a mini-white gene targeting cassette (Fig. 2a). Homozygotes of noboKO were embryonic lethal and, like other Halloween mutants, did not produce a differentiated cuticle structure (Fig. 2b,c). We also found that noboKO homozygotes displayed abnormal morphogenetic movements that involve the failure of head involution, defects in dorsal closure and abnormal gut looping (Fig. 2d–g). Moreover, the epidermal expression of both IMP-E1 and IMP-L1, which are ecdysteroid-inducible genes, was greatly reduced or absent in noboKO mutants (Fig. 2h–k). These phenotypic characteristics are very similar to the feature of Halloween mutants6,23. We also confirmed that the lethality and phenotype of noboKO mutants were solely due to the loss of nobo function, as shown by the fact that the lethality was rescued by nobo transgene expression driven by phm-GAL4#22 (Table 1).
Figure 2. Generation and phenotypic analysis of noboKO homozygous mutant embryos.
(a) Genomic and exon-intron structures of the nobo (CG4688) loci of the wild-type and nobo knock-out (noboKO) strains. White and black boxes indicate the coding sequence and the untranslated regions, respectively. CG4649 is a gene located next to nobo. Dashed arrows indicate orientations of the genes. In the noboKO allele, 1,033 bp of the nobo gene region was replaced with a gene-targeting construct including the hsp70::mini-white marker cassette with loxP sites, resulting in a 680 bp deletion in the entire (696 bp) nobo coding sequence. Arrows ‘F' and ‘R' indicate the positions of the genotyping primers used in Supplementary fig. 2 (also see Methods and Supplementary table S3). (b,c) Dark-field images of embryonic cuticles from (b) noboKO heterozygous (noboKO/+) and (c) homozygous (noboKO/noboKO) embryos. (d–g) Anti-FasIII antibody staining to visualise overall embryo morphology. (d,f) noboKO/+ embryos. (e, g) noboKO/noboKO embryos. (e) The bracket indicates defective head involution. (g) The arrow and the arrowhead indicate the dorsal open phenotype and abnormal gut looping, respectively. (h–k) Expression patterns of (h, i) IMP-E1 and (j, k) IMP-L1 in stage 14 embryos. (h, j) noboKO/+ embryos. (i, k) noboKO/noboKO embryos. These data show that the nobo mutant exhibited severe reductions of these 20E-inducible genes. Scale bars: 100 µm for all images.
Table 1. Viability of noboKO and nobo RNAi animals with the expression of nobo and other GST genes. The number of viable noboKO/noboKO and nobo RNAi adults was scored. Transgenes were driven by phm-GAL4#22 driver. Details of genetic crosses for this experiment are described in Supplemental table 1. Values in parentheses indicate the number of viable control non-noboKO homozygous or non-RNAi progeny from the parental strains in the same experimental batches (see Supplemental table S1).
| Background | Transgene | Number of adults |
|---|---|---|
| noboKO | nobo#1 | 101 (220) |
| nobo#2 | 124 (255) | |
| nobo#3 | 46 (102) | |
| nobo-Bm#1 | 85 (377) | |
| nobo-Bm#5 | 77 (348) | |
| CG6673B | 0 (105) | |
| GSTe4 | 0 (102) | |
| GSTe12 | 0 (117) | |
| nobo RNAi | nobo-Bm#1 | 120 (498) |
| nobo-Bm#2 | 105 (350) | |
| GSTe4 | 0 (92) | |
| GSTe12 | 0 (105) | |
| Sepia | 0 (249) | |
| CG6673A | 0 (120) | |
| CG6673B | 0 (278) | |
| CG6662 | 0 (107) |
We next determined whether the lethality of the noboKO mutant was rescued by delivering 20E midway through embryogenesis. With control ethanol treatment, no noboKO homozygous embryos developed into first instar larvae (Table 2). By contrast, with 100 μM 20E application, some noboKO mutant embryos hatched into first instar larvae (Table 2), whereas the rescued animals died at the first instar larvae and did not grow into the second instar stage on a normal diet (100%; N = 46). These results suggest that nobo is required for embryonic ecdysteroid biosynthesis and is indeed classified as a Halloween gene. On the basis of the Halloween-class naked cuticle phenotype, we named CG4688 ‘noppera-bo' after a legendary Japanese faceless ghost.
Table 2. Rescue of homozygous noboKO embryos by incubation with 20E. The numbers of the first instar larvae are indicated. Embryos of the indicated genotype were incubated with or without 100 µM 20E. Genotypes were assessed by the presence of GFP signal.
| Number of the 1st instar larvae | ||
|---|---|---|
| Steroid | noboKO/noboKO | noboKO/CyO Act-GFP |
| None | 0 | 96 |
| 20E | 64 | 145 |
Noppera-bo is also crucial for ecdysteroid biosynthesis during larval development
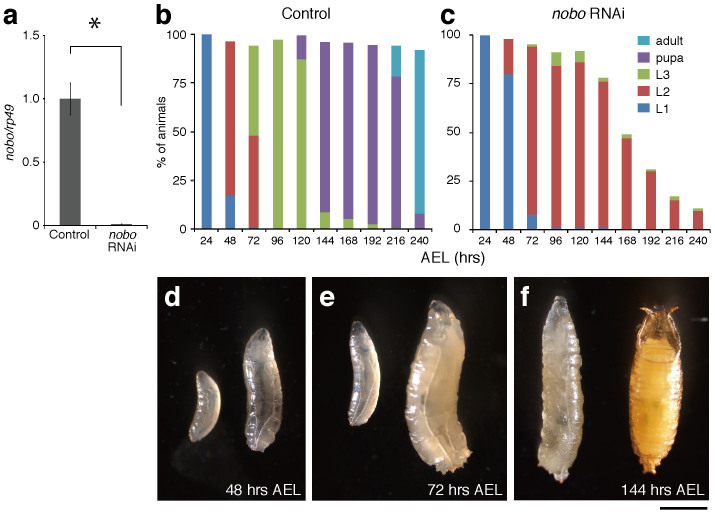
To investigate the importance of nobo during D. melanogaster larval development, we examined the phenotypes of either the overexpression or the knockdown of the nobo gene in the PG using the GAL4/UAS system. In a wild-type background, the overexpression of nobo using any GAL4 lines that we tested had no visible effect on development (data not shown). To knock down nobo, we performed transgenic RNAi experiments26 using a transgenic line carrying an inverted repeat construct corresponding to the nobo mRNA (nobo-IR) under the control of the UAS promoter. When the UAS-nobo-IR strains were crossed with the PG-specific driver phm-GAL4, the RNAi animals caused larval lethality. The larval lethality was observed with two independent UAS-nobo-IR constructs (see Methods), each of which targeted a different region of the nobo mRNA (data not shown). Hereafter, we refer to the animals in which the nobo RNAi was driven by phm-GAL4#22 with UAS-nobo-IR#40316 (see Methods) as ‘nobo RNAi animals' for simplicity.
The lethal phase for nobo RNAi animals was examined in more detail. In our experimental conditions, nobo RNAi animals exhibited a ~1% reduction in the level of the nobo mRNA at the first instar stage (Fig. 3a). nobo RNAi animals completed embryogenesis, hatched normally, and most nobo RNAi animals showed no apparent morphological or behavioural defects until 24 hours after egg laying (AEL), i.e., until the first instar larval stage (Fig. 3b,c). However, at ~48 hours AEL, nobo RNAi animals showed apparent growth arrest compared with control animals (Fig. 3b,d). At 72 hours AEL, while about half of control animals grew to the third instar larval stage (Fig. 3b,e), almost all nobo RNAi animals were the second instar larvae (Fig. 3c,e). At 144 hours AEL, the majority of control animals became pupae (Fig. 3b,f), but nobo RNAi animals were still at the second instar stage and gradually died (Fig. 3c,f). At 240 hours AEL, almost all control animals became adults (Fig. 3b). At this time point, a majority of nobo RNAi animals had already died, and the few larvae that were still alive were arrested at the second instar larval stage (Fig. 3c).
Figure 3. Larval lethality and developmental arrest phenotype of nobo RNAi larvae.
(a) Expression levels of control and nobo RNAi first instar larvae collected at 36 hours AEL. Each error bar represents the s.e.m. from three independent samples. The normalised nobo expression level in the control was set as 1. *, P<0.05 with Student's t-test. (b,c) The survival rate and developmental progression of (b) control (N = 150) and (c) nobo RNAi animals (N = 100). L1, L2 and L3 indicate the first, second and third instar larvae, respectively. (d–f) Comparison of body size and developmental stage between control (right) and nobo RNAi (left) animals. Typically, control animals became the second instar larva, the third instar larva and pupa at (d) 48 hours (hrs), (e) 72 hours and (f) 144 hours AEL, respectively. In contrast, nobo RNAi animals in these photos are the first, second and second instar larvae, which were collected at each of the same time points, respectively. Scale bar: 1 mm.
We also confirmed that the larval lethality and larval arrest phenotype of nobo RNAi animals was due to the loss of ecdysteroids. We first examined ecdysteroid titre in the second instar larvae (60 hours AEL) of control and nobo RNAi animals by mass-spectrometric analysis. In the control larvae, we detected 1.55 ± 0.27 pg of 20 E/mg of wet weight (mean ± s.e.m., N = 5). In contrast, ecdysteroid titre in nobo RNAi animals (N = 5) was below the quantifiable limit in the same experimental conditions (see Methods), suggesting that nobo RNAi impairs ecdysteroid biosynthesis during larval stages. Moreover, we also found that nobo RNAi animals fed yeast paste containing 20E just after hatching grew to the later larval, pupal and even adult stages (Fig. 4a, inset). These results demonstrate that, in addition to embryogenesis, nobo is also crucial for larval development via the regulation of ecdysteroid biosynthesis.
Figure 4. Feeding rescue experiments and abnormal cholesterol accumulation in nobo loss-of-function animals.
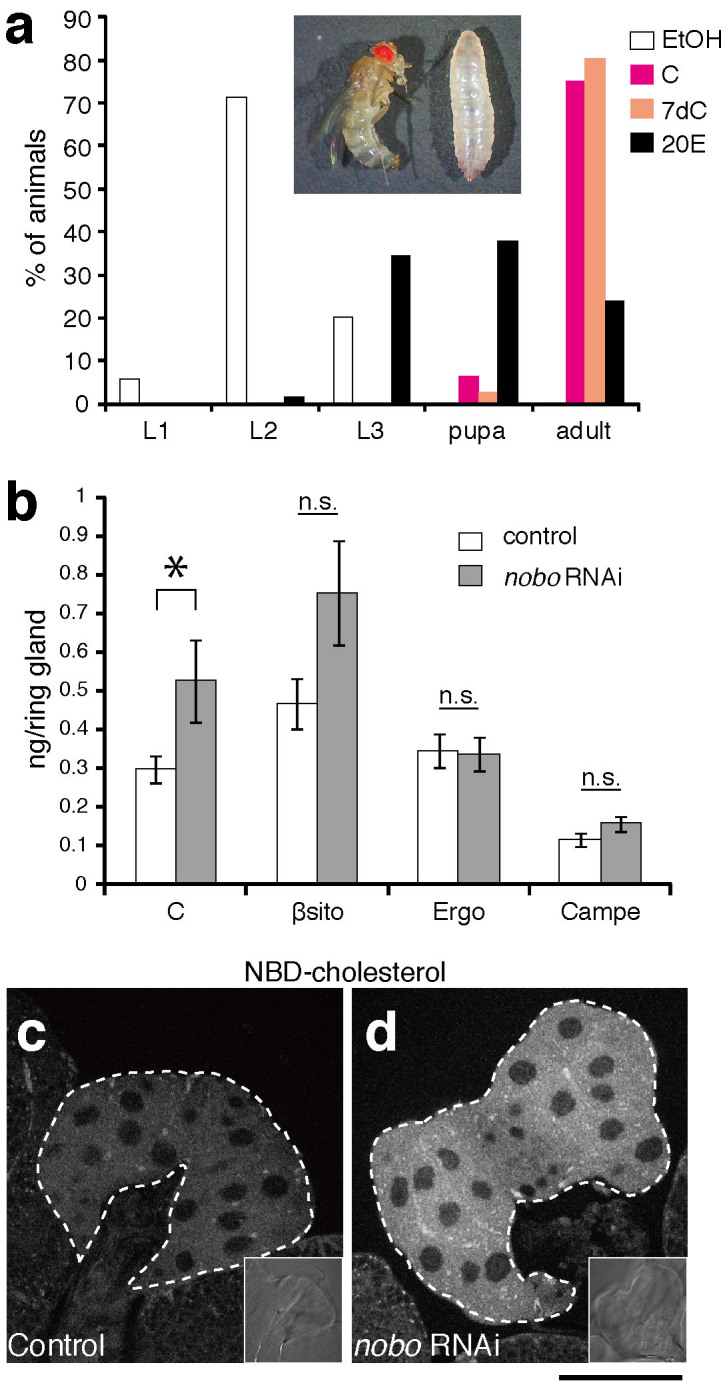
(a) Feeding rescue experiments for nobo RNAi larvae. nobo RNAi and control larvae were fed yeast paste supplemented with ethanol (EtOH, for negative control), cholesterol (C), 7-dehydrocholesterol (7dC) or 20E throughout their larval development. The concentration of each supplemented steroid in yeast paste was 0.5%(w/w) (See Methods for more details). The percentage of living animals at 240 hours AEL in each experimental condition was scored. N>30 for each experiments. Inset photo shows nobo RNAi animals at 240 hours AEL, which were raised on food with EtOH- (right) and 20E-supplemented (left) food, respectively. The larva (right) was at the 2nd instar stage. (b) Sterol amounts in the RG isolated from control and nobo RNAi larvae. C, cholesterol; βsito, β-sitosterol; Ergo, ergosterol; Campe, campesterol. 7dC amounts were under the detectable level in our experimental conditions, and thus, the 7dC data were not included in this graph. N = 10 for each genotype. *, P<0.05 with Student's t-test. n.s., not significant. Note that the higher level of β-sitosterol was observed in nobo RNAi PG cells, but the difference was not statistically significant. (c,d) Fluorescence and bright-field (inset) images of the PG from (c) control and (d) nobo RNAi animals incubated with 22-NBD-cholesterol. White dotted lines indicate the PG area. Scale bar: 50 µm.
Administration of cholesterol rescues noppera-bo loss-of-function phenotypes
To determine which step of ecdysteroid biosynthesis is affected by the loss of nobo function, we performed a feeding experiment with various precursors of 20E. If Nobo is involved in a certain ecdysteroid biosynthesis step, we expected that the larval arrest phenotype of the loss of nobo function animals would be rescued by an exogenous administration of intermediate(s) downstream of the biosynthesis step. We have applied the same logic to confirm the conversion steps of some ecdysteroidogenic enzymes, such as Sro16 and Nvd11,27. Intriguingly, we found that noboKO mutants were almost completely rescued and reached the first instar stage when their mothers were fed yeast paste supplemented with 0.5%(w/w) cholesterol or 7dC (Table 3). We confirmed the homozygosity of the rescued noboKO first instar larvae by PCR genotyping (Supplementary fig. S2). Similarly, the nobo RNAi larvae on 0.5%(w/w) cholesterol- or 7dC-supplemented food moulted normally and grew into the adult stage (Fig. 4a). Considering the conventional view that cholesterol is the most upstream precursor of ecdysteroids in the PG3,28, these results suggest that Nobo may not convert identified ecdysteroid intermediates but may rather play a role in transport and/or metabolism in the PG.
Table 3. Rescue of homozygous noboKO embryos by maternal sterol administration. The numbers of the first instar larvae that were offspring of noboKO/CyO Act-GFP mothers, which were fed standard cornmeal food with yeast pastes containing 0.5%(w/w) each sterol, are indicated. Genotype was assessed by the presence of a GFP signal and genomic PCR results (See Supplementary fig. S2).
| Number of first instar larvae | ||
|---|---|---|
| Sterol | noboKO/noboKO | noboKO/CyO Act-GFP |
| None | 0 | 44 |
| C | 79 | 129 |
| 7dC | 63 | 118 |
We also examined the developmental phenotype of the homozygous noboKO first instar larvae, which were derived by the maternal application of cholesterol described above. On a normal diet, all of the rescued noboKO homozygous larvae exhibited a developmental arrest phenotype at the first or second instar larval stage, and none of them reached the third instar larval stage (Table 4). This larval arrest phenotype was due to ecdysteroid deficiency because the rescued noboKO homozygous first instar larvae grew to the third instar larval stage on a diet supplemented with 20E (Table 4). In contrast to the RNAi animals, the larval arrest phenotype of the noboKO larvae was not rescued when they were fed cholesterol- or 7dC-supplemented food (Table 4). These results suggest that the PG cells that completely lack nobo function cannot utilise cholesterol for ecdysteroid biosynthesis during larval development, whereas nobo RNAi PG cells, which are partial nobo loss-of-function cells, can. This point is argued in the Discussion below.
Table 4. Rescue of homozygous noboKO larvae by oral sterol administration. The numbers of the first, second and third instar larvae of the noboKO homozygous larvae, which were fed standard cornmeal food with yeast pastes containing 0.5%(w/w) sterol/steroid supplement, are indicated. Homozygous noboKO larvae were obtained by maternal administration of cholesterol to homozygous noboKO embryos as shown in Table 3. Larval instars were scored at 72 hours AEL.
| Larval instar | |||
|---|---|---|---|
| Sterol/steroid | First | Second | Third |
| None | 16 | 2 | 0 |
| C | 18 | 4 | 1 |
| 7dC | 18 | 6 | 0 |
| 20E | 1 | 4 | 19 |
Noppera-bo plays a crucial role in cholesterol transport and/or metabolism
We then examined whether cellular cholesterol in the PG cells was affected in nobo RNAi animals. A mass-spectrometric study revealed that the RG from the third instar larvae of conditional nobo RNAi significantly accumulated cholesterol compared to the RG from control animals (Fig. 4b). There were no statistically significant differences in the amounts of other plant and fungal sterols, contained in our regular diet such as β-sitosterol, ergosterol and campesterol, between control and nobo RNAi RG cells (Fig. 4b). We could not detect 7dC in either control or RNAi RG cells by our experimental method. To further confirm the abnormal accumulation of cholesterol, we dissected the RGs from the third instar larvae and incubated them in vitro with 22-NBD cholesterol, a fluorescent analogue of cholesterol29. We observed a significant elevation in fluorescence in the PG cells of nobo RNAi larvae compared to control animals (Fig. 4c,d). These results suggest that Nobo plays an essential role in the appropriate transport and/or metabolism of cholesterol for synthesising ecdysone.
A subfamily of noppera-bo GST genes is well conserved in dipteran and lepidopteran species
As classical phase II detoxification enzymes, GSTs are thought to have rapidly evolved in response to toxins and insecticides; thus, each insect genome encodes multiple GST genes30. nobo is found in the genomes of not only D. melanogaster but also other species of the genus Drosophila31. We further examined the evolutionarily conservation of nobo by analysing the phylogenetic relationship among Nobo and the other 277 GST proteins from 12 insects, the nematode Caenorhabditis elegans and Homo sapiens (Supplementary fig. 3a). Nobo belongs to the epsilon subclass, one of the six subclasses of insect cytosolic GSTs14. In the epsilon cluster, our phylogenetic analysis revealed that D. melanogaster Nobo is included in an evolutionarily conserved subclade, which also includes GSTs from dipteran species other than Drosophilidae species, such as the mosquitoes Aedes aegypti, Anopheles gambiae, and Culex quinquefasciatus, as well as lepidopteran species, such as the silkworm Bombyx mori and the monarch butterfly Danaus plexippus (Supplementary fig. 3a,b). The orthologous relationships between A. gambiae, B. mori and D. melanogaster are consistent with previous studies32,33. This conservation feature is in contrast to many other cytosolic GSTs, which are duplicated within a certain species (Supplementary fig. 3a). On the other hand, no clear orthologs of nobo were found in other insects than dipteran and lepidopteran species. These results suggest that nobo is evolutionarily conserved in Diptera and Lepidoptera.
According to the current GST nomenclature system, the orthologues of nobo from D. melanogaster, A. gambiae and B. mori are designated as GSTe1414, GSTe832 and GSTe733, respectively. As described later, we succeeded in demonstrating that B. mori GSTe7 was functionally orthologous to D. melanogaster nobo. To avoid the further confusing numberings to represent the same functional orthologues among insect species, we would like to propose a unique subfamily name, noppera-bo (nobo), for these orthologues. Hereafter, we refer to B. mori GSTe7 as nobo-Bm in this manuscript.
For the phylogenetic analysis, we also included human GSTA subclass members because previous studies have reported that some GSTA proteins are involved in steroidogenesis in mammals34,35,36,37. We found that the mammalian GSTA proteins were clustered in a clade completely different from the Noppera-bo subclade (Supplementary fig. 3a).
Noppera-bo GSTs play a conserved and specific role in insects
To examine whether other insect orthologues of nobo also play conserved roles in ecdysteroid biosynthesis, we investigated whether the nobo gene from the silkworm B. mori (nobo-Bm) can compensate for D. melanogaster nobo loss-of-function during development. Indeed, the phm-GAL4-driven expression of nobo-Bm allowed both noboKO homozygous mutants and nobo RNAi animals to complete their development and grow to adult stages (Table 1, Supplementary table S1 and S2). These results suggest that the nobo genes are truly functionally orthologous between Diptera and Lepidoptera. These data also confirm that the effect of the RNAi was specific to nobo and was not an off-target effect.
We further utilised the overexpression system to examine the functional specificity of nobo among other GST genes. A BLAST search indicated that the nobo gene is most similar to two epsilon-class GSTs in D. melanogaster, GSTe4 and GSTe12. However, overexpression of neither GSTe4 nor GSTe12 could rescue the lethality of nobo loss-of-function animals (Table 1). We also obtained previously reported transgenic lines to overexpress other D. melanogaster GST genes, including sepia, CG6662, CG6673A and CG6673B38. In particular, Sepia and CG6673A are known to be involved in eye pigment synthesis and a neurodegeneration process, respectively, and their substrates have already been identified38,39. However, the overexpression of none of these genes rescued the lethality of nobo loss-of-function animals (Table 1). These results support the idea that nobo GSTs plays a unique role in controlling ecdysteroid biosynthesis.
Discussion
In this study, we identified the noppera-bo (nobo) subfamily of GST proteins as components of the ecdysteroid biosynthetic pathway. All of our results indicate that Nobo plays a crucial role in ecdysteroid biosynthesis in Drosophila melanogaster. It is worth noting that a role of D. melanogaster nobo (GSTe14) in ecdysteroid biosynthesis has been independently demonstrated by Payre, Kageyama and their colleagues40.
GSTs are enzymes that conjugate the reduced form of glutathione (GSH) to various substrates. In general, GSTs are best known as cellular Phase II metabolic enzymes that catalyse the conjugation of GSH to xenobiotic substrates, including pollutants and drugs, for the purpose of detoxification in eukaryotes because GSH-conjugated substrates are easily transported out of cells41,42. In insects, GSTs are also essential for detoxifying endogenous and exogenous compounds. In particular, interest in insect GSTs has focused on their role in insecticide resistance43 and phytochemical detoxification44,45.
However, in addition to the role in detoxification processes, some GSTs have indispensable functions in other essential cellular processes, such as the regulation of cellular signal transduction cascades and the production of essential metabolites in insects38,39 and vertebrates46,47. In particular, previous studies have reported that the human GST A3-3 and its counterparts in other mammals are involved in mammalian steroid hormone biosynthesis34,35,36,37,48. Human GST A3-3 is selectively expressed in steroidogenic organs and catalyses the isomerisation of the Δ5-ketosteroid precursor in the biosynthesis of progesterone and testosterone. In conjunction with the studies in mammals, our study raises an interesting possibility that, similar to the families of cytochrome P450s and short-chain dehydrogenase/reductases5, the GST family is also involved in steroid hormone biosynthesis across animal phyla. It would be intriguing to examine whether the steroid hormone biosynthesis pathways in other eukaryotes require GST proteins.
This study provides several lines of genetic evidence showing that Nobo is required for ecdysteroid biosynthesis via the regulation of cholesterol transport and/or metabolism. First, nobo is exclusively expressed in ecdysteroid-producing organs including the PG and the ovary. Second, noboKO mutants exhibit a typical Halloween-class embryonic phenotype. Third, the larval developmental arrest phenotype is caused by the PG-specific knockdown of nobo. Finally, administration of 20E or cholesterol rescues nobo loss-of-function animals. However, compared to the known ecdysteroidogenic enzymes, Nobo is unique because Nobo seems not to be involved in the conversion of canonical ecdysteroid intermediates but to regulate the dynamics of cholesterol, which is normally thought to be the most upstream precursor of ecdysteroid biosynthesis. As far as we know, this is the first report suggesting that the behaviour of cholesterol in ecdysteroid biosynthesis is controlled by a specific factor whose expression and function are restricted to the ecdysteroidogenic organs.
As insects are unable to synthesize cholesterol de novo49, the increased cholesterol in nobo RNAi PG suggests that uptake of extracellular cholesterol, or intracellular transport and/or metabolism of cholesterol could be disordered in the PG. Using a fluorescent analogue of cholesterol, we observed a significant elevation in fluorescence in the PG cells of nobo RNAi larvae compared to control animals (Fig. 4c,d). This result raises two possibilities for Nobo function in wild-type PG: (1) Nobo inhibits the uptake of extracellular cholesterol in wild-type cells and thus may negatively regulate ecdysteroid biosynthesis or (2) Nobo is required for consumption of cholesterol via controlling transport and/or metabolism of intracellular cholesterol and thus may positively regulate ecdysteroid biosynthesis. Whereas we cannot completely rule out either of these possibilities, it appears the latter possibility is more likely because all of our genetic data strongly suggest that nobo is a positive regulator for promoting ecdysteroid biosynthesis. Related to this point, the loss-of-function phenotype of nobo is significantly similar to that of Niemann-Pick type C disease-1a (NPC1a)50,51,52, whose mammalian orthologues are essential for intracellular transport of cholesterol53. The shared phenotypic characteristics include the larval arrest phenotype due to the low ecdysteroid titre, the aberrant accumulation of sterols and the fact that the phenotype is rescued by dietary application of cholesterol and 7dC50,51,52. This consistency also supports our hypothesis that Nobo controls ecdysteroid biosynthesis by regulating the behaviour of intracellular cholesterol in the PG.
Our data show that the embryonic phenotype of noboKO homozygotes is rescued by maternal application of cholesterol, whereas the larval arrest phenotype of the rescued noboKO homozygote larvae is not rescued by oral cholesterol administration. We propose that these two phenomena are not paradoxical. In insects including D. melanogaster, the earlier embryonic stage of development is the syncytial stage, where an embryo possesses multiple nuclei without complete cell membranes in a single cytoplasm. Thus, maternally loaded excessive cholesterol can penetrate throughout the syncytial cytoplasm, resulting in the penetration of cholesterol throughout the blastodermal cells after cellularisation, even without Nobo function. In contrast, the larval PG cells take up cholesterol extracellularly and, thus, require Nobo to transport and/or metabolise this cholesterol. Therefore, unlike the embryos, noboKO homozygote larvae cannot become final instar larvae even when they are fed cholesterol-supplemented food. If this scenario is correct, however, it actually raises another question: what endogenous sterol/steroidal precursor is transported and/or metabolised by Nobo for embryonic ecdysteroid biosynthesis in wild-type embryos. Currently, reliable precursor materials for embryonic ecdysteroids have not been reported.
It is actually puzzling that exogenously applied cholesterol can rescue the developmental arrest phenotype of the loss of nobo function animals even though cellular cholesterol abnormally accumulates. One speculation is that most endogenous cellular cholesterol might be somehow sequestered in an ‘unavailable' form in loss of nobo function animals. This would be consistent with the accumulation and explain the reason why the administration of exogenous ‘available' cholesterol rescues the nobo RNAi phenotype. Currently it remains to be delineated why cholesterol is accumulated in the loss of nobo function PG cells. To clarify this point, identifying substrate(s) of Nobo and pathway(s) affected by Nobo is obviously important.
Thus far, we have not been able to find a specific substrate for Nobo. Considering the differences in the chemical structures of steroid hormones and their biosynthesis pathways in mammals and insects, it is unlikely that Nobo catalyses the isomerisation of steroidal substrates as human GST A3-3 does. The only hint regarding the substrate(s) of Nobo from the transgenic rescue experiments using several types GST proteins (Table 1) is that Nobo might have a relatively narrow substrate specificity. One possibility is that Nobo conjugates GSH directly to small bioactive compound(s), such as cholesterol and/or other lipids to modulate the ecdysteroid biosynthesis pathway by an unknown mechanism. In the plant Arabidopsis thaliana, 12-oxo-phytodienoic acid (OPDA), the precursor of jasmonic acid, is conjugated with GSH, which is important for the transportation of OPDA into the vacuole54. By way of analogy to the observation in Arabidopsis, sterol-GSH conjugates may be necessary for a proper subcellular localisation and utilisation of sterols in the PG. An alternative possibility is that Nobo might conjugate GSH to a protein substrate that is essential for the behaviour of cholesterol transport and/or metabolism. In mammals, a growing amount of evidence suggests that glutathionylation of certain proteins is essential for modulating their functions in cells47. Previous studies have identified some essential proteins for regulating cholesterol metabolism and homeostasis in D. melanogaster28,55, including NPC1a. It is therefore worth examining whether Nobo conjugates GSH to these known regulators and modulates their functions in cholesterol metabolism.
Our phylogenetic analysis indicates that the nobo subfamily of GST proteins is well conserved in Diptera and Lepidoptera. We also demonstrate that the in vivo function of D. melanogaster nobo can be replaced with B. mori nobo (nobo-Bm), suggesting the functional orthologous relationship of nobo between Diptera and Lepidoptera. However, we could not find any clear orthologues of nobo in any other insect taxons (Supplementary fig. 3a). Because the GST family has great intra- and interspecies functional and structural diversity, it is possible that a simple BLAST search strategy and the neighbour-joining method failed to discover the true “functional” GST orthologues in other insect species. Alternatively, our data also raise the possibility that ecdysteroid biosynthesis is differentially regulated among insect species. Curiously, the essential ecdysteroidogenic enzyme genes spookier and Cyp6t3 have so far only been found in the genomes of Drosophilidae18,56. In the future, it would be very interesting to study not only the evolutionarily conservation of ecdysteroidogenic enzymes in arthropods57,58 but also the specific molecular mechanisms of ecdysteroid biosynthesis in certain insects.
Methods
Fly strains
Drosophila melanogaster flies were reared on standard agar-cornmeal medium at 25°C under a 12 h/12 h light/dark cycle. Oregon R was used as the wild-type strain for the in situ RNA hybridisation and immunohistochemistry shown in Fig. 1, and w1118 was used as the wild-type (control) strain for all genetic experiments. The UAS-nobo-IR (stock numbers #40316 and #101884) and UAS-torso-IR (stock number #101154) strains were obtained from the Vienna Drosophila RNAi Center. yw; P{CaryP}attP4059 was obtained from BestGene, Inc. ptth-GAL4;UAS- grim, in which the ptth gene-expressing neurons were ablated20, phm-GAL4#2220,56 and UAS-dicer2 were kindly gifted by M. B. O'Connor (University of Minnesota, USA). UAS-sepia, UAS-CG6673A, UAS-CG6673B and UAS-CG666238 were kind gifts from J. Yim (National University Seoul, Korea). yw; P{70FLP}23 P{70I-SceI}4A/TM624 and w; P{70FLP}1024 were obtained from the Bloomington Drosophila Stock Center.
Quantitative reverse transcription (qRT)-PCR
Total RNA was isolated using the RNAiso Plus reagent (TaKaRa). Genomic DNA was digested using Recombinant DNaseI (TaKaRa). cDNA was synthesised using the ReverTra Ace qRT RT Kit (TOYOBO). qRT-PCR was performed using the THUNDERBIRD SYBR qPCR Mix (TOYOBO) with a Thermal Cycler Dice TP800 system (TaKaRa). Serial dilutions of a plasmid containing the ORF of each gene were used as a standard. The expression levels were normalised to rp49 in the same sample. The primers for quantifying D. melanogaster nobo are described in Supplementary table S3. Primers amplifying rp49 were previously described60.
in situ RNA hybridisation
To generate a template for synthesising sense and antisense nobo RNA probes, the nobo ORF region was isolated from the pUAST-nobo-HA vector (described below) and inserted into pBluescriptII digested with EcoRI and NotI, whose sites are positioned in the multicloning site of the pUAST-HA vector. Plasmids for synthesising the IMP-E1 and IMP-L1 probes have been previously described7. Synthesis of DIG-labelled RNA probes and in situ hybridisation were performed as previously described7.
Generation of anti-Nobo antibody and immunohistochemistry
Antibodies against the Nobo protein were raised in guinea pig. A synthetic peptide (NH2-MSQPKPILYYDERSPPVRSC-COOH) corresponding to residues 1–20 of the Nobo amino acid sequence (GenBank accession number AAF58397) was used for immunisation. Immunostaining for embryos, the brain-ring gland complex in third instar larvae and the ovary in female adults was performed as previously described7,61. The antibodies used were anti-Nobo antiserum (1:200 dilution), anti-FasIII 7G10 (obtained from the Developmental Studies Hybridoma Bank, Univ. of Iowa; 1:20 dilution), anti-guinea pig IgG antibody conjugated with Alexa488 (Life Technologies; 1:200 dilution), and anti-mouse IgG antibody conjugated with Alexa488 (Life Technologies; 1:200 dilution).
UAS vectors, overexpression of genes and generation of transgenic strains
The GAL4/UAS system62 was used to overexpress genes in D. melanogaster. To generate pUAST vectors to overexpress nobo and nobo-Bm (B. mori GSTe7), specific primers (Supplementary table S3) were used for PCR to add BglII and NotI sites to the 5′ and 3′ ends, respectively, of each of the cDNA fragments corresponding to CDSs. Template cDNAs were reverse transcribed using total RNAs of the ring gland from D. melanogaster and the PG from B. mori (KINSHU x SHOWA F1 hybrid) using Prime Script Reverse Transcriptase (TaKaRa). PCR was performed using Prime Star HS DNA polymerase (TaKaRa). The amplified CDS regions of nobo and nobo-Bm were digested with BglII and NotI, and then ligated into a pUAST-HA vector carrying a sequence coding three tandem HA tags at the C terminal7. To generate overexpression vectors of GSTe12 and GSTe4, each CDS region was ligated into the pWALIUM10-moe vector (purchased from Harvard RNAi Center; http://www.flyrnai.org/TRiP-HOME.html). Specific primers (Supplementary table S3) were used for PCR to add BglII and XbaI sites to the 5′ and 3′ ends, respectively, of each of the cDNA fragments corresponding to CDSs. Transformants were generated using the phiC31 integrase system in the P{CaryP}attP40 strain. The w+ transformants of pUAST and pWALIUM10-moe were established using standard protocols.
Generation of the gene-targeted noboKO allele
Gene-targeting of nobo was carried out by the ends-out method24,25 using the pP{EndsOut2} and pBSII-70w63 vectors provided by the Drosophila Genomics Resource Center and Dr. T. Matsuo (Tokyo Metropolitan Univ.), respectively. 5′ upstream and 3′ downstream regions of nobo were amplified by PCR with specific primer pairs (Supplementary table S3). Both PCR fragments were subcloned into pP{EndsOut2} with a hsp70-white mini-gene fragment excited from the pBSII-70w with NotI and HindIII. The nobo targeting vector was injected into the w- strain using standard protocols. Targeting crosses were carried out as described by the Sekelsky Lab (http://sekelsky.bio.unc.edu/Research/Targeting/Targeting.html). The nobo knock-out strain was back-crossed to the w1118 strain for five generations.
Embryonic cuticle preparation
Embryonic cuticle preparation was carried out as previously described64.
20E rescue of noboKO embryos
The 20E rescue experiment was performed as previously described16,18. 20E was purchased from Sigma. Homozygous noboKO (noboKO/noboKO) embryos were obtained as offspring from noboKO/CyO Act5C-GFP parents. Homozygous noboKO/noboKO embryos were distinguished from heterozygous noboKO (noboKO/CyO Act5C-GFP) embryos by assessing the presence or absence of a GFP signal under a fluorescence dissection microscope (Leica MZFLIII).
Rescue experiments with ecdysteroid intermediates
Cholesterol and 7-dehydrocholesterol (7dC) were purchased from Wako and Sigma, respectively. For maternal sterol rescue experiments, we used a noboKO strain balanced with the CyO Act5C-GFP balancer chromosome, which carries a GFP expression construct. Female flies were kept on standard cornmeal food with yeast pastes containing 0.5%(w/w) sterol in 3.3%(w/w) ethanol (50 mg of yeast paste, 0.75 mg of sterol, 95 µl of water and 5 µl of ethanol) for 3 days. Then female flies were crossed with male flies of the same strain, which were reared on standard cornmeal food without steroidal supplement, and were left to lay eggs on grape agar plates for 1 day. At 24 hours AEL, the genotype of each hatched larva was scored by assessing the presence or absence of a GFP signal under a fluorescence dissection microscope MZFLIII (Leica). The noboKO allele was also distinguished from its wild-type sequence by genomic PCR with a specific primer pair (Fig. 2a and Supplementary table S3). Feeding rescue experiments for rescued noboKO and nobo RNAi larvae were conducted as previously described11,16.
Transgenic RNAi experiment and scoring of developmental progression
UAS-nobo-IR and w1118 flies were crossed with UAS-dicer2; UAS-phm-GAL4#22 flies. Eggs were laid on grape plates with yeast pastes at 25°C for 4 hours. Fifty hatched first instar larvae were transferred into a single vial with standard cornmeal food. Every 24 hours, developmental stages were scored by tracheal morphology as previously described16.
Fluorescence analysis of cholesterol distribution by 22-NBD-cholesterol
To assess the incorporation of cholesterol and to visualise its distribution, we conducted in vitro incubation of the brain-ring gland complexes dissected from third instar larvae with 22-(N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-23,24-bisnor-5-cholen-3β-ol (22-NBD-cholesterol; Life Technologies). 22-NBD-cholesterol was dissolved in 100% ethanol at a 2 mM concentration for a stock solution. To avoid the lethality of nobo RNAi at the earlier larval stages, we utilised the GAL80ts technique65 to conditionally suppress GAL4 transcriptional activity during first and second instar larval development. In this experiment, control (w1118; UAS-dicer2/+; phm-GAL4#22 tubP-GAL80ts/+) and conditional nobo RNAi flies (w1118; UAS-dicer2/UAS-nobo-IR; phm-GAL4#22 tubP-GAL80ts/+) were used. The first instar larvae were transferred into standard cornmeal food and reared at 21°C for 2 days. After 2 days, larvae were then reared at 25°C for an additional 3 days, allowing the larvae to reach the third instar stage. The third instar larvae were dissected in PBS, and the brain-ring gland complexes were transferred into Schneider's Drosophila Medium (Life Technologies) containing 10% fatal bovine serum, 100 U/ml penicillin (Wako) and 100 µg/ml Streptomycin (Wako). After an incubation at 25°C for 10 min, the medium was replaced with a fresh medium containing 0.5% 22-NBD-cholesterol stock solution, which achieved a 10 µM 22-NBD-cholesterol with 0.5% final ethanol concentration. Then, tissues were incubated at 25°C for 6 hours in a dark condition. Tissues were washed with PBS twice and mounted. A 488 nm laser was used for excitation of 22-NBD-cholesterol fluorescence, and fluorescence emission was selected by a 490–555 nm-band pass filter. Fluorescence images were obtained with an LSM 700 laser-scanning confocal microscope (Zeiss).
A mass-spectrometric quantification of 20E and sterols
For the measurement of 20E in whole bodies of control (w1118; UAS-dicer2/+; UAS-phm-GAL4#22/+) and nobo RNAi flies (w1118; UAS-dicer2/UAS-nobo-IR; UAS-phm-GAL4#22/+), the second instar larvae (56–64 hours AEL) of each genotype were collected. Then the wet weight of each sample was measured, and the samples were frozen with liquid nitrogen and stored at -80°C until measurement. For the measurement of sterol levels in the RG, the RG samples from the control and nobo RNAi larvae were collected as described above in “Fluorescence analysis of cholesterol distribution by 22-NBD-cholesterol”. Ten ring glands were collected from the third instar larvae and then transferred into a single glass vial on dry ice. All samples were stored at -80°C until measurement. For each genotype, 10 independent samples, each containing 10 ring glands, were used for the analysis. Extraction of steroids, HPLC fractionation and mass-spectrometric analyses were previously described66,67, except a minor modification of the sterol quantification and the mobile phase conditions (run time: 0–12 min, acetonitrile isocratic, flow rate: 300 μl/min). In the mass-spectrometric analyses, the exact quantification range was 0.1221–31.25 ng/mL. In this experimental condition, a limit of the quantification of 20E was 0.916 pg of 20E/mg of wet weight sample.
Author Contributions
S.E. and R.N. designed the study; S.E., T.A., F.I., M.I., T.S. and R.N. performed the experiments; S.E., F.I., M.I., H.K., T.S. and R.N. contributed and developed reagents; S.E., T.A., F.I., M.I., T.S. and R.N. analysed the data; and S.E. and R.N. wrote the paper.
Supplementary Material
Supplementary figures S1, S2 and S3; Supplementary tables S1, S2, S3 and S4
Acknowledgments
We are grateful to Drs. Takashi Matsuo, Michael B. O'Connor, Ryosuke L. Ohniwa, and Jeongbin Yim and the Bloomington Drosophila Stock Center, the Drosophila Genetic Resource Center in Kyoto, the Vienna Drosophila RNAi Center and the Developmental Studies Hybridoma Bank for stocks and reagents. We also thank Dr. Satomi Takeo for helpful advice for creating knock-out strains; Drs. Yuko Shimada-Niwa and DeMar Taylor for critical reading of the manuscript; and Drs. François Payre and Yuji Kageyama for a helpful and constructive discussion on this work. S.E. was a recipient of a fellowship from the Japan Society for the Promotion of Science. This work was supported in part by Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science and Technology of the Japanese government. This work was also supported by JSPS KAKENHI Grant Number 25712010 and MEXT KAKENHI Grant Number 23116701 on Innovative Areas ‘Regulatory Mechanism of Gamete Stem Cells' to R.N, and by JSPS KAKENHI Grant Number 25252023 to H.K.
References
- Spindler K. D. et al. Ecdysteroid hormone action. Cell Mol Life Sci 66, 3837–3850 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka N., Rewitz K. F. & O'Connor M. B. Ecdysone control of developmental transitions: lessons from Drosophila research. Annu Rev Entomol 58, 497–516 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert L. I., Rybczynski R. & Warren J. T. Control and biochemical nature of the ecdysterroidogenic pathway. Annu Rev Entomol 47, 883–916 (2002). [DOI] [PubMed] [Google Scholar]
- Niwa Y. S. & Niwa R. Neural control of steroid hormone biosynthesis during development in the fruit fly Drosophila melanogaster. Genes Genet. Syst. 89, 27–34 (2014). [DOI] [PubMed] [Google Scholar]
- Enzymes for ecdysteroid biosynthesis: Their biological functions in insects and beyond. Biosci Biotechnol Biochem 78, 1283–1292 (2014). [DOI] [PubMed] [Google Scholar]
- Rewitz K. F., Rybczynski R., Warren J. T. & Gilbert L. I. The Halloween genes code for cytochrome P450 enzymes mediating synthesis of the insect moulting hormone. Biochem Soc Trans 34, 1256–1260 (2006). [DOI] [PubMed] [Google Scholar]
- Niwa R. et al. CYP306A1, a cytochrome P450 enzyme, is essential for ecdysteroid biosynthesis in the prothoracic glands of Bombyx and Drosophila. J. Biol. Chem. 279, 35942–35949 (2004). [DOI] [PubMed] [Google Scholar]
- Yamanaka N. et al. Identification of a novel prothoracicostatic hormone and its receptor in the silkworm Bombyx mori. J. Biol. Chem. 280, 14684–14690 (2005). [DOI] [PubMed] [Google Scholar]
- Niwa R. et al. The ecdysteroidogenic P450 Cyp302a1/disembodied from the silkworm, Bombyx mori, is transcriptionally regulated by prothoracicotropic hormone. Insect Mol Biol 14, 563–571 (2005). [DOI] [PubMed] [Google Scholar]
- Namiki T. et al. Cytochrome P450 CYP307A1/Spook: a regulator for ecdysone synthesis in insects. Biochem Biophys Res Commun 337, 367–374 (2005). [DOI] [PubMed] [Google Scholar]
- Yoshiyama T., Namiki T., Mita K., Kataoka H. & Niwa R. Neverland is an evolutionally conserved Rieske-domain protein that is essential for ecdysone synthesis and insect growth. Development 133, 2565–2574 (2006). [DOI] [PubMed] [Google Scholar]
- Namiki T. et al. A basic-HLH transcription factor, HLH54F, is highly expressed in the prothoracic gland in the silkworm Bombyx mori and the fruit fly Drosophila melanogaster. Biosci Biotechnol Biochem 73, 762–765 (2009). [DOI] [PubMed] [Google Scholar]
- Niwa R. et al. Expressions of the cytochrome P450 monooxygenase gene Cyp4g1 and its homolog in the prothoracic glands of the fruit fly Drosophila melanogaster (Diptera: Drosophilidae) and the silkworm Bombyx mori (Lepidoptera: Bombycidae) - Springer. Appl Entomol Zool 46, 533–543 (2011). [Google Scholar]
- Saisawang C., Wongsantichon J. & Ketterman A. J. A preliminary characterization of the cytosolic glutathione transferase proteome from Drosophila melanogaster. Biochem J 442, 181–190 (2012). [DOI] [PubMed] [Google Scholar]
- Maróy P., Kaufmann G. & Dübendorfer A. Embryonic ecdysteroids of Drosophila melanogaster. J Insect Physiol 34, 633–637 (1988). [Google Scholar]
- Niwa R. et al. Non-molting glossy/shroud encodes a short-chain dehydrogenase/reductase that functions in the ‘Black Box' of the ecdysteroid biosynthesis pathway. Development 137, 1991–1999 (2010). [DOI] [PubMed] [Google Scholar]
- Warren J. T. et al. Phantom encodes the 25-hydroxylase of Drosophila melanogaster and Bombyx mori: a P450 enzyme critical in ecdysone biosynthesis. Insect Biochem Mol Biol 34, 991–1010 (2004). [DOI] [PubMed] [Google Scholar]
- Ono H. et al. Spook and Spookier code for stage-specific components of the ecdysone biosynthetic pathway in Diptera. Dev. Biol. 298, 555–570 (2006). [DOI] [PubMed] [Google Scholar]
- Yamanaka N. et al. Differential regulation of ecdysteroidogenic P450 gene expression in the silkworm, Bombyx mori. Biosci Biotechnol Biochem 71, 2808–2814 (2007). [DOI] [PubMed] [Google Scholar]
- McBrayer Z. et al. Prothoracicotropic hormone regulates developmental timing and body size in Drosophila. Dev Cell 13, 857–871 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewitz K. F., Yamanaka N., Gilbert L. I. & O'Connor M. B. The insect neuropeptide PTTH activates receptor tyrosine kinase torso to initiate metamorphosis. Science 326, 1403–1405 (2009). [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C. & Wieschaus E. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster I. Zygotic loci on the second chromosome. Roux's Arch. Dev. BIol. 193, 267–282 (1984). [DOI] [PubMed] [Google Scholar]
- Chávez V. M. et al. The Drosophila disembodied gene controls late embryonic morphogenesis and codes for a cytochrome P450 enzyme that regulates embryonic ecdysone levels. Development 127, 4115–4126 (2000). [DOI] [PubMed] [Google Scholar]
- Rong Y. S. & Golic K. G. Gene targeting by homologous recombination in Drosophila. Science 288, 2013–2018 (2000). [DOI] [PubMed] [Google Scholar]
- Radford S. J., Goley E., Baxter K., McMahan S. & Sekelsky J. Drosophila ERCC1 is required for a subset of MEI-9-dependent meiotic crossovers. Genetics 170, 1737–1745 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerdell J. R. & Carthew R. W. Heritable gene silencing in Drosophila using double-stranded RNA. Nat Biotechnol 18, 896–898 (2000). [DOI] [PubMed] [Google Scholar]
- Yoshiyama-Yanagawa T. et al. The conserved Rieske oxygenase DAF-36/Neverland is a novel cholesterol-metabolizing enzyme. J. Biol. Chem. 286, 25756–25762 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa R. & Niwa Y. S. The Fruit Fly Drosophila melanogaster as a Model System to Study Cholesterol Metabolism and Homeostasis. Cholesterol 2011, 176802 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G. Cholesterol-protein interaction: methods and cholesterol reporter molecules. Subcell. Biochem. 51, 1–45 (2010). [DOI] [PubMed] [Google Scholar]
- Friedman R. Genomic organization of the glutathione S-transferase family in insects. Mol Phylogenet Evol 61, 924–932 (2011). [DOI] [PubMed] [Google Scholar]
- Clark A. G. et al. Evolution of genes and genomes on the Drosophila phylogeny. Nature 450, 203–218 (2007). [DOI] [PubMed] [Google Scholar]
- Yu Q. et al. Identification, genomic organization and expression pattern of glutathione S-transferase in the silkworm, Bombyx mori. Insect Biochem Mol Biol 38, 1158–1164 (2008). [DOI] [PubMed] [Google Scholar]
- Ayres C. et al. Comparative genomics of the anopheline glutathione S-transferase epsilon cluster. PLoS ONE 6, e29237 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson A. S. & Mannervik B. Human glutathione transferase A3-3, a highly efficient catalyst of double-bond isomerization in the biosynthetic pathway of steroid hormones. J. Biol. Chem. 276, 33061–33065 (2001). [DOI] [PubMed] [Google Scholar]
- Raffalli-Mathieu F., Orre C., Stridsberg M., Hansson Edalat M. & Mannervik B. Targeting human glutathione transferase A3-3 attenuates progesterone production in human steroidogenic cells. Biochem J 414, 103–109 (2008). [DOI] [PubMed] [Google Scholar]
- Tars K., Olin B. & Mannervik B. Structural basis for featuring of steroid isomerase activity in alpha class glutathione transferases. J Mol Biol 397, 332–340 (2010). [DOI] [PubMed] [Google Scholar]
- Fedulova N., Raffalli-Mathieu F. & Mannervik B. Porcine glutathione transferase Alpha 2-2 is a human GST A3-3 analogue that catalyses steroid double-bond isomerization. Biochem J 431, 159–167 (2010). [DOI] [PubMed] [Google Scholar]
- Kim J. et al. Identification and characteristics of the structural gene for the Drosophila eye colour mutant sepia, encoding PDA synthase, a member of the omega class glutathione S-transferases. Biochem J 398, 451–460 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. K., Kim S.-H. S., Kim J. J., Kim H. H. & Yim J. J. Glutathione S-transferase omega 1 activity is sufficient to suppress neurodegeneration in a Drosophila model of Parkinson disease. J. Biol. Chem. 287, 6628–6641 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanut-Delalande H. et al. Pri peptides are mediators of ecdysone for the temporal control of development. Nat Cell Biol 10.1038/ncb3052 (2014) in press. [DOI] [PubMed] [Google Scholar]
- Frova C. Glutathione transferases in the genomics era: new insights and perspectives. Biomol Eng 23, 149–169 (2006). [DOI] [PubMed] [Google Scholar]
- Tew K. D. & Townsend D. M. Glutathione-S-transferases as determinants of cell survival and death. Antioxid. Redox Signal. 17, 1728–1737 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enayati A. A., Ranson H. & Hemingway J. Insect glutathione transferases and insecticide resistance. Insect Mol Biol 14, 3–8 (2005). [DOI] [PubMed] [Google Scholar]
- Sun X.-Q. et al. Glutathione S-transferase of brown planthoppers (Nilaparvata lugens) is essential for their adaptation to gramine-containing host plants. PLoS ONE 8, e64026 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabab M., Khan S. A., Vogel H., Heckel D. G. & Boland W. OPDA isomerase GST16 is involved in phytohormone detoxification and insect development. FEBS J 281, 2769–2783 (2014). [DOI] [PubMed] [Google Scholar]
- Laborde E. Glutathione transferases as mediators of signaling pathways involved in cell proliferation and cell death. Cell Death Differ 17, 1373–1380 (2010). [DOI] [PubMed] [Google Scholar]
- Tew K. D. et al. The role of glutathione S-transferase P in signaling pathways and S-glutathionylation in cancer. Free Radic Biol Med 51, 299–313 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffalli-Mathieu F. & Mannervik B. Human Glutathione Transferase A3-3 Active as Steroid Double-Bond Isomerase. Methods Enzymol 401, 265–278 (2005). [DOI] [PubMed] [Google Scholar]
- Svoboda J. A., Kaplanis J. N., Robbins W. E. & Thompson M. J. Recent developments in insect steroid metabolism. Annu Rev Entomol 20, 205–220 (1975). [DOI] [PubMed] [Google Scholar]
- Huang X., Suyama K., Buchanan J., Zhu A. J. & Scott M. P. A Drosophila model of the Niemann-Pick type C lysosome storage disease: dnpc1a is required for molting and sterol homeostasis. Development 132, 5115–5124 (2005). [DOI] [PubMed] [Google Scholar]
- Fluegel M. L., Parker T. J. & Pallanck L. J. Mutations of a Drosophila NPC1 gene confer sterol and ecdysone metabolic defects. Genetics 172, 185–196 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Warren J. T., Buchanan J., Gilbert L. I. & Scott M. P. Drosophila Niemann-Pick Type C-2 genes control sterol homeostasis and steroid biosynthesis: a model of human neurodegenerative disease. Development 134, 3733–3742 (2007). [DOI] [PubMed] [Google Scholar]
- Rosenbaum A. I. & Maxfield F. R. Niemann-Pick type C disease: molecular mechanisms and potential therapeutic approaches. J Neurochem 116, 789–795 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkama-Ohtsu N. et al. 12-oxo-phytodienoic acid-glutathione conjugate is transported into the vacuole in Arabidopsis. Plant Cell Physiol 52, 205–209 (2011). [DOI] [PubMed] [Google Scholar]
- Talamillo A. et al. Scavenger Receptors Mediate the Role of SUMO and Ftz-f1 in Drosophila Steroidogenesis. PLoS Genet 9, e1003473 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Q., Magico A. & King-Jones K. Nuclear receptor DHR4 controls the timing of steroid hormone pulses during Drosophila development. PLoS Biol 9, e1001160 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewitz K. F., Styrishave B., Løbner-Olsen A. & Andersen O. Marine invertebrate cytochrome P450: emerging insights from vertebrate and insects analogies. Comp Biochem Physiol C Toxicol Pharmacol 143, 363–381 (2006). [DOI] [PubMed] [Google Scholar]
- Rewitz K. F., O'Connor M. B. & Gilbert L. I. Molecular evolution of the insect Halloween family of cytochrome P450s: phylogeny, gene organization and functional conservation. Insect Biochem Mol Biol 37, 741–753 (2007). [DOI] [PubMed] [Google Scholar]
- Markstein M., Pitsouli C., Villalta C., Celniker S. E. & Perrimon N. Exploiting position effects and the gypsy retrovirus insulator to engineer precisely expressed transgenes. Nat Genet 40, 476–483 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley K. P., Leonard M. W. & Engel J. D. Quantitation of RNA using the polymerase chain reaction. Trends Genet 9, 380–385 (1993). [DOI] [PubMed] [Google Scholar]
- Shimada Y., Burn K. M., Niwa R. & Cooley L. Reversible response of protein localization and microtubule organization to nutrient stress during Drosophila early oogenesis. Dev. Biol. 355, 250–262 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A. H. & Perrimon N. Targeted Gene-Expression as a Means of Altering Cell Fates and Generating Dominant Phenotypes. Development 118, 401–415 (1993). [DOI] [PubMed] [Google Scholar]
- Matsuo T., Sugaya S., Yasukawa J., Aigaki T. & Fuyama Y. Odorant-binding proteins OBP57d and OBP57e affect taste perception and host-plant preference in Drosophila sechellia. PLoS Biol 5, e118 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieschaus E. & Nüsslein-Volhard C. in Drosophila: A Practical Approach (Roberts, D. B.) 179–214 (Oxford Univesity Press., 1998).
- McGuire S. E., Le P. T., Osborn A. J., Matsumoto K. & Davis R. L. Spatiotemporal rescue of memory dysfunction in Drosophila. Science 302, 1765–1768 (2003). [DOI] [PubMed] [Google Scholar]
- Hikiba J. et al. Simultaneous quantification of individual intermediate steroids in silkworm ecdysone biosynthesis by liquid chromatography-tandem mass spectrometry with multiple reaction monitoring. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 915–916, 52–56 (2013). [DOI] [PubMed] [Google Scholar]
- Igarashi F. et al. A highly specific and sensitive quantification analysis of the sterols in silkworm larvae by high performance liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry. Anal Biochem 419, 123–132 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures S1, S2 and S3; Supplementary tables S1, S2, S3 and S4