Abstract
The role of amyloid-β (Aβ) neuropathology and its significant changes in biofluids after traumatic brain injury (TBI) is still debated. We used ultrasensitive digital ELISA approach to assess amyloid-β1-42 (Aβ42) concentrations and time-course in cerebrospinal fluid (CSF) and in plasma of patients with severe TBI and investigated their relationship to injury characteristics, neurological status and clinical outcome. We found decreased CSF Aβ42 levels in TBI patients acutely after injury with lower levels in patients who died 6 months post-injury than in survivors. Conversely, plasma Aβ42 levels were significantly increased in TBI with lower levels in patients who survived. A trend analysis showed that both CSF and plasma Aβ42 levels strongly correlated with mortality. A positive correlation between changes in CSF Aβ42 concentrations and neurological status as assessed by Glasgow Coma Scale (GCS) was identified. Our results suggest that determination of Aβ42 may be valuable to obtain prognostic information in patients with severe TBI as well as in monitoring the response of the brain to injury.
Studies in Alzheimer disease (AD) have highlighted the utility of CSF amyloid-β peptide (Aβ) as a ‘state marker' of the disease, reliably reflecting AD pathology1,2. Recently, an increasing body of literature has shown potential links between traumatic brain injury (TBI) and forms of neurodegeneration such as Alzheimer disease3,4,5. Recent studies have also shown significant changes in brain extracellular amyloid-β dynamics in patients with severe brain injury, either in fluids or tissue6,7,8. The 42–amino acid form of amyloid-β1–42 (Aβ42) is of special interest; this form appears to have the greatest propensity to deposit into insoluble plaques, one of the pathological hallmarks of Alzheimer's disease, as well as to aggregate into oligomeric Aβ species and is deemed to underlie the neurodegeneration/neurotoxicity observed in AD in combination with other molecular targets and biomarkers such as tau9. In this study we have used a novel ultrasensitive digital ELISA (Single Molecule Arrays, SiMoA) to assess amyloid-β1-42 (Aβ42) concentrations in CSF and matching plasma samples of patients with severe traumatic brain injury (TBI) and correlated results with injury characteristics, neurological status and clinical outcome. The developed ELISA for Aβ42 has been analytically qualified and validated and shows no matrix interference and good precision and accuracy.
Results
Study population
A total of 12 patients with severe TBI and 20 controls were included for analyses. The clinical and demographic characteristics of the patients are summarized in Table 1. Patients with severe TBI had similar percentage of diffuse injury and focal mass lesion as well as survival/mortality rate (Table 1). In the control population (n = 20), 100% were men, and the average age was 26 ± 4 years. Except for the age (p = 0.001), there were no significant differences in the characteristics between control subjects and TBI patients.
Table 1. Summary of Demographic and Clinical Data for Severe Traumatic Brain Injury cases included in the study.
| sTBI (n = 12) | |
|---|---|
| Age, years, mean (SD) | 49 (17.6) |
| F/M, n (%) | 1/11 (8/92) |
| GCS, median (range)* | 7 (3–8) |
| Time to first sample withdrawal, h, median (range) | 15 (5–24) |
| Mechanism of injury, n (%) | |
| Motor vehicle | 4 (33) |
| Motor cycle | 1 (8) |
| Fall | 5 (42) |
| Other | 2 (17) |
| CT classification, n (%)* | |
| Diffuse injury | 6 (50) |
| I | - |
| II | 5 (42) |
| III | - |
| IV | 1 (8) |
| Focal Mass Lesion | 6 (50) |
| V | 1 (8) |
| VI | 5 (42) |
| Outcome GOSE 6 mo, n (%) | |
| Poor outcome | 6 (50) |
| 1 | 6 (50) |
| Good outcome | 6 (50) |
| 5 | 2 (17) |
| 7 | 1 (8) |
| 8 | 3 (25) |
Abbreviations: GCS = Glasgow Coma Scale; CT = Computed Tomography; GOSE = Glasgow Outcome Score Extended.
*At the time of admission.
CSF and plasma concentrations of Aβ42 acutely after injury
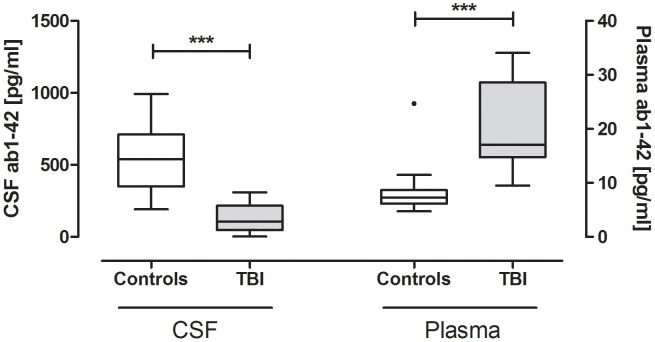
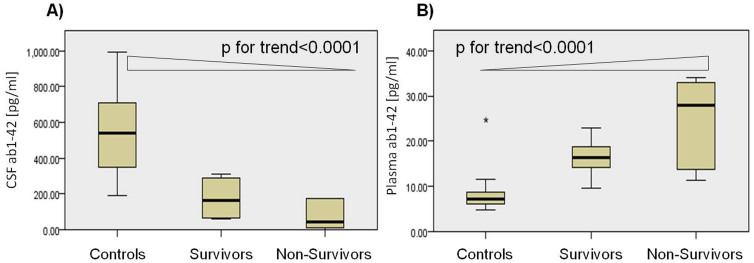
The median CSF and plasma concentrations of Aβ42 acutely after injury for patients with severe TBI and for controls are shown in Table 2. Aβ42 concentrations in CSF were significantly lower in TBI patients than in controls (p < 0.0001); in contrast, plasma concentrations of Aβ42 were significantly lower in controls than in TBI patients (p < 0.0001) (Table 2, Fig. 1). CSF and plasma levels of Aβ42 did not correlate with age, time to sample withdrawal and severity of injury as assessed by GCS and motor GCS. There was no correlation between CSF and plasma levels of Aβ42 (Spearman correlation coefficients = −0.02, p = 0.946). CSF and plasma Aβ42 concentrations did not differ between patients with diffuse injury and focal mass lesion (p = 0.93 and p = 0.49, respectively). CSF Aβ42 concentrations were lower in patients who died compared to patients who survived; conversely, plasma concentrations were lower in survivors than non-survivors (Table 2). CSF Aβ42 significantly decreased from normal through TBI survivors to TBI non-survivors (P for trend = p < 0.0001; Jonckheere-Terpstra test), while plasma Aβ42 significantly increased from normal through TBI survivors to TBI non-survivors (P for trend = p < 0.0001; Jonckheere-Terpstra test) (Fig. 2).
Table 2. CSF and plasma Aβ42 levels in patients with severe TBI acutely after injury and in controls. Data are given as median (interquartile range).
| N | CSF Aβ42 (pg/mL) | N | Plasma Aβ42 (pg/mL) | ||
|---|---|---|---|---|---|
| TBI | Admission | 12 | 105.9 (46.02–216.2)† | 12 | 17.02 (14.75–28.59)† |
| Diffuse Injury (Admission) | 6 | 105.9 (52.45–227.6) | 6 | 19.65 (14.91–32.90) | |
| Focal Mass Lesion (Admission) | 6 | 118.7(16.59–239.3) | 6 | 16.61 (13.21–27.35) | |
| Survivors (Admission) | 6 | 161.1 (65.67–286.9)° | 6 | 16.29 (14.13–18.88)° | |
| Non-Survivors (Admission) | 6 | 46.02 (11.55–172.4)‡ | 6 | 27.97 (13.66–32.90)‡ | |
| Controls | 15 | 537.6 (350.8–710.0) | 20 | 7.289 (6.126–8.668) |
†p < .0001 (p values of the Mann-Whitney test for differences between the groups [TBI versus Controls]).
°p < .05, or ‡ p < .0001 (p values of the post-hoc test for differences between Survivors and Non-survivors versus Controls).
Figure 1. CSF and plasma Aβ42 concentrations acutely after injury in patients and controls.
Aβ42 concentrations acutely after injury (24 hrs) in CSF (A) and in plasma (B) of patients with severe TBI and controls. The horizontal line in each box represents the median and the boxes representing 25th to 75th quartile. Significant differences are indicated with *** (P < 0.0001) (Mann–Whitney U-test).
Figure 2. Box-and-whisker plots demonstrating median CSF (A) and plasma (B) Aβ42 concentrations acutely after injury in TBI patients who died and in TBI patients who survived, and in controls.
Jonckheere-Terpstra test demonstrates an increase in CSF (A) Aβ42 in patients across the groups and a decrease in plasma (B) Aβ42 in patients across the groups. The black horizontal line in each box represents the median, with the boxes representing the interquartile range.
Description of Longitudinal CSF and plasma Aβ42 Levels
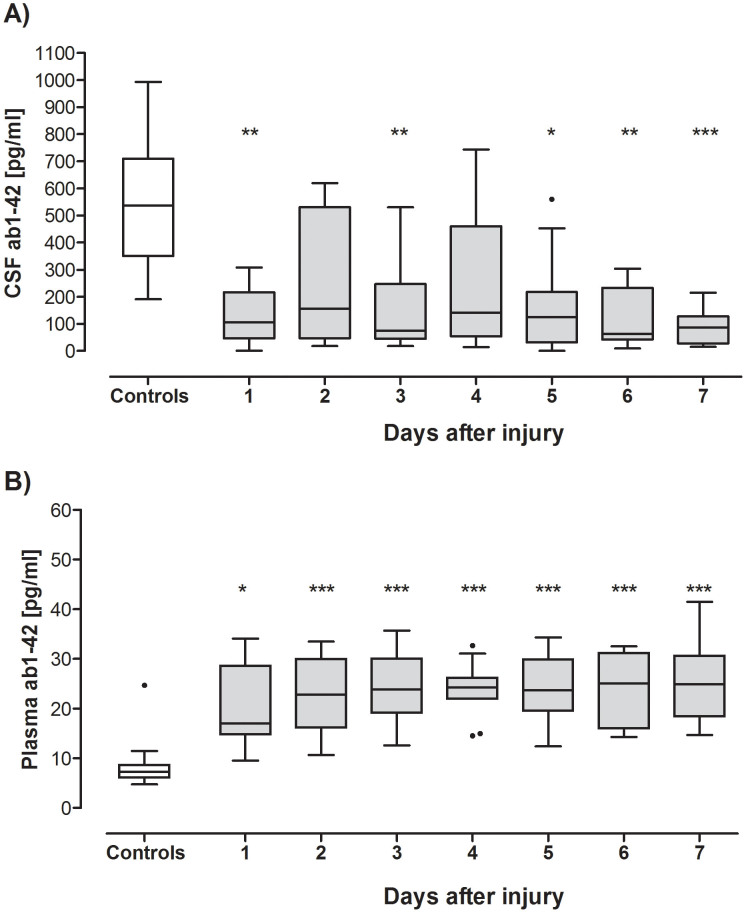
CSF Aβ42 levels were decreased in TBI patients compared with controls over the study period (Fig. 3). CSF Aβ42 concentrations were significantly lower on day 1 and 3 and from day 5 to day 7 (Fig. 3). CSF Aβ42 nadir level was on day 6 after injury (62.62 pg/mL [42.73–233.4]). Figure 3B shows daily plasma Aβ42 concentrations that, in contrast, were significantly and persistently elevated in TBI subjects compared to controls. Plasma Aβ42 levels peaked 6 days after injury (25.03 pg/mL [16.01–31.11]). Within-subjects comparison in the temporal analysis window showed that CSF and plasma Aβ42 levels did not vary significantly over the study period (p = 0.09 and p = 0.55, respectively, Friedman test). Plasma Aβ42 concentrations did not correlate with CSF Aβ42 concentrations at any of the time points examined.
Figure 3. Longitudinal CSF and plasma Aβ42 Levels in TBI patients and controls.
The concentration of CSF Aβ42 (A) was significantly decreased in TBI patients on day 1 and 3 and from day 5 to day 7 after injury compared to controls. Plasma Aβ42 (B) was significantly elevated over the study period, compared to controls. The horizontal bar represents median concentration. Significant differences are indicated with * (P < 0.05), ** (P < 0.01) or *** (P < 0.001) (p values of the post-hoc Dunn's Test for differences between the groups [TBI versus Controls]).
CSF Aβ42 Levels in Relation to Neurological Status
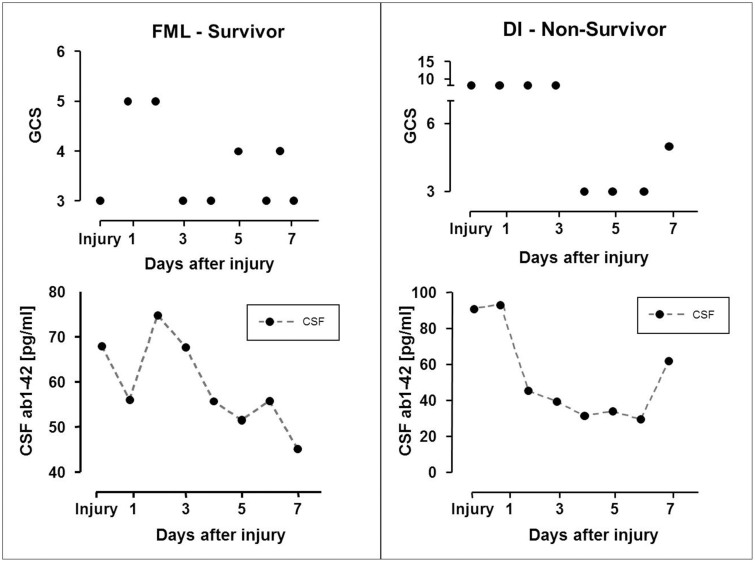
In several patients, CSF Aβ42 was associated with the global neurological status, as assessed with the Glasgow Coma Score (GCS); higher concentrations of CSF Aβ42 were associated with patient neurological status improvement, whereas reduced Aβ42 levels correlated with patient deterioration/worsening. CSF Aβ42 changes appeared to track, and in some cases even precede neurological status changes (Fig. 4).
Figure 4. CSF Aβ42 levels and neurological status.
Graph showing the time course of CSF Aβ42 concentrations and changes in neurological status, as reflected by GCS, in 2 severely brain injured patients (FML, Focal Mass Lesion; DI, Diffuse Injury).
Discussion
In this investigation, we assessed and monitored CSF and plasma Aβ42 values in matched longitudinal samples of patients with severe TBI using the SiMoA Aβ42 assay. This breakthrough technology allowed highly sensitive and precise quantification of Aβ42, improving overall diagnostic accuracy, in a small but clinically well-characterized TBI cohort.
We found that initial Aβ42 levels presented opposite dynamics in CSF and plasma of patients with severe TBI, with significant reductions in CSF Aβ42 concentrations and increases in plasma Aβ42 levels early after injury compared to controls. The reduction of CSF Aβ42 concentrations in TBI patients might suggest deposition of aggregated Aβ42 and plaque formation10 in the brain early after injury, as reported in previous neuropathological studies10,11. Nonetheless, it might also result from Aβ42 leakage across an impaired blood-brain barrier (BBB) into the blood. This later hypothesis is supported by a substantial rise of Aβ42 in plasma.
Interestingly, TBI patients who died were characterized by more marked decrease in CSF Aβ42 and highly elevated levels of plasma Aβ42 compared to controls, while CSF and plasma Aβ42 values in survivors were intermediate between these 2 groups (Fig. 2). This suggests that the magnitude of both CSF reduction and plasma elevation in Aβ42 increases with increasing brain injury severity. These findings may be explained by the fact that severely injured patients doomed to die had a more extensive BBB damage/breakdown and consequently higher Aβ42 levels entering the peripheral circulation as compared to individuals with mild injures. Supporting these observations, substantial evidence has now accumulated showing the direct influence of BBB disruption on the clinical outcome after TBI12. A sensitive marker that can predict outcome, and capture TBI-induced BBB disruption could be extremely useful; further studies assessing the relationships between TBI, BBB disruption and levels of Aβ42 are warranted.
Overall, our findings complement and extend previous results on the topic of amyloid peptides in biofluids following TBI13. Consistently with our observations, several studies have shown decreased CSF Aβ42 concentrations after TBI14,15 and an association with poor clinical outcome14. On the other hand, in a study by Olsson and colleagues16, marked increase in ventricular CSF Aβ42 and unchanged level of plasma Aβ42 were observed in patients after severe TBI. However, this discrepancy may be due to different collection protocols, lack of control group, patient characteristics and outcome, or different determination techniques. In particular, in our study the ultrasensitive digital immunoassay for quantification of the Aβ42 in plasma enables measurement of this marker at concentrations not reliably detected with prior generations of commercial assay and might explain the conflicting results with the earlier report.
Within this study, we did not found differences between the Aβ levels in patients with diffuse brain injury compared with focal TBI. This finding stands in contrast to previous microdialysis (MD) studies, which found increased interstitial fluid (ISF) Aβ42 levels in patients who sustained diffuse brain injury8,17. However, MD data are not directly comparable to CSF and plasma owing to the fact that interstitial fluid comes from a relatively restricted area of the brain and its composition depends on the microdialysis catheter location. An alternative explanation is that CT and the broad distinction between focal TBI and diffuse axonal injury (DAI) based on the Marshall classification18 may underestimate the extent of components of axonal injury in patients with predominately focal TBI19. Advanced neuroradiological tools such as MRI and ideally diffusion tensor imaging (DTI) could be appropriate approach to solve this question.
In line with previous investigations in patients with mild cognitive impairment or AD20,21, no significant correlation between the levels of Aβ42 in CSF and plasma was identified in our study. These findings might indicate a delayed release of Aβ42 into the blood after TBI. However, the relation between brain ISFAβ CSFAβ42 and plasma Aβ42 is fairly complex21 possibly involving BBB, paravascular pathways and astrocytic water transport (aquaporin4-dependent bulk flow). However, the role of such structures in the clearance of biomarkers has only recently been explored22 and will be a critical area for future investigation.
The longitudinal study showed persistent reduced levels of CSF Aβ42 and elevated levels of plasma Aβ42 over time suggesting that levels of this marker reflect and characterize pathophysiological processes that start with the primary injury, evolve over the acute period and encompass the subacute and chronic phases. It is of note that, the pattern of changes in CSF levels of this protein observed over time correlated with neurological outcome, as reported by others6.
Our study has several limitations. First, it consists of a relatively small sample size that did not allow multivariate analyses including significant clinical and demographic variables. Second, Aβ is produced by many different cells in the body20,23, therefore, increased release of Aβ42 from extracerebral origin cannot be excluded. Nonetheless, the exclusion of multiple injuries makes it likely that the changes in plasma levels of this marker fundamentally reflect the brain injury and associated BBB disruption. Third, there was a significant age difference between TBI patients and controls. However, we did not find any significant correlation between age and Aβ42 concentrations in CSF and plasma. In addition, the prognostic value of Aβ42 levels was unrelated to age. Furthermore, these findings are in agreement with those of 2 recent studies demonstrating a diagnostic and prognostic value of the Aβ42 levels in patients with acute and chronic intracerebral hemorrhage that was independent of age24,25. Finally, in future studies it might be worthwhile to analyze the influence of other relevant clinical variables, such as polytrauma, renal function and APOE ε4 status, on plasma Aβ42 concentrations.
In conclusion, our data show an opposite dynamics of Aβ42 in CSF and plasma and a stepwise decrease and increase in CSF and plasma Aβ42 concentrations, respectively, occurring with increasing severity of injury. Importantly, our work indicates for the first time a potential clinical relevance in TBI of plasma Aβ42 as detected by novel ultrasensitive digital immunoassay. Future studies that include a larger sample size will be required to validate these findings and to determine whether combined information from CSF and plasma β42 levels might be effective predictors of outcome and BBB disruption after severe TBI.
Methods
Patients
This study is part of the BANDITS (Biomarker Assessment for Neurotrauma Diagnosis and Improved Triage System) Feasibility Study, an observational study on the association between brain damage markers and demographic and clinical variables, neuroimaging, and clinical outcome of patients with severe TBI. Other biomarker analyses from this project have previously been reported elsewhere26,27. In the current study we focused on a pilot cohort of 12 patients in whom paired CSF and plasma samples were available. Severe traumatic brain injury was defined as a Glasgow Coma Score (GCS) of 8 or less on the hospital admission. Exclusion criteria were no informed consent, age < 18 years, known history of neurological and/or autoimmune disease, multiple injuries and pregnancy. All patients underwent insertion of an intracranial pressure (ICP) monitor using a ventriculostomy catheter that was placed as part of the routine medical care for patients with severe TBI. The study protocol was approved by the local ethics committee of the two sites involved (Pecs, Szeged) and by the Western Institutional Review Board (WIRB) and Human Research Protection Office (HRPO). Next of kin or legal representatives provided written informed consent for study participation. The study was conducted in accordance with the approved guidelines and regulations, in line with the tenets of the Declaration of Helsinki.
Initial computed tomography (CT) scans obtained on admission were classified according to the classification of Marshall et al. For the purpose of our analysis, Marshall score was further categorized into two groups (diffuse injury versus focal mass lesion), as previously described28. Outcome was assessed at 6 months post-injury using the Glasgow Outcome Score Extended (GOSE). Patient characteristics are shown in Table 1.
Because there are no certified reference standards for Aβ42 and values vary depending on the assay used, we included a control population consisting of 20 individuals who underwent lumbar puncture (LP) to exclude possible peripheral nervous system disorders, suspicion of subarachnoid hemorrhage or meningitis and with proven negative results. Exclusion criteria were antecedents of neurologic disease and any contraindication for lumbar puncture.
Sample Collection and Handling
In patients with severe TBI, CSF for biomarker analysis was collected on admission after the insertion of an intracranial pressure monitoring device (median 12.5 hrs, range 5–24 hrs) and daily up to 7 days. In control subjects CSF was collected by LP. Blood and CSF samples were drawn at the same time. Approximately 4–5 mL of CSF and plasma were collected from each subject at each sample point. The samples were immediately centrifuged for 10 min at 4000 rpm, frozen and stored at −80°C until assayed.
Measurement of Aβ42
Quantification of CSF and plasma Aβ42concentrations was performed at Quanterix Corporation, Cambridge, Massachusetts, USA. All samples were blinded to case identity and assayed in triplicate. Samples from individual patients were tested within a single plate. Aβ42 was measured using SiMoA technology. This method involves performing a paramagnetic bead–based ELISA, followed by isolation of individual capture beads in arrays of femtoliter-sized reaction wells29. Singulation of capture beads within microwells permits buildup of fluorescent product from an enzyme label, so that signal from a single immunocomplex can be readily detected with a CCD camera. At very low Aβ42 concentrations, Poisson statistics predict that bead containing microwells in the array will contain either a single labeled Aβ42 molecule or no Aβ42 molecules, resulting in a digital signal of either “active” or “inactive” wells. At higher Aβ42 concentrations, when all wells become occupied by at least 1 labeled Aβ42 molecule, digital measurements transition to non-digital (analog) measurements of total fluorescence intensity. With single molecule sensitivity, concentrations of labeling reagents can be lowered, resulting in reduced nonspecific background. This effect enables high signal- to-background ratios at extremely low analyte concentrations.
Arrays of femtoliter-volume wells were prepared as described. In brief, the ends of bundles of 50,000 optical fibers were polished with diamond lapping films and etched one end of each bundle in mild acid solution. Differential etch rates of the optical fiber core and cladding glass of the bundles causes 4.5 μm–diameter, 3.5 μm –deep wells to be formed, giving an array of 50 000 microwells across the bundle. Optical fiber arrays were mounted in linear groups of 8 within glass holders for bead loading and imaging, to correspond with microtiter plate columns of 8 wells, which were used as rinse troughs. Paramagnetic capture beads were comprised of a monoclonal anti-Aβ42 antibody (Covance, 6E10) directed to the N-terminus. Biotinylated detector reagent was comprised of a monoclonal anti Aβ42 antibody (Invitrogen H31L21) directed to the C-terminus. Streptavidin: β-galactosidase (SβG) was prepared by covalent conjugation of purified streptavidin (Thermo Scientific) and βG (Sigma) using standard coupling chemistry. Bead-sample incubations and labeling of immunocomplexes in conical 96 well plates (Axygen) were conducted. The assay was performed in three steps, starting with analyte capture, incubation with biotinylated detector, and labeling of the immunocomplexes with SβG. Following assay and bead collection with a magnet, beads were loaded onto the arrays for imaging in a loading buffer comprised of PBS and 0.01% Tween-20, MgCl2, and sucrose. Wells containing beads with labeled Aβ42 were visualized by the hydrolysis of enzyme substrate (resorufin β-D-galactopyranoside, RGP, Invitrogen) by βG into fluorescent product. RGP was introduced to the wells during sealing of the arrays with a silicon gasket. Enzyme-containing wells were imaged by fluorescence microscope fitted with a CCD camera. The images were analyzed to determine the average number of label enzymes/bead (AEB) as described. At <70% active beads relative to total beads (low Aβ42), the signal output is a count of active beads corrected for a low statistical probability of multiple enzymes/bead. At >70% active beads (higher Aβ42), the probability of multiple enzymes/bead increases, and average fluorescence of the wells is converted to AEB based on the average intensities of wells containing single enzymes determined at lower concentrations.
The assay was calibrated with Aβ42 obtained from Covance and a stock solution prepared by dilution to 3.5 ng/mL in PBS/Tween-20. Assay calibrators and controls were prepared by dilution of the stock solution in PBS diluent containing a surfactant and BSA (PBS/BSA). Calibrators were prepared by serial 3-fold dilution to give a calibration range of 0–250 pg/mL. Limits of detection (LoD) were estimated as three standard deviations above the zero calibrator across calibration curves on six separate days (n = 3 replicates per curve). LoDs ranged from 0.014 to 0.032 pg/mL, with an average of 0.020 pg/mL. The limit of quantification (LoQ) was estimated to be 0.038 pg/mL from repeated measurement of immunodepleted plasma, as previously described30. Assay imprecision was estimated at low levels of Aβ42 as total coefficients of variation (CV) from six days of repeated testing of three plasma samples. CVs ranged from 6.1 to 10.0% at Aβ42 concentrations of 0.5 to 4.8 pg/mL. Specificity for Aβ42 was evaluated by assaying 0.5, 1.0, 5.0, 10 and 50 pg/mL of the peptide variants Aβ38, Aβ40, and Aβ43 in PBS/BSA (Merck). No detectable cross reactivity was noted for the shorter Aβ38 and Aβ40 peptides, while the longer Aβ43 variant exhibited a cross reactivity of 11–16%. Linearity of the assay has been described previously30.
Statistical analyses
Statistical analyses were carried out using the SPSS 20.0 software package (SPSS Inc, Chicago, Illinois, USA) and JMP version 10.0 (SAS Institute, Inc, Cary, NC). Data normality was assessed. For descriptive analyses, continuous variables are presented as median and interquartile range; differences were tested using the Mann–Whitney U test. Distributions of categorical variables are presented as frequencies and percentages. The significance of differences in proportions was assessed using chi-square or Fisher's exact test where appropriate. To test for significant trends in biomarker concentrations across groups, the Jonckheere-Terpstra test for non-parametric trend analysis was used. When trends were significant (p < 0.05), pairwise between-group comparisons was applied (post-hoc Wilcoxon signed-rank test). The statistical significance of within-subject longitudinal change in CSF and plasma Aβ42 concentration was analyzed using the non-parametric Friedman test followed by post-hoc comparisons applying Dunn's test. The relation between quantitative variables was assessed by bivariate correlations (Spearman rank correlation test). All statistical tests were two-tailed. P values less than 0.05 were considered significant.
Author Contributions
S.M. performed data analysis and interpretation and drafted the manuscript. A.B. and P.B. performed clinical work and contributed to the interpretation of the results. J.R., G.P., D.H., D.W. and F.K. participated in the laboratory work. A.J. contributed to the design of the study and participated in the interpretation of the analytical results. All authors contributed substantially to the revision of the manuscript and have approved the article for publication.
Acknowledgments
This work was supported by Clinical Neuroscience Image Center of Hungarian Academy of Sciences (HAS) (SROP-4.2.2.A-11/1/KONV-2012-0017 and Hungarian Brain Research Program - Grant No. KTIA_13_NAP-A-II/8). We thank the patients and their families for their invaluable contributions.
Footnotes
Drs. Mondello, Buki, Barzo and Kobeissy declare no competing financial interests. Drs. Randall, Provuncher, Hanlon, Wilson and Jeromin are employees and receive salaries from Quanterix Corporation.
References
- Masters C. L. et al. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A 82, 4245–9 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K., Hampel H., Weiner M. & Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol 6, 131–44 (2010). [DOI] [PubMed] [Google Scholar]
- Shively S., Scher A. I., Perl D. P. & Diaz-Arrastia R. Dementia resulting from traumatic brain injury: what is the pathology? Arch Neurol 69, 1245–51 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee A. C. et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 136, 43–64 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern R. A. et al. Long-term consequences of repetitive brain trauma: chronic traumatic encephalopathy. PM R 3, S460–7 (2011). [DOI] [PubMed] [Google Scholar]
- Brody D. L. et al. Amyloid-beta dynamics correlate with neurological status in the injured human brain. Science 321, 1221–4 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnoni S. & Brody D. L. New perspectives on amyloid-beta dynamics after acute brain injury: moving between experimental approaches and studies in the human brain. Arch Neurol 67, 1068–73 (2010). [DOI] [PubMed] [Google Scholar]
- Marklund N. et al. Monitoring of beta-Amyloid Dynamics after Human Traumatic Brain Injury. J Neurotrauma (2013). [DOI] [PubMed] [Google Scholar]
- Jack C. R. Jr et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol 9, 119–28 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G. W., Gentleman S. M., Lynch A. & Graham D. I. beta A4 amyloid protein deposition in brain after head trauma. Lancet 338, 1422–3 (1991). [DOI] [PubMed] [Google Scholar]
- Strozyk D., Blennow K., White L. R. & Launer L. J. CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology 60, 652–6 (2003). [DOI] [PubMed] [Google Scholar]
- Shlosberg D., Benifla M., Kaufer D. & Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol 6, 393–403 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsitsopoulos P. P. & Marklund N. Amyloid-beta Peptides and Tau Protein as Biomarkers in Cerebrospinal and Interstitial Fluid Following Traumatic Brain Injury: A Review of Experimental and Clinical Studies. Front Neurol 4, 79 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz G. et al. Amyloid beta 1–42 and tau in cerebrospinal fluid after severe traumatic brain injury. Neurology 60, 1457–61 (2003). [DOI] [PubMed] [Google Scholar]
- Kay A. D. et al. Alterations in cerebrospinal fluid apolipoprotein E and amyloid beta-protein after traumatic brain injury. J Neurotrauma 20, 943–952 (2003). [DOI] [PubMed] [Google Scholar]
- Olsson A. et al. Marked increase of beta-amyloid((1–42)) and amyloid precursor protein in ventricular cerebrospinal fluid after severe traumatic brain injury. J Neurol 251, 870–876 (2004). [DOI] [PubMed] [Google Scholar]
- Marklund N. et al. Monitoring of brain interstitial total tau and beta amyloid proteins by microdialysis in patients with traumatic brain injury. J Neurosurg 110, 1227–37 (2009). [DOI] [PubMed] [Google Scholar]
- Marshall L. F. et al. A new classification of head injury based on computerized tomography. J Neurosurg 75 (SUPPL.) S14–S20 (1991). [Google Scholar]
- Skandsen T. et al. Prevalence and impact of diffuse axonal injury in patients with moderate and severe head injury: a cohort study of early magnetic resonance imaging findings and 1-year outcome. J Neurosurg 113, 556–63 (2010). [DOI] [PubMed] [Google Scholar]
- Mehta P. D., Pirttila T., Patrick B. A., Barshatzky M. & Mehta S. P. Amyloid beta protein 1-40 and 1–42 levels in matched cerebrospinal fluid and plasma from patients with Alzheimer disease. Neurosci Lett 304, 102–6 (2001). [DOI] [PubMed] [Google Scholar]
- Hansson O. et al. Evaluation of plasma Abeta(40) and Abeta(42) as predictors of conversion to Alzheimer's disease in patients with mild cognitive impairment. Neurobiol Aging 31, 357–67 (2010). [DOI] [PubMed] [Google Scholar]
- Iliff J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 4, 147ra111 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderstichele H. et al. Standardization of measurement of beta-amyloid(1–42) in cerebrospinal fluid and plasma. Amyloid 7, 245–58 (2000). [DOI] [PubMed] [Google Scholar]
- Marti-Fabregas J. et al. Prognostic value of plasma beta-amyloid levels in patients with acute intracerebral hemorrhage. Stroke 45, 413–7 (2014). [DOI] [PubMed] [Google Scholar]
- Hernandez-Guillamon M. et al. Plasma beta-amyloid levels in cerebral amyloid angiopathy-associated hemorrhagic stroke. Neurodegener Dis 10, 320–3 (2012). [DOI] [PubMed] [Google Scholar]
- Mondello S., Buki A., Italiano D. & Jeromin A. alpha-Synuclein in CSF of patients with severe traumatic brain injury. Neurology (2013). [DOI] [PubMed] [Google Scholar]
- Mondello S. et al. Neuronal and glial markers are differently associated with computed tomography findings and outcome in patients with severe traumatic brain injury: a case control study. Crit Care 15, R156 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raabe A. et al. Correlation of computed tomography findings and serum brain damage markers following severe head injury. Acta Neurochir 140, 787–792 (1998). [DOI] [PubMed] [Google Scholar]
- Rissin D. M. et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol 28, 595–9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg H. et al. Hypoxia due to cardiac arrest induces a time-dependent increase in serum amyloid beta levels in humans. PLoS One 6, e28263 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]