Abstract
Here we report the cloning and functional characterization of the cyclin D-dependent kinase 4 and 6 (Cdk4/6) inhibitory protein Cdkn2d/p19Ink4d of Xenopuslaevis (Xl-Ink4d). Xl-Ink4d is the only Ink4 family gene highly expressed during Xenopus development and its transcripts were detected maternally and during neurulation. The Xl-Ink4d protein has 63% identity to mouse and human Cdkn2d/p19Ink4d and its function as a negative regulator of cell cycle traverse is evolutionary conserved. Indeed, Xl-lnk4d can functionally substitute for mouse Cdkn2d in binding to mouse Cdk4 and inhibiting cyclin-D1-dependent CDK4 kinase activity. Further, enforced expression of Xl-lnk4d arrests mouse fibroblasts in the G1 phase of the cell cycle. These findings indicate that CDKN2d/p19Ink4d is conserved through vertebrate evolution and suggest Xl-lnk4d may contribute to the development of Xenopuslaevis.
Keywords: Xenopuslaevis, Cyclin-dependent kinase inhibitor, Ink4d, Cdkn2d, Cell cycle
Introduction
Regulation of the cell cycle is intimately involved in development, where cell cycle arrest is coordinately controlled with terminal differentiation. Progression through the cell cycle is driven by cyclin-dependent kinases (Cdks) and their obligate binding partners, the cyclins. Cyclin/Cdk complexes are regulated by two families of Cdk inhibitory proteins (CKIs), the Cip/Kip family (Cdkn1) and the Inhibitors of Cdk4 (Ink4) family (Cdkn2) [1]. Enforced expression of CKIs induces cell cycle arrest by binding to and inhibiting cyclin-dependent Cdk activity. Specifically, Cip/Kip family members negatively regulate cyclin E-Cdk2, cyclin A-Cdk2, and cyclin B-Cdk1 complexes, whereas Ink4 proteins bind to and inhibit Cdk4 and Cdk6 kinases, by preventing their binding to D-type cyclins [1]. In turn, the retinoblastoma tumor suppressor protein Rb cannot be phosphorylated, and this prevents the release of E2F transcription factors that regulate the expression of genes that are necessary for entry and progression through the DNA synthetic (S) phase of the cell cycle [2].
Orthologues of Ink4 genes are conserved throughout vertebrate evolution. Fugu rubripes, the most evolutionary distant organism analyzed, harbors two Ink4 genes; CDKN2b/Ink4b and CDKN2d/Ink4d [3]. In the amphibian species Xenopustropicalis, three Ink4 genes have been annotated in the draft genome sequence, CDKN2b/Ink4b, CDKN2c/Ink4c and CDKN2d/Ink4d (Joint Genome Institute, genome. jgi-psf.organd www.metazome.net). These three Ink4 orthologues are likely conserved in X. laevis because of the high degree of similarity between the two Xenopus species. In mammals there are four Ink4 genes, CDKN2a/p16Ink4a, CDKN2b/p15Ink4b, CDKN2c/p18Ink4c and CDKN2d/p19Ink4d [4–7].
In mice, Cdkn2d/p19Ink4d and Cdkn2c/p18Ink4c are the only two Ink4 family members expressed during embryonic development, where p19Ink4d is first detected at embryonic day (E) 11.5 and where p18Ink4c expression initiates at E13.5 [8,9]. Targeted deletion of either or both genes does not disrupt mouse development; thus, alone neither is essential for embryogenesis [10–12]. However, p19Ink4d and p18Ink4c are required to regulate cell cycle arrest and quiescence in specific cell contexts. For example, loss of Ink4c and Ink4d in the mouse induces male sterility due to a block in meiosis-I [12]. In the Central Nervous System (CNS) Ink4d-null mice progressively lose hearing due to cell cycle re-entry of sensory hair cells within the organ of Corti, followed by apoptosis [13]. Moreover, p19Ink4d is required for proper mouse tooth development [14,15]. Further, Ink4d and Cdkn1b/p27Kip1 together are necessary to maintain cerebral cortex neurons and retinal progenitor cells in a post-mitotic state, and for postnatal survival [16,17]. In the mouse, p18Ink4c is induced during myogenic differentiation [18], is transiently expressed in granule neuron progenitors to time their exit from the cell cycle [19], and is required to maintain the hematopoietic stem cell progenitor pool [20]. Thus, in mice both p19Ink4d and p18Ink4c contribute to the induction and/or maintenance of a post-mitotic state in differentiated tissues.
Here we evaluated the expression and function of Ink4 genes in early Xenopuslaevis development. Only one Ink4 gene is highly expressed during Xenopus development and this encodes a protein, Xl-Ink4d, that was highly similar to the mouse orthologuep 19Ink4d, where Xl-Ink4d can bind to and inhibit mouse Cdk4 kinase activity on Rb, and is sufficient to provoke G1 arrest in mouse fibroblasts.
Materials and Methods
Cloning of Xenopus Ink4 genes
A 139 base pair fragment was amplified from Xenopuslaevis genomic DNA by PCR using published degenerate oligonucleotide primers predicted to amplify the first 139 bp region of exon 2 of Ink4-like genes [3]. This fragment was used as a probe to screen a Xenopuslaevis adult spleen library (Lamda Zap Express) using standard methods [21]. Several clones were obtained and sequenced; however, only a single Ink4c DNA sequence was found (noted Xl-Ink4d1) that was similar to mouse and human Cdkn2d/p19Ink4d. Sequence analysis was performed using the DNA star software package (Lazergene).
Production of GST-Xl-Ink4d and GST-Mm-Ink4d fusion proteins and Cdk4 binding assays
The Xl-Ink4d1 and mouse Ink4d(Mm-Ink4d) coding sequences were cloned in frame with an N-terminal GST-tag into pGEX-5X-1 and pGEX-2T, respectively (Amersham). The pGEX plasmids were transformed into BL21-D bacteria and their expression was induced with Isopropyl β-D-Thiogalactoside (IPTG) (0.1 mM) for 2 hour according to the manufacturer’s instructions. GST-tagged proteins were purified using glutathione-sepharose (Amersham) according to the manufacturer’s instructions. The coding sequence of Xenopuslaevis Cdk4 (Xl-Cdk4) was cloned into pCMVTNT™ (Promega) and transcribed and translated in the presence of [35S]-methionine using TNT® Coupled Reticulocyte Lysate Systems according to the manufacturer’s instructions (Promega).
GST pull down assays were performed as described [22]. Briefly, in vitro transcribed and translated Xl-Cdk4 (20 μl) was incubated with 1 μg of purified GST, GST-Xl-Ink4d1 (Xenopus), or GST-Mm-Ink4d (mouse) proteins immobilized on glutathione sepharose. The mixture was incubated at 4°C for 2 hour and washed several times in IP kinase buffer (50 mM HEPES pH 7.5, 10 mM MgCl2, 1 mM DTT, 2.5 mM EGTA, 10 mM β-glycerophosphate, 0.1 mM sodium orthovanadate, 1 mMNaF). Bound proteins were denatured and separated on a 12% (w/v) polyacrylamide-SDS gel and visualized by autoradiography [23].
In vitro kinase assays
In vitro Cdk4 kinase assays were performed as described [22,24], with minor modifications. Briefly, Spodoptera frugiperda Sf9 cells were infected with baculo viruses encoding mouse Cdk4 and cyclin D1. Lysates from these cells were immuno-precipitated with Protein A-Sepharose pre-adsorbed to a Cdk4 antibody (C-22, Santa Cruz Biotechnology). After overnight incubation at 4°C, increasing amounts of GST-Xl-Ink4d1, GST-Mm-Ink4d, or GST proteins were added to the reactions and incubated for 2 hour at 4°C. Immuno-precipitations were washed in IP kinase buffer (50 mM HEPES pH 7.5, 10 mM MgCl2, 1 mM DTT, 2.5 mM EGTA, 10 mM β-glycerophosphate, 0.1 mM sodium orthovanadate, 1 mMNaF) and in vitro kinase assays were performed using GST-Rb as substrate and [γ-32P]-ATP. The reactions were resolved by electrophoresis on 12.5% (w/v) poly-acrylamide-SDS gels, and analyzed by autoradiography [23].
Virus infection and cell cycle analysis
Retroviruses were generated as described [25], including control MSCV-IRES-GFP virus as well as a MSCV-Xl-Ink4d1-IRES-GFP and MSCV-Mm-Ink4d-IRES-GFP viruses. NIH-3T3 mouse fibroblast cells were infected with retroviruses and cultured for 24 hour. Cells were trypsinized, permeabilized and stained with propidium iodide to stain DNA. The DNA content of GFP-positive cells was measured by fluorescence-activated cell sorting (FAC scaliber) and the data were analyzed using Cell quest software (Becton Dickinson).
Xenopus embryo manipulations
Xenopuslaevis embryos were obtained, fertilized and microinjected as described [26]. Briefly, female frogs were induced to lay eggs by gonadotropin injection, fertilized in vitro with macerated testis and de-jellied with 3% (w/v) cysteine hydrochloride. Embryos were staged according to the Xenopuslaevis normal tables of development[27].
Reverse transcription PCR and quantitative RT-PCR
Total RNA was isolated from Xenopus embryos at stages 2–41 using Qiashredder and Qiaeasy RNA isolation kit (Qiagen). RNA was reverse transcribed with Superscript II polymerase primed with oligod T and PCR amplified with Hot Star Taq DNA polymerase (Qiagen). For RT-PCR trace [α32P]-dCTP was included in the reaction to allow detection of the PCR product by autoradiography. Reactions were separated on pre-cast 10% (w/v) polyacrylamide Tris–Borate–EDTA gels (Bio-Rad), fixed, dried and exposed to X-ray film. For relative quantitative RT-PCR, reactions were performed on an Cycler thermocycler using iQ SYBR Green Supermix (Bio-Rad) and primers for Ink4d1 and ODC (as an internal control). ODC CT values were subtracted from Ink4d1 CT values (ΔCT) to normalize for input cDNA. Relative RNA levels were calculated by subtracting the ΔCT stage40 from ΔCT (ΔΔCT) and using the calculation 2−ΔΔCT. Primers used to amplify Xenopus Ink4d1(forward; 5′-TTGTAGGGATGCACGGAATC-3′, reverse; 5′-ATGAACCGAATCCTTTGCAC-3′), Ink4d-Q (quantitative) (forward; 5′-TCCTGTCATTACCTTCCTTGCCCT-3′, reverse; 5′-TGGACAAGGTTGGGTGTTTCCTCT-3′), Cyclin-D1 (forward; 5′-ATCTGGACAGGAACCTCATCACG-3′, reverse; 5′-GGACTCAATCTGTTCTTGGCACG-3′) [28], CDK4 (forward; 5′-CACTGTGACCG ACGAAAGAT-3′, reverse; 5′-TTCCGTGGATCCCTAGTGG-3′), and ODC(forward; 5′-GTCAA TGATGGAGTGTATGGATC-3′, reverse; 5′-CCATTCCGCTCTCCTGAGCAC-3′).
Immunoblotting
Embryos were lysed in ice-cold lysis buffer (120 mM NaCl, 50 mM Tris-HCl [pH 8.0], 0.5% NP-40, 1 mM EDTA, and Complete protease inhibitors [Roche]). Lysates were cleared of lipid and yolk by Freon extraction (http://spot.colorado.edu/~klym/) and protein concentration was determined using a BCA Protein Assay Kit (Pierce). Equal amount of protein was resolved on 15% (w/v) polyacrylamide-SDS gels and transferred onto nitrocellulose membranes. To detect Xl-Ink4d protein, we raised a rabbit polyclonal antibody to the C-terminal peptide of Xl-Ink4d1 (amino acid sequence: SQLAAILDPRLASIFELST) and affinity-purified the antibody using the same peptide. This peptide is unique to the predicted Xenopus Xl-Ink4d protein and its sequence is shared between Xl-Ink4d1, Xl-Ink4d2 and the Ink4d of X-Tropical is but not those of Fugu, Mouse or Human. Thus the antibody does not cross react with mouse p19Ink4d protein (negative data not shown). Membranes were probed with an antibody against α-tubulin (Sigma) as a control for protein loading. Goat anti-rabbit HRP-conjugated secondary antibodies (Amersham) and Super Signal Dura (Pierce) were used to develop the blots.
In situ hybridization of whole embryos
In situ hybridization was performed as described [26]. Anti-sense probes were generated as described by Kelley et al. [29]. Briefly, anti-sense probes were synthesized with digoxigenin-coupled UTP (Roche) and detected with alkaline phosphatase coupled to anti-digoxigenin Fab fragments (Roche) followed by the chromogenic reaction with NBT/BCIP (Vectstain).
Results and Discussion
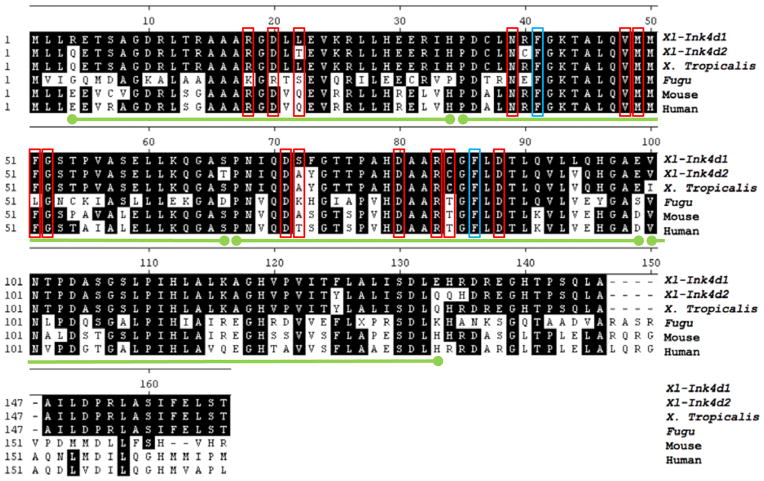
We identified a cDNA, Xl-Ink4d1 that was most similar to mouse p19Ink4d (Mm-Ink4d). Blast search of the NCBI EST database using the Xl-Ink4d1 sequence identified a second allele (Xl-Ink4d2) and the predicted amino acid sequences of these proteins was compared to the Ink4d proteins from other species (Figure 1). Alignment of the Ink4d proteins from Homo sapiens (human), Musmusculus (mouse), Xenopustropicalis, and Fugurubripes showed a high degree of amino acid conservation (63% amino acid identity between mouse and Xenopus Ink4d), suggesting a conserved function for this gene throughout evolution.
Figure 1.
Predicted amino acid sequence alignments of Ink4d proteins. The predicted amino acid sequences of Ink4d proteins from Xenopus tropicalis, Fugu (Fugu rubrides), mouse (Mus musculus) and human (Homo sapiens) were compared to the two Ink4d proteins of Xenopus laevis. The Xl-Ink4d1 cDNA was isolated from a library made from Xenopus laevis adult spleen, whereas Xl-Ink4d2 was identified by blasting the NCBI database for similar expressed sequences in Xenopus laevis and represents a second allele of Ink4d. Xl-Ink4d1 protein shares 63% amino acid identity with both the human and mouse Ink4d protein. The predicted four ankyrin repeats (green lines) as well as many of the amino acids that make contacts with CDK6 are conserved between mammals and Xenopus (red box = hydrogen bond interaction, blue box = non-polar interaction).
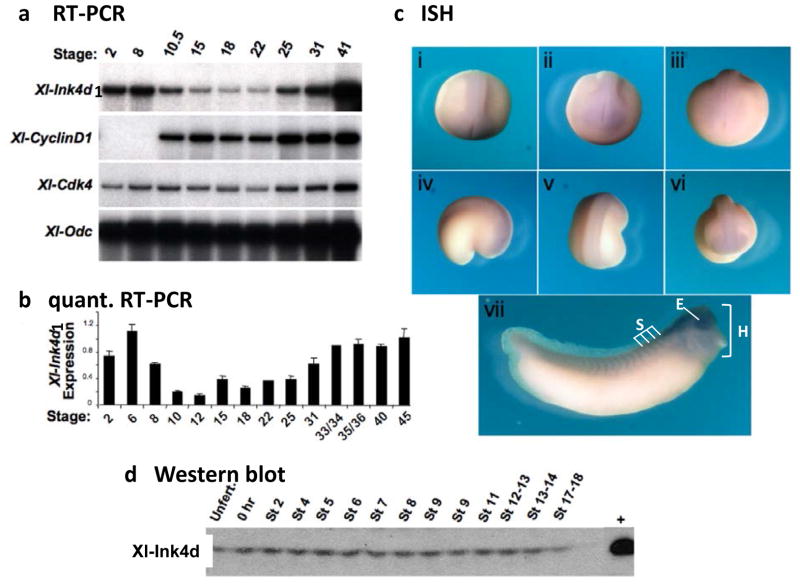
Two Ink4 genes are expressed during mouse embryogenesis, Ink4c and Ink4d, whereas Ink4a and Ink4b are only expressed in adult and aging animals [8,9]. As three Ink4 genes were identified in Xenopustropicalis, we evaluated their expression by RT-PCR and real time PCR in developmentally staged Xenopuslaevis embryos (Figure 2a and 2b). Xl-Ink4d1 transcripts were readily detected by RT-PCR and real time PCR indicating high-levels of this transcript during early development (Figure 2a and 2b). Xl-Ink4b transcripts were detected only by real-time PCR and their detection required five additional amplification cycles compared to that of Xl-Ink4d1 mRNA; thus, significantly lower levels of Xl-Ink4bvs. Xl-Ink4d1 are expressed during early Xenopuslaevis development. Moreover, Xl-Ink4c was undetectable by RT-PCR at any stage of embryonic development (not shown). This finding is in accord with the lack ofXl-Ink4c transcripts in publically available Xenopus tropicalis embryonic tissue EST libraries (Unigene (www.ncbi.nih.gov/Unigene) and in the Gurdon Institute Xenopus tropicalis EST database (http://informatics.gurdon.com.ad.uk)). Collectively, these data indicate that Xl-Ink4d1 is the predominant Ink4 gene expressed during Xenopus development.
Figure 2.
Expression of Xl-Ink4d during Xenopus laevis development. Embryos were staged according Xenopus laevis normal tables of development [27] and RNA was harvested at the indicated stages. (a) Reverse transcription PCR was performed on total RNA from staged embryos to amplify Xl-Ink4d1, cyclin-D1, Cdk4 and ODC transcripts. Trace [α32P]-dCTP was added to the reactions and the PCR products were separated by gel electrophoresis. (b) Relative quantitative real-time PCR analysis of Xl-Ink4d1 mRNA levels was performed using iQ SYBR Green Supermix (Bio-Rad), run and detected using an iCycler thermocycler (Bio-Rad). (c) In situ hybridization (ISH) for Xl-Ink4d1 expression during early embryonic development. Xl-Ink4d1 expression is detected at low levels in the dorsal anterior region of the developing tadpole. Stage 19 (i–iii); (i) dorsal view, (ii) anterior view, (iii) posterior view. Stage 22 (iv–vi); (iv) lateral view, (v) dorsal view, (vi) anterior view. Stage 32 (vii), trunk somites (S), eye (E) and head (H). (d) Immunoblotting of Xl-Ink4d from whole embryo lysates at the indicated stages using a polyclonal antibody directed against the C-terminus of the protein. Embryos microinjected with synthesized Xl-Ink4d1 mRNA were harvested and run as a positive control (+).
The Xl-Ink4d1 transcript was maternally expressed in the egg and was evident before the mid-blastula transition (MBT, stage 8) when zygotic transcription begins. Xl-Ink4d1 transcript levels decreased at the beginning of gastrulation (stage 10.5), remained low through neurulation (stage 22) and then increased at the end of neurulation (stage 25) (Figure 2a and 2b). In situ hybridization performed with in vitro transcribed antisense riboprobes revealed low levels of Xl-Ink4d1 expression in the dorsal anterior region of the developing tadpole (Figure 2c). At neurula stages, Xl-Ink4d1 was expressed in the neural plate (Figure 2c i–vi). By the late tail bud stage (stage 32a) Xl-Ink4d1 expression was most prevalent in the brain, somite field, and eye and throughout the head (Figure 2c vii). In situ analysis using a probe that selectively detects the Xl-Ink4d2 allele established that its expression was identical to that of Xl-Ink4d1 indicating that expression of the two alleles is regulated in a similar manner (data not shown). Interestingly, mouse p19Ink4d is expressed in the brain, spinal cord and dorsal root ganglia at embryonic day 13.5[9], suggesting a conserved function for Ink4d in neural development.
A rabbit polyclonal antibody generated against the C-terminus of Xl-Ink4d1, which is conserved between Xl-Ink4d1 and Xl-Ink4d2, detected the Xenopus protein by immuno-blotting with high specificity (Figure 2d). Immunoblotting of whole embryo extracts from staged embryos demonstrated equal levels of Xl-Ink4d protein in the unfertilized egg and through early development (up to stage 18) indicating that although mRNA levels fluctuated during development the protein levels did not change.
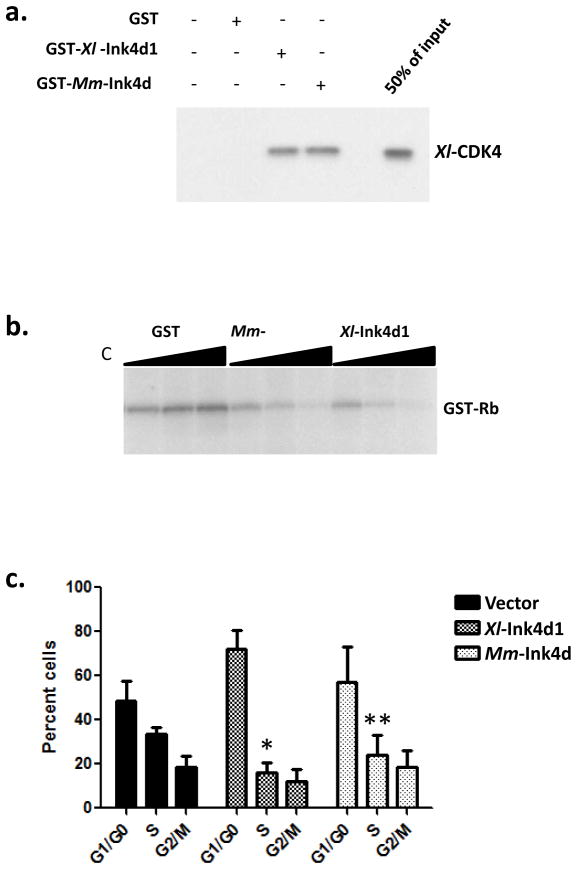
To determine if Xl-Ink4d functioned in a manner akin to that of mouse p19Ink4d, we tested its activity in a series of in vitro and ex vivo experiments. Mammalian Ink4 proteins inhibit the kinase activity of Cdk4 by binding to Cdk4 and preventing its interactions with D-type cyclins [30]. We assessed the binding of Xl-Ink4d1, and of mouse Ink4d (Mm-Ink4d), to Xenopuslaevis Cdk4 (Xl-Cdk4) by GST-pull down experiments (Figure 3a). Both GST-Xl-Ink4d1 and GST-Mm-Ink4d fusion proteins bound to Xl-Cdk4, whereas, as expected, GST alone failed to interact with Xl-Cdk4 (Figure 3a).
Figure 3.
Xl-Ink4d is a cyclin D-dependent Cdk4 inhibitor. (a) The indicated GST fusion proteins were expressed in bacteria and purified on glutathione sepharose. The adsorbed sepharose was incubated with in vitro transcribed and translated [35S]-labeled Xenopus Cdk4 (Xl-Cdk4), washed and separated on an SDS-polyacrylamide gel. 50% of the input Xl-Cdk4 that was used for pull-downs was run to estimate the percent of starting material that bound to the GST fusion protein (50% input). (b) Cdk4 kinase reactions were run with increasing amounts of purified GST-Xl-Ink4d1 and GST-Mm-Ink4d (mouse Ink4d) fusion proteins. Active mouse Cdk4/cyclinD1 kinase complexes were generated from SF9 cells infected with baculoviruses encoding mouse Cdk4 and cyclin-D1. Kinase assays were performed with [γ-32P]-ATP using purified GST-Rb as substrate. (c) The cell cycle profile of NIH-3T3 mouse fibroblasts overexpressing vector-alone (black bars), Xenopus Xl-Ink4d1 (dark grey bars) or mouse Mm-Ink4d (light grey bars). NIH-3T3 cells were infected with retroviruses that co-express the indicated Ink4d genes and GFP, and the DNA content of GFP-positive cells was measured by propidium iodide staining and FACS analysis. The percentage of cells in G1/G0, S and G2/M phase is presented. Expression of either Xl-Ink4d1 (* p=0.006) or Mm-Ink4d (** p=0.05) shows a significant decrease in S phase compared to vector alone.
Cdk4 phosphorylates and inactivates the function of the Rb tumor suppressor [1]. To test the ability of the Xl-Ink4d1 protein to inhibit Cdk4 kinase activity, mouse Cdk4-cyclin-D1 complexes were immuno-precipitated from Sf9 insect cell lysate that were co-infected with baculoviruses expressing mouse cyclin-D1 and Cdk4 [31], and these complexes were then incubated with increasing concentrations of the purified GST-Ink4d1 fusion proteins. Both Xl-Ink4d1 and Mm-Ink4d inhibited Rb phosphorylation in a dose-dependent fashion (Figure 3b). Therefore, Xl-Ink4d1 can inhibit the kinase activity of mouse cyclin-D1/Cdk4 complexes.
Cdk4 kinase activity drives cell cycle progression through the G1 phase of the cell cycle and inhibition of Cdk4 activity by overexpression of mouse Ink4d arrests cells in this phase [22]. To test if Xl-Ink4d1 could also arrest mouse fibroblasts in G1 phase we infected NIH-3T3 mouse fibroblasts with retroviruses encoding Xl-Ink4d1 or Mm-Ink4d under the control of the MSCV promoter together with GFP, which is expressed from the same transcript through an Internal Ribosomal Entry Site (IRES) present in the MSCV-IRES-GFP retroviral vector [32]. Infected cells were analyzed 32 hours after infection by Fluorescence Activated Cell Sorting (FACS) and the DNA content of GFP-positive cells was determined by propidium iodide staining. Overexpression of either Xl-Ink4d1 or Mm-Ink4d significantly reduced the percentage of cells in S phase compared to cells infected with control retrovirus (from 34% to 16% and 24%, respectively) and led to corresponding increases in the percentage of cells in G1 phase (from 49% to 72% and 57%, respectively), indicative of an accumulation of cells arrested in G1 (Figure 3c). Therefore, the Xenopuslaevis Xl-Ink4d1 protein can functionally substitute for the mouse Mm-Ink4d protein by binding to mouse Mm-Cdk4, inhibiting its cyclin D-dependent kinase activity and arresting mouse fibroblasts in G1 phase.
Altogether, our data demonstrate that Xl-Ink4d1 is a bona fide Xenopus orthologue of mammalian p19Ink4d that is remarkably conserved throughout evolution. This is underscored by the facts that, similar to mouse Mm-Ink4d, Xl-Ink4d1 is expressed in neural regions during embryonic development and that Xl-Ink4d1 can substitute for mouse Mm-Ink4d in regulating the G1 phase of the cell cycle of mouse fibroblasts. The high levels of Ink4d expression during Xenopuslaevis development in neuronal tissues suggests that, akin to its mammalian homolog [16,17], Xl-Ink4d serves an important function in Xenopus embryonic brain development, and this deserves further examination.
Acknowledgments
We thank the SJCRH Flow Cytometry Laboratory for cell cycle analysis, the Hartwell Center for Bioinformatics and Biotechnology at SJCRH for sequencing, primer synthesis and bioinformatics support, and Dr. Robert G. Hawley for kindly providing the MSCV-IRES-GFP retroviral vector. This work was funded in part by NIH/NCI grant CA-71907, CA-096832 (MFR), NIH/NIDDK grant DK-44158 (JLC), Cancer Center Core Grant CA-21765 (MFR, PEM), by monies from the State of Florida to Scripps Florida (JLC) and by the American Lebanese Syrian Associated Charities (ALSAC) of St. Jude Children’s Research Hospital.
References
- 1.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 2.Sherr CJ. The Pezcoller lecture: cancer cell cycles revisited. Cancer Res. 2000;60:3689–3695. [PubMed] [Google Scholar]
- 3.Gilley J, Fried M. One INK4 gene and no ARF at the Fugu equivalent of the human INK4A/ARF/INK4B tumour suppressor locus. Oncogene. 2001;20:7447–7452. doi: 10.1038/sj.onc.1204933. [DOI] [PubMed] [Google Scholar]
- 4.Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 5.Hannon GJ, Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994;371:257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 6.Guan KL, Jenkins CW, Li Y, Nichols MA, Wu X, et al. Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function. Genes Dev. 1994;8:2939–2952. doi: 10.1101/gad.8.24.2939. [DOI] [PubMed] [Google Scholar]
- 7.Okuda T, Hirai H, Valentine VA, Shurtleff SA, Kidd VJ, et al. Molecular cloning, expression pattern, and chromosomal localization of human CDKN2D/INK4d, an inhibitor of cyclin D-dependent kinases. Genomics. 1995;29:623–630. doi: 10.1006/geno.1995.9957. [DOI] [PubMed] [Google Scholar]
- 8.Zindy F, Quelle DE, Roussel MF, Sherr CJ. Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene. 1997;15:203–211. doi: 10.1038/sj.onc.1201178. [DOI] [PubMed] [Google Scholar]
- 9.Zindy F, Soares H, Herzog KH, Morgan J, Sherr CJ, et al. Expression of INK4 inhibitors of cyclin D-dependent kinases during mouse brain development. Cell Growth Differ. 1997;8:1139–1150. [PubMed] [Google Scholar]
- 10.Franklin DS, Godfrey VL, Lee H, Kovalev GI, Schoonhoven R, et al. CDK inhibitors p18(INK4c) and p27(Kip1) mediate two separate pathways to collaboratively suppress pituitary tumorigenesis. Genes Dev. 1998;12:2899–2911. doi: 10.1101/gad.12.18.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zindy F, van Deursen J, Grosveld G, Sherr CJ, Roussel MF. INK4d-deficient mice are fertile despite testicular atrophy. Mol Cell Biol. 2000;20:372–378. doi: 10.1128/mcb.20.1.372-378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zindy F, den Besten W, Chen B, Rehg JE, Latres E, et al. Control of spermatogenesis in mice by the cyclin D-dependent kinase inhibitors p18(Ink4c) and p19(Ink4d) Mol Cell Biol. 2001;21:3244–3255. doi: 10.1128/MCB.21.9.3244-3255.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen P, Zindy F, Abdala C, Liu F, Li X, et al. Progressive hearing loss in mice lacking the cyclin-dependent kinase inhibitor Ink4d. Nat Cell Biol. 2003;5:422–426. doi: 10.1038/ncb976. [DOI] [PubMed] [Google Scholar]
- 14.Han J, Ito Y, Yeo JY, Sucov HM, Maas R, et al. Cranial neural crest-derived mesenchymal proliferation is regulated by Msx1-mediated p19(INK4d) expression during odontogenesis. Dev Biol. 2003;261:183–196. doi: 10.1016/s0012-1606(03)00300-2. [DOI] [PubMed] [Google Scholar]
- 15.Zhao M, Gupta V, Raj L, Roussel M, Bei M. A network of transcription factors operates during early tooth morphogenesis. Mol Cell Biol. 2013;33:3099–3112. doi: 10.1128/MCB.00524-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zindy F, Cunningham JJ, Sherr CJ, Jogal S, Smeyne RJ, et al. Postnatal neuronal proliferation in mice lacking Ink4d and Kip1 inhibitors of cyclin-dependent kinases. Proc Natl Acad Sci U S A. 1999;96:13462–13467. doi: 10.1073/pnas.96.23.13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunningham JJ, Levine EM, Zindy F, Goloubeva O, Roussel MF, et al. The cyclin-dependent kinase inhibitors p19(Ink4d) and p27(Kip1) are coexpressed in select retinal cells and act cooperatively to control cell cycle exit. Mol Cell Neurosci. 2002;19:359–374. doi: 10.1006/mcne.2001.1090. [DOI] [PubMed] [Google Scholar]
- 18.Franklin DS, Xiong Y. Induction of p18INK4c and its predominant association with CDK4 and CDK6 during myogenic differentiation. Mol Biol Cell. 1996;7:1587–1599. doi: 10.1091/mbc.7.10.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uziel T, Zindy F, Xie S, Lee Y, Forget A, et al. The tumor suppressors Ink4c and p53 collaborate independently with Patched to suppress medulloblastoma formation. Genes Dev. 2005;19:2656–2667. doi: 10.1101/gad.1368605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan Y, Shen H, Franklin DS, Scadden DT, Cheng T. In vivo self-renewing divisions of haematopoietic stem cells are increased in the absence of the early G1-phase inhibitor, p18INK4C. Nat Cell Biol. 2004;6:436–442. doi: 10.1038/ncb1126. [DOI] [PubMed] [Google Scholar]
- 21.Deconinck AE, Mead PE, Tevosian SG, Crispino JD, Katz SG, et al. FOG acts as a repressor of red blood cell development in Xenopus. Development. 2000;127:2031–2040. doi: 10.1242/dev.127.10.2031. [DOI] [PubMed] [Google Scholar]
- 22.Hirai H, Roussel MF, Kato JY, Ashmun RA, Sherr CJ. Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6. Mol Cell Biol. 1995;15:2672–2681. doi: 10.1128/mcb.15.5.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harlow E, Lane D. Using Antibodies: A Laboratory Manual. Cold Sprin Harbor Laboratory Press; 1999. [Google Scholar]
- 24.Matsushime H, Quelle DE, Shurtleff SA, Shibuya M, Sherr CJ, et al. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zindy F, Eischen CM, Randle DH, Kamijo T, Cleveland JL, et al. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sive HL, Grainger RM, Harland RM. Early Development of Xenopus laevis: a laboratory manual. Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- 27.Nieuwkoop PD, Faber J. Normal Table of Xenopus Laevis (Daudin): A Systematical and Chronological Survey of the Development from the Fertilized Egg Till the End of Metamorphosis. Garland Pub, INC; 1994. [Google Scholar]
- 28.Tanaka T, Kubota M, Shinohara K, Yasuda K, Kato JY. In vivo analysis of the cyclin D1 promoter during early embryogenesis in Xenopus. Cell Struct Funct. 2003;28:165–177. doi: 10.1247/csf.28.165. [DOI] [PubMed] [Google Scholar]
- 29.Kelley C, Yee K, Harland R, Zon LI. Ventral expression of GATA-1 and GATA-2 in the Xenopus embryo defines induction of hematopoietic mesoderm. Dev Biol. 1994;165:193–205. doi: 10.1006/dbio.1994.1246. [DOI] [PubMed] [Google Scholar]
- 30.Harper JW, Elledge SJ. Cdk inhibitors in development and cancer. Curr Opin Genet Dev. 1996;6:56–64. doi: 10.1016/s0959-437x(96)90011-8. [DOI] [PubMed] [Google Scholar]
- 31.Kato J, Matsushime H, Hiebert SW, Ewen ME, Sherr CJ. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993;7:331–342. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- 32.Hawley RG, Lieu FH, Fong AZ, Hawley TS. Versatile retroviral vectors for potential use in gene therapy. Gene Ther. 1994;1:136–138. [PubMed] [Google Scholar]